Does HCV eradication with DAAs affect the rate

















- Slides: 80

Does HCV eradication with DAAs affect the rate of HCC in HCV cirrhosis? Antonio Craxì Gastroenterologia & Epatologia, Di. Bi. M. I. S. , Università di Palermo antonio. craxi@unipa. it

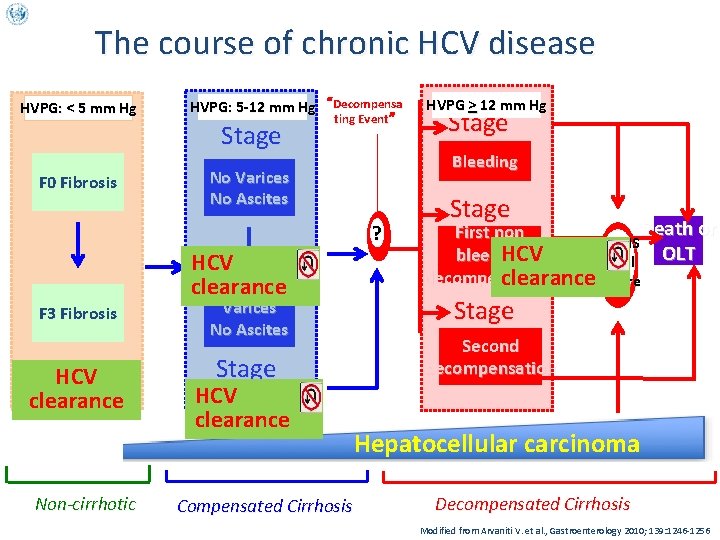
The course of chronic HCV disease HVPG: < 5 mm Hg F 0 Fibrosis HVPG: 5 -12 mm Hg “Decompensa Stage 1 No Varices ting Event” No Ascites ? HCV clearance F 3 Fibrosis HCV clearance Non-cirrhotic Varices No Ascites Stage HCV 2 clearance Compensated Cirrhosis HVPG > 12 mm Hg Stage 3 Bleeding Stage First non 4 HCV bleeding Death or OLT SEPSIS Renal decompensation clearance. Failure Stage 5 Second decompensation Hepatocellular carcinoma Decompensated Cirrhosis Modified from Arvaniti V. et al. , Gastroenterology 2010; 139: 1246 -1256

HCV clearance and residual risk of HCC in the age of IFN

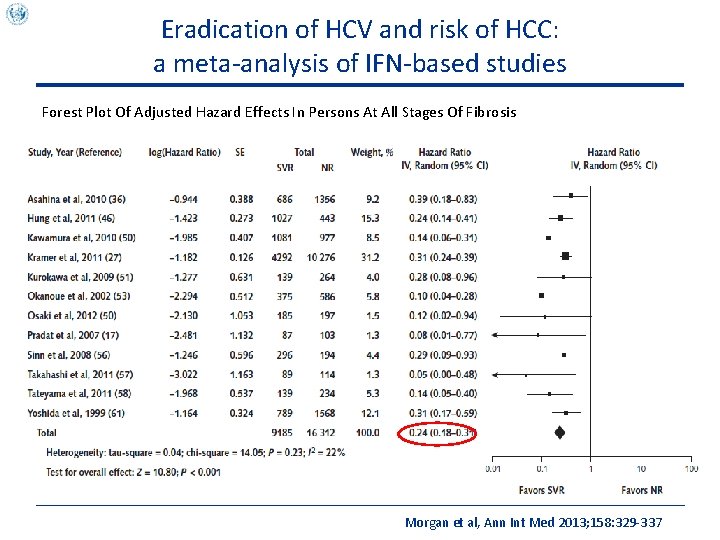
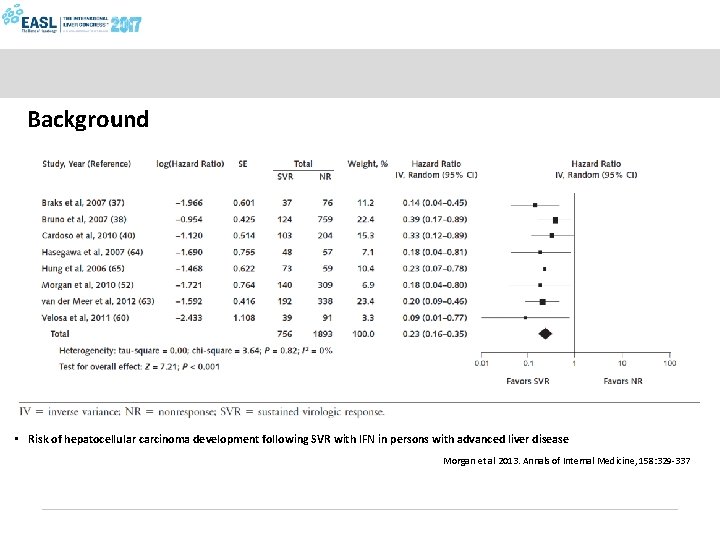
Eradication of HCV and risk of HCC: a meta-analysis of IFN-based studies Forest Plot Of Adjusted Hazard Effects In Persons At All Stages Of Fibrosis Morgan et al, Ann Int Med 2013; 158: 329 -337

