EFFECTS OF PRIMERS CONTAINING SULFUR AND PHOSPHATE MONOMERS










- Slides: 28


EFFECTS OF PRIMERS CONTAINING SULFUR AND PHOSPHATE MONOMERS ON BONDING TYPE IV GOLD ALLOY Yohsuke Taira , Kohji Kamada Department of Applied Prosthodontics, Graduate School of Biomedical Sciences, Nagasaki University, 1 -7 -1 Sakamoto, Nagasaki 852 -8588, Japan Journal of Dentistry, Volume 36, Issue 8, August 2008 http: //www. sciencedirect. com/science? _ob=Article. URL&_udi=B 6 T 86 -4 SJR 2 KF 1&_user=3931974&_rdoc=1&_fmt=&_orig=search&_sort=d&view=c&_acct=C 000054426&_version=1&_url. Version=0&_userid=3931 974&md 5=1402 fc 035 d 5855626 b 9 ea 53 faa 0 c 836 a

Au-Cu-Ag • Resin-bonded fixed partial denture • Suitable biocompatibility • Excellent corrosion resistance • Easy procedure


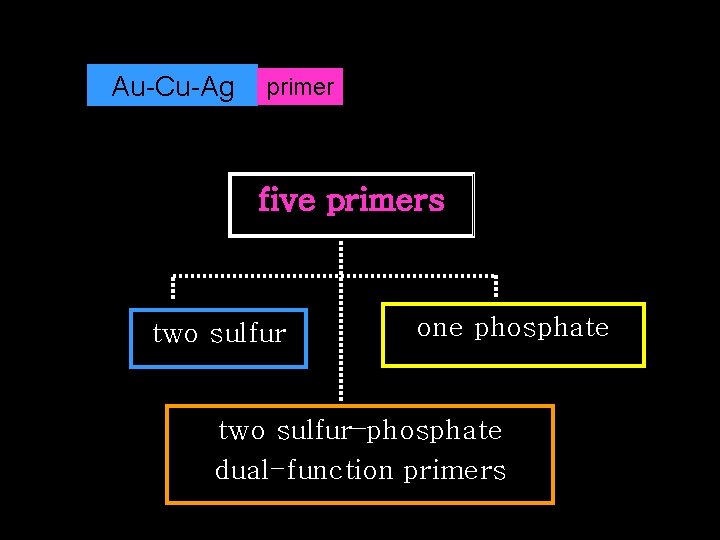
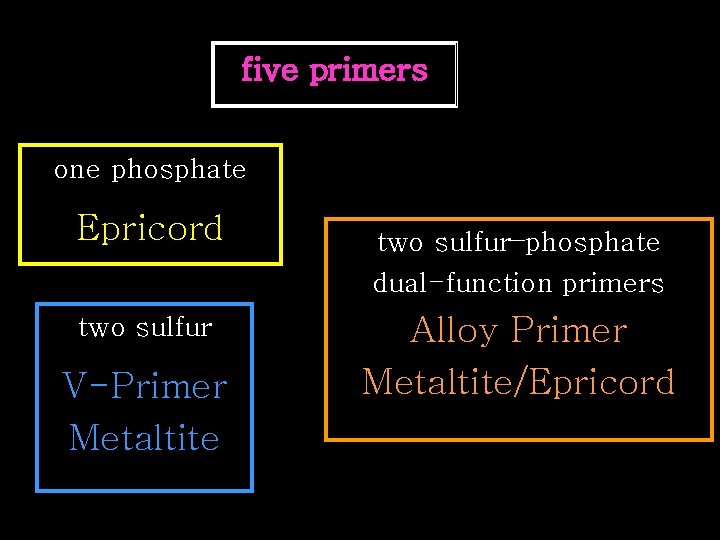
Au-Cu-Ag primer five primers two sulfur one phosphate two sulfur–phosphate dual-function primers