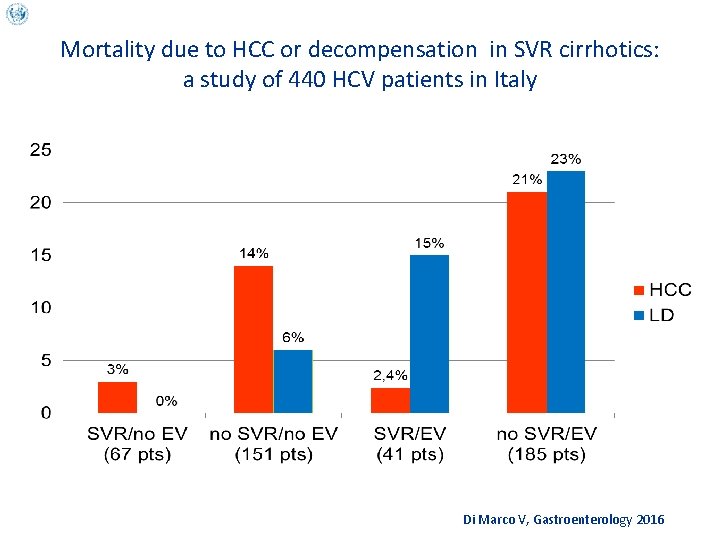
Mortality due to HCC or decompensation in SVR cirrhotics: a study of 440 HCV patients in Italy Di Marco V, Gastroenterology 2016