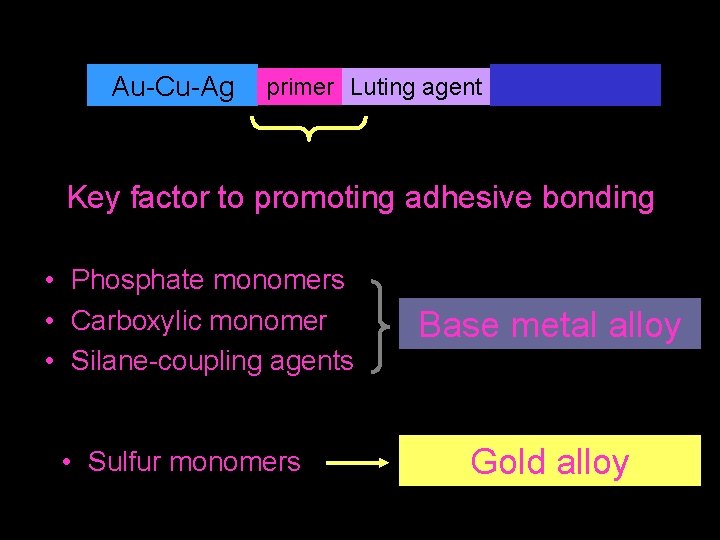
Au-Cu-Ag primer Luting agent Key factor to promoting adhesive bonding • Phosphate monomers • Carboxylic monomer • Silane-coupling agents • Sulfur monomers Base metal alloy Gold alloy

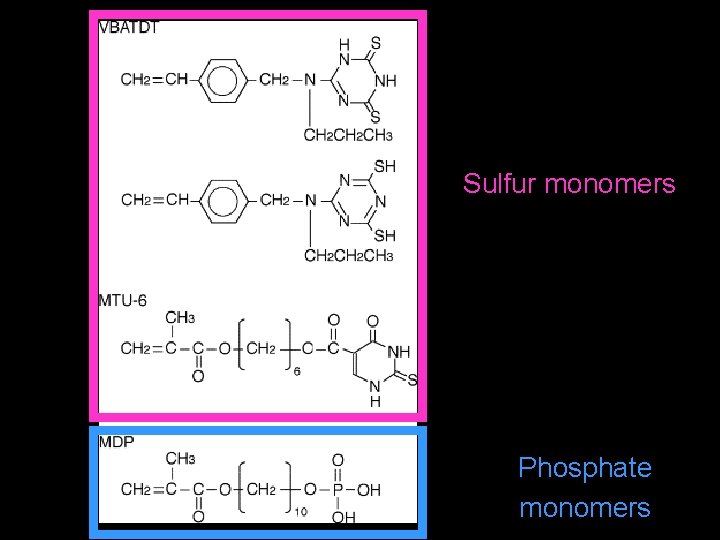
Sulfur monomers Phosphate monomers

five primers one phosphate Epricord two sulfur V-Primer Metaltite two sulfur–phosphate dual-function primers Alloy Primer Metaltite/Epricord


Shear bond test X-ray photoelectron spectroscopy (XPS) analysis

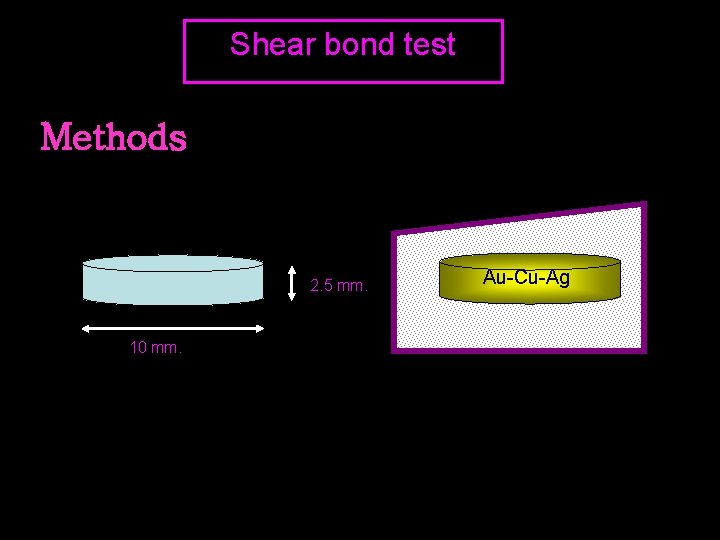
Shear bond test Methods 2. 5 mm. 10 mm. Au-Cu-Ag