HCV clearance and residual risk of HCC in the age of DAAs

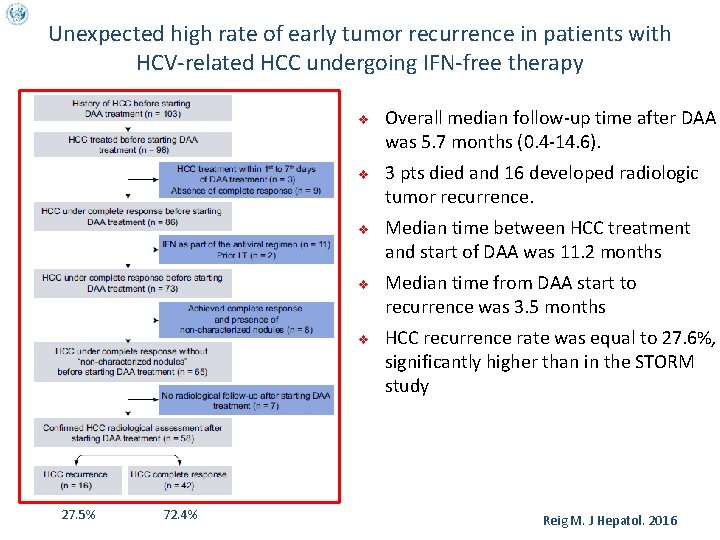
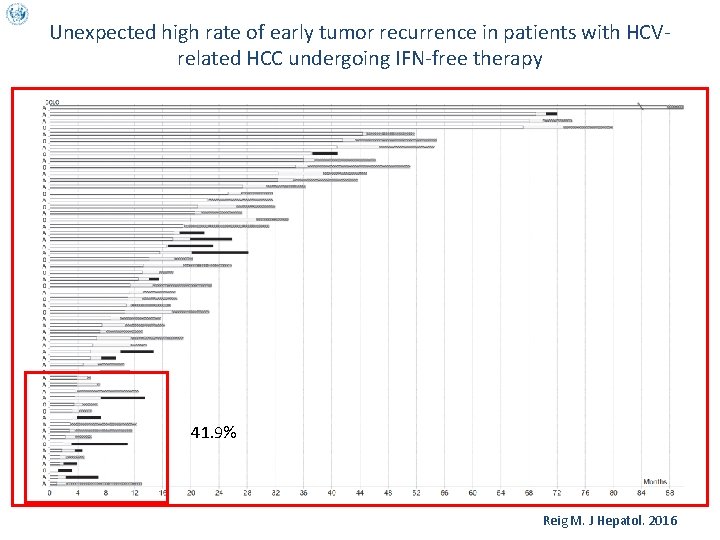
Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing IFN-free therapy v v v 27. 5% 72. 4% Overall median follow-up time after DAA was 5. 7 months (0. 4 -14. 6). 3 pts died and 16 developed radiologic tumor recurrence. Median time between HCC treatment and start of DAA was 11. 2 months Median time from DAA start to recurrence was 3. 5 months HCC recurrence rate was equal to 27. 6%, significantly higher than in the STORM study Reig M. J Hepatol. 2016

Unexpected high rate of early tumor recurrence in patients with HCVrelated HCC undergoing IFN-free therapy 41. 9% Reig M. J Hepatol. 2016

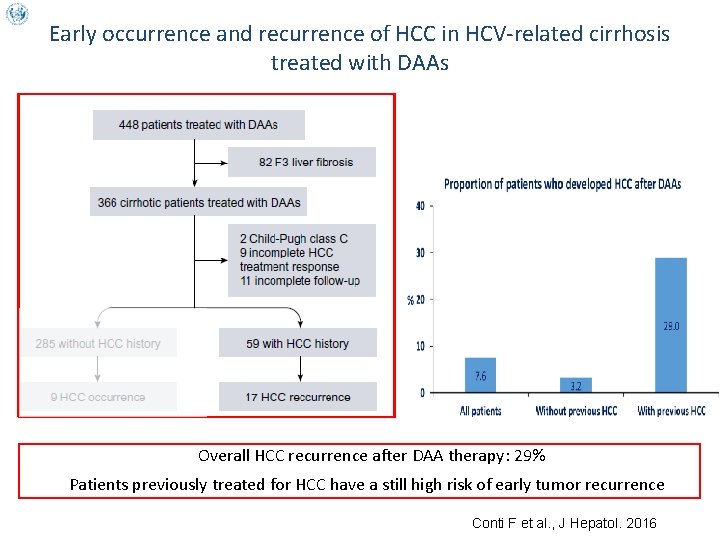
Early occurrence and recurrence of HCC in HCV-related cirrhosis treated with DAAs Overall HCC recurrence after DAA therapy: 29% Patients previously treated for HCC have a still high risk of early tumor recurrence Conti F et al. , J Hepatol. 2016