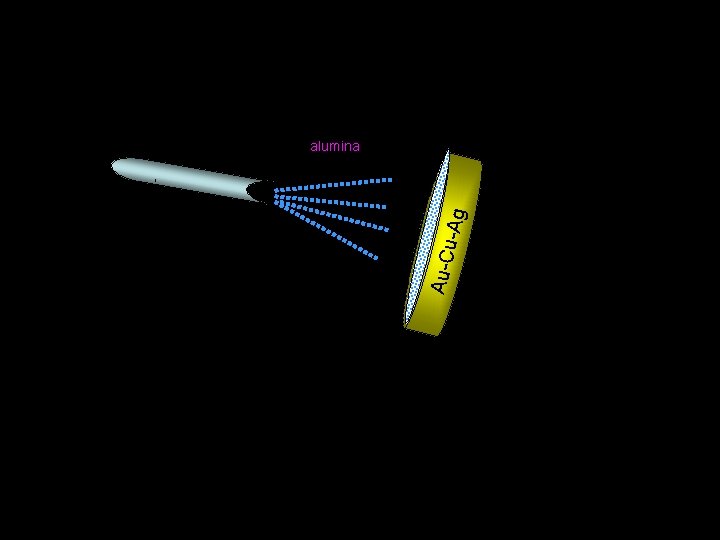
Au-C u-Ag alumina Au-Cu-Ag

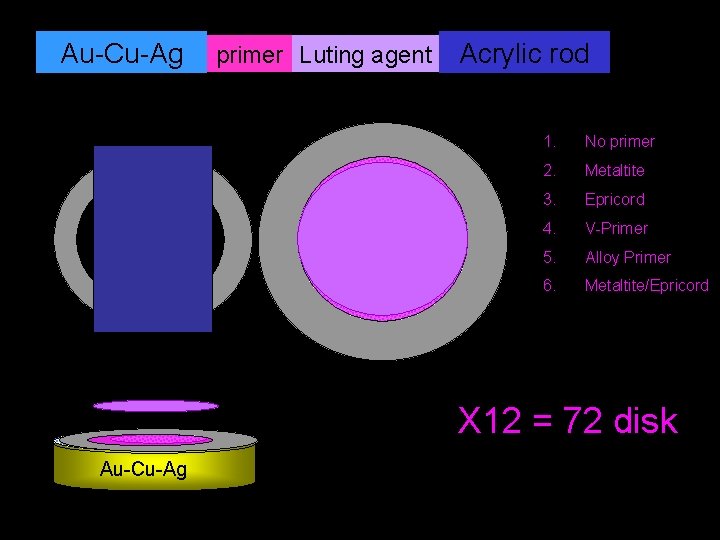
Au-Cu-Ag primer Luting agent Acrylic rod 1. No primer 2. Metaltite 3. Epricord 4. V-Primer 5. Alloy Primer 6. Metaltite/Epricord X 12 = 72 disk Au-Cu-Ag

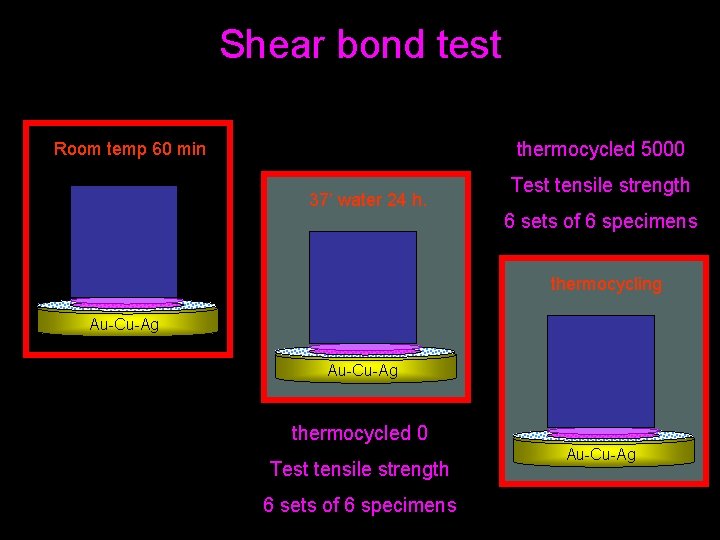
Shear bond test thermocycled 5000 Room temp 60 min 37’ water 24 h. Test tensile strength 6 sets of 6 specimens thermocycling Au-Cu-Ag thermocycled 0 Test tensile strength 6 sets of 6 specimens Au-Cu-Ag

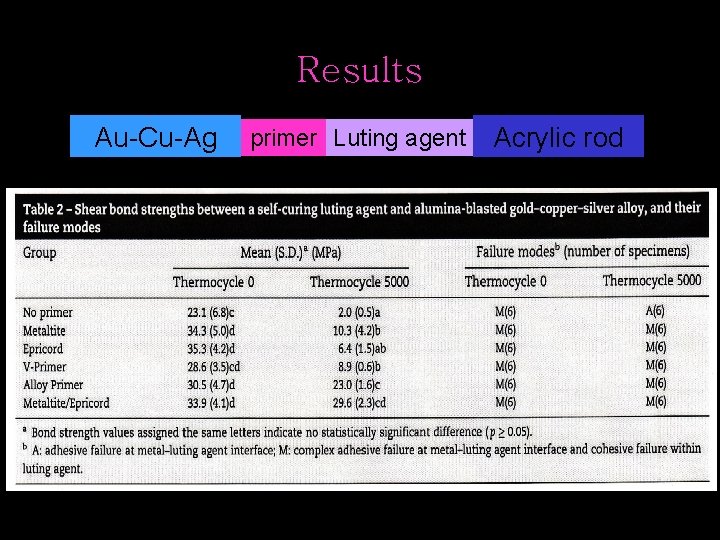
Results Au-Cu-Ag primer Luting agent Acrylic rod

X-ray photoelectron spectroscopy (XPS) analysis Methods 2. 5 mm. 10 mm. Au-Cu-Ag

Au-C u-Ag alumina Au-Cu-Ag

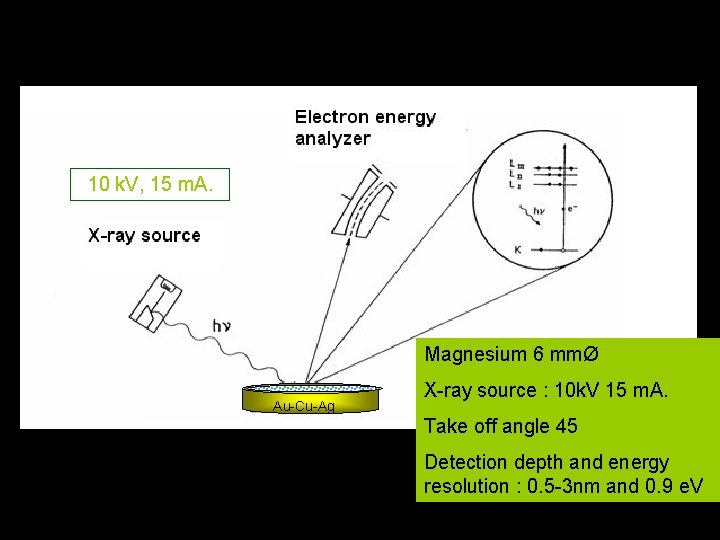
10 k. V, 15 m. A. Magnesium 6 mmØ Au-Cu-Ag X-ray source : 10 k. V 15 m. A. Take off angle 45 Detection depth and energy resolution : 0. 5 -3 nm and 0. 9 e. V