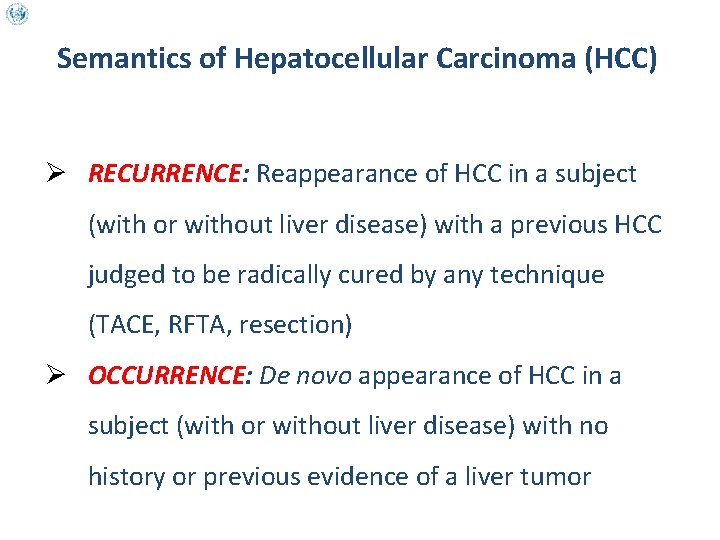
Semantics of Hepatocellular Carcinoma (HCC) Ø RECURRENCE: Reappearance of HCC in a subject (with or without liver disease) with a previous HCC judged to be radically cured by any technique (TACE, RFTA, resection) Ø OCCURRENCE: De novo appearance of HCC in a subject (with or without liver disease) with no history or previous evidence of a liver tumor

HCV clearance on DAAs and recurrence of HCC

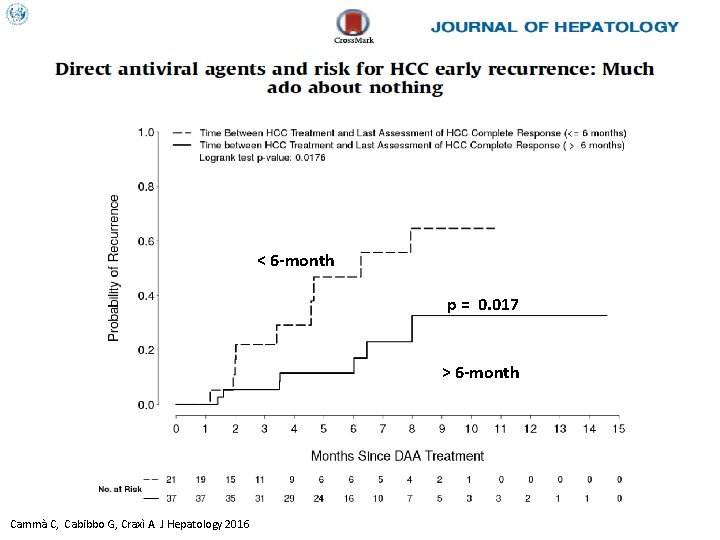
< 6 -month p = 0. 017 > 6 -month Cammà C, Cabibbo G, Craxì A. J Hepatology 2016

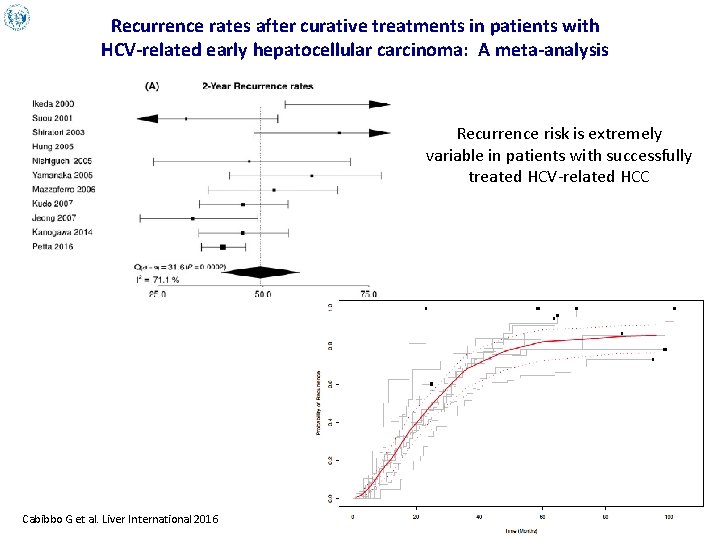
Recurrence rates after curative treatments in patients with HCV-related early hepatocellular carcinoma: A meta-analysis Recurrence risk is extremely variable in patients with successfully treated HCV-related HCC Cabibbo G et al. Liver International 2016