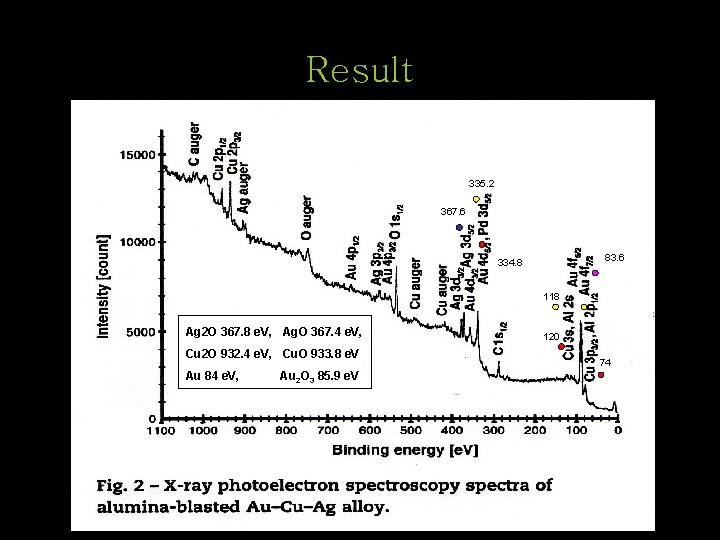
Result 335. 2 367. 6 83. 6 334. 8 118 Ag 2 O 367. 8 e. V, Ag. O 367. 4 e. V, Cu 2 O 932. 4 e. V, Cu. O 933. 8 e. V Au 84 e. V, Au 2 O 3 85. 9 e. V 120 74

Discussion • success of the dental treatment with resin-bonded prostheses is related to the durability of adhesive bonding • the greatest bond strength after thermocycling was achieved with Alloy Primer or Metaltite/Epricord

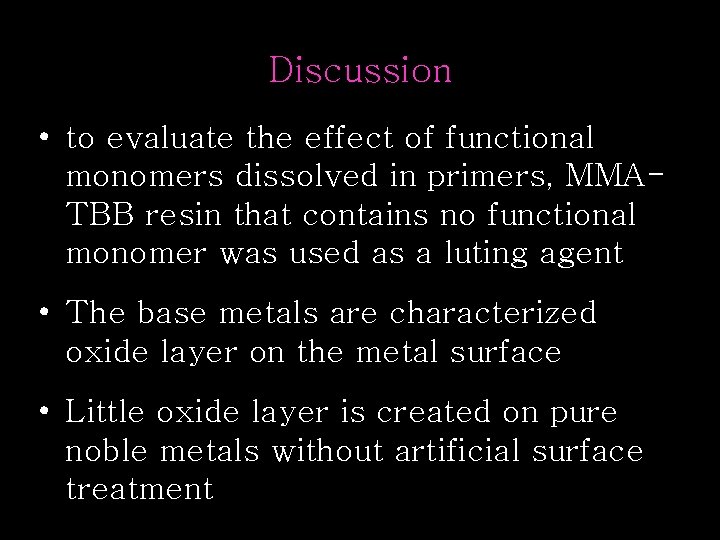
Discussion • to evaluate the effect of functional monomers dissolved in primers, MMATBB resin that contains no functional monomer was used as a luting agent • The base metals are characterized oxide layer on the metal surface • Little oxide layer is created on pure noble metals without artificial surface treatment

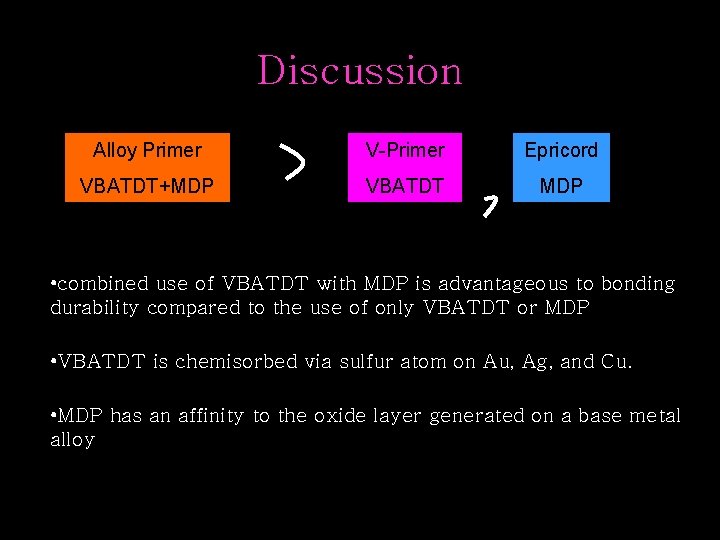
Discussion Alloy Primer V-Primer Epricord VBATDT+MDP VBATDT MDP • combined use of VBATDT with MDP is advantageous to bonding durability compared to the use of only VBATDT or MDP • VBATDT is chemisorbed via sulfur atom on Au, Ag, and Cu. • MDP has an affinity to the oxide layer generated on a base metal alloy

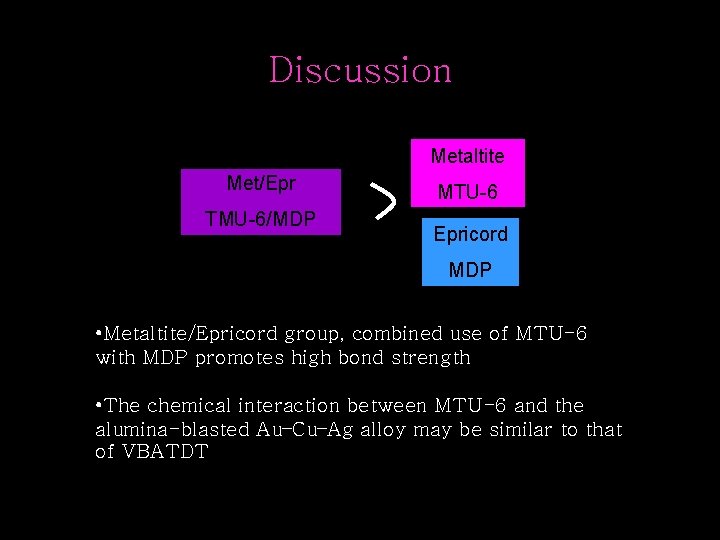
Discussion Metaltite Met/Epr TMU-6/MDP MTU-6 Epricord MDP • Metaltite/Epricord group, combined use of MTU-6 with MDP promotes high bond strength • The chemical interaction between MTU-6 and the alumina-blasted Au–Cu–Ag alloy may be similar to that of VBATDT

Discussion • thermocycling test resulted in decreased bond strength, and the relationship between primer type and bond strength • Thermal stress , such as mechanical stress, hydrolysis of resin, water diffusion into the bonded interface, and corrosion of substrate materials, as well as can clinically affect bonding durability

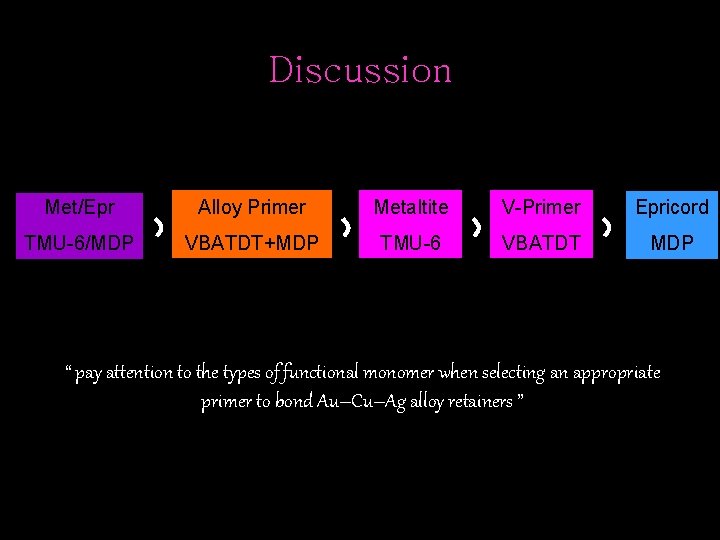
Discussion Met/Epr Alloy Primer Metaltite V-Primer Epricord TMU-6/MDP VBATDT+MDP TMU-6 VBATDT MDP “ pay attention to the types of functional monomer when selecting an appropriate primer to bond Au–Cu–Ag alloy retainers ”

Conclusion • the primers (Metaltite/Epricord and Alloy Primer) containing a sulfur monomer with a phosphate monomer significantly improved the bond strength after 5000 thermocycles between a self-curing resin and Au–Cu–Ag alloy. • No significant difference was found between Metaltite/Epricord and Alloy Primer.

• X-ray Photoelectron Spectroscopy/Electron Spectroscopy for Chemical Analysis (XPS/ESCA)Xray Photoelectron Spectroscopy (XPS), also known as Electron Spectroscopy for Chemical Analysis (ESCA), is used to determine quantitative atomic composition and chemistry. It is a surface analysis technique with a sampling volume that extends from the surface to a depth of approximately 50 -70 Angstroms. Alternatively, XPS can be utilized for sputter depth profiling to characterize thin films by quantifying matrix-level elements as a function of depth. XPS is an elemental analysis technique that is unique in providing chemical state information of the detected elements, such as distinguishing between sulfate and sulfide forms of the element sulfur. The process works by irradiating a sample with monochromatic x-rays, resulting in the emission of photoelectrons whose energies are characteristic of the elements within the sampling volume. • Evans Analytical Group® (EAG) uses this technique in a variety of applications to help customers, across a range of industries, with R&D, as well as process development/improvement. Examples include: • • • Identifying stains and discolorations Characterizing cleaning processes Analyzing the composition of powders and debris Determining contaminant sources Examining polymer functionality before and after processing to identify and quantify surface changes Measuring lube thickness on hard disks Obtaining depth profiles of thin film stacks (both conducting and non-conducting) for matrix level constituents Assessing the differences in oxide thickness between samples These insights into a product’s chemical makeup allow you to make product and process improvements more quickly, enabling you to reduce cycle time and save money. • • • With EAG, you also have access to the best facilities, instruments, and scientists available for performing an XPS analysis. We handle many different materials from multiple industries, giving us a wide variety of experience. Plus, our person-to-person service ensures that you will receive answers to all of your questions.

 Phosphate monomer
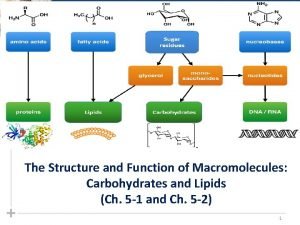
Phosphate monomer Macromolecules and their monomers
Macromolecules and their monomers Nombres naturals exercicis
Nombres naturals exercicis Dimeros de primers
Dimeros de primers Diseño de primers degenerados
Diseño de primers degenerados Els primers freds
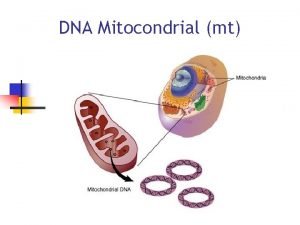
Els primers freds Las mitocondrias
Las mitocondrias Minipcr
Minipcr Escultura unifacial
Escultura unifacial Temperatura de melting primers
Temperatura de melting primers Lambda phage dna
Lambda phage dna Dna primers
Dna primers Fawaz aldabbagh
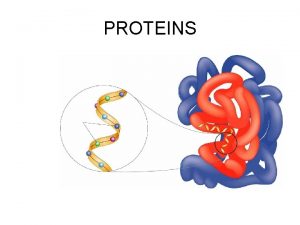
Fawaz aldabbagh Protein monomers
Protein monomers Are monomers building blocks
Are monomers building blocks Monomer in lipids
Monomer in lipids Cellulose starch and glycogen
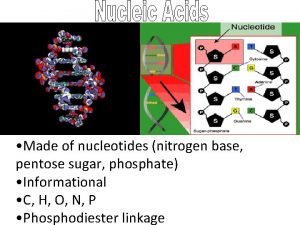
Cellulose starch and glycogen Building block of nucleic acids
Building block of nucleic acids A vs b glucose
A vs b glucose Specialty monomers
Specialty monomers Dogma of biology
Dogma of biology Monomers that make up proteins
Monomers that make up proteins Mixtures separated by distillation
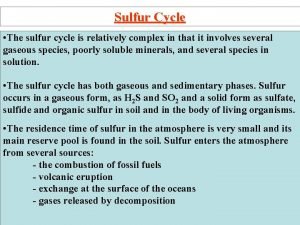
Mixtures separated by distillation Sulfur atomic number and mass
Sulfur atomic number and mass Sulfur number of neutrons protons and electrons
Sulfur number of neutrons protons and electrons Limestone and sulfur dioxide
Limestone and sulfur dioxide Gluconeogenesis of amino acids
Gluconeogenesis of amino acids Sugar phosphate and nitrogenous base
Sugar phosphate and nitrogenous base Calcitonin vitamin d3
Calcitonin vitamin d3