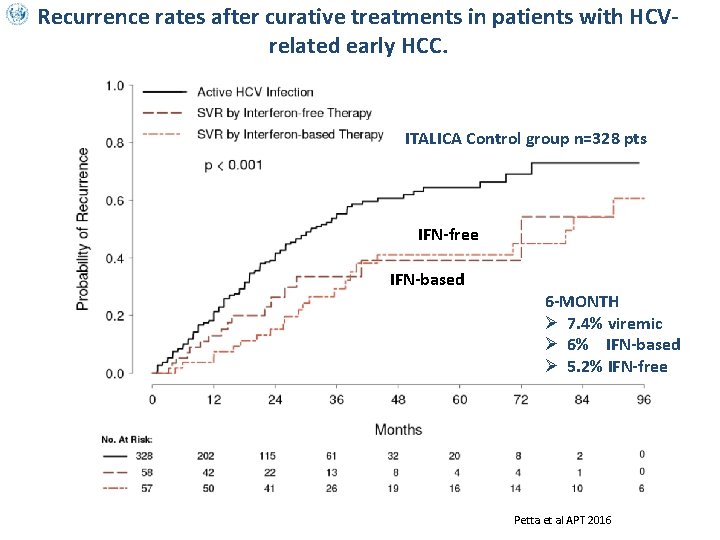
Recurrence rates after curative treatments in patients with HCVrelated early HCC. ITALICA Control group n=328 pts IFN-free IFN-based 6 -MONTH Ø 7. 4% viremic Ø 6% IFN-based Ø 5. 2% IFN-free Cammà et. Petta et al APT 2016 ITALICA submitted

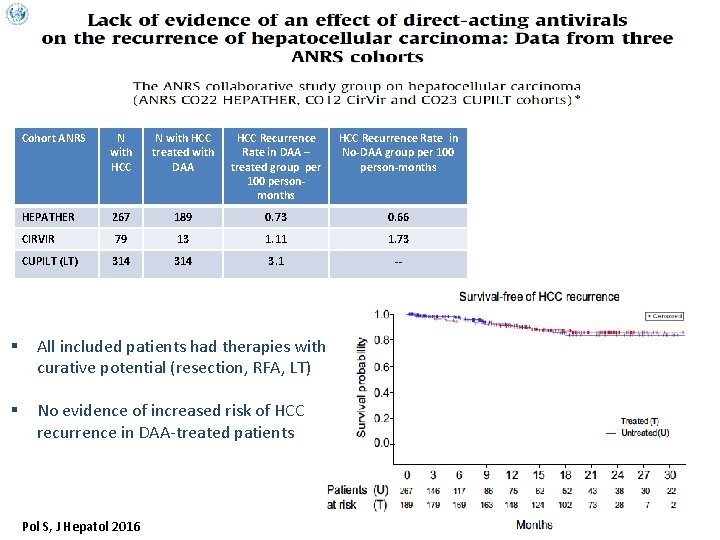
Cohort ANRS N with HCC treated with DAA HCC Recurrence Rate in DAA – treated group per 100 personmonths HCC Recurrence Rate in No-DAA group per 100 person-months HEPATHER 267 189 0. 73 0. 66 CIRVIR 79 13 1. 11 1. 73 CUPILT (LT) 314 3. 1 -- § All included patients had therapies with curative potential (resection, RFA, LT) § No evidence of increased risk of HCC recurrence in DAA-treated patients Pol S, J Hepatol 2016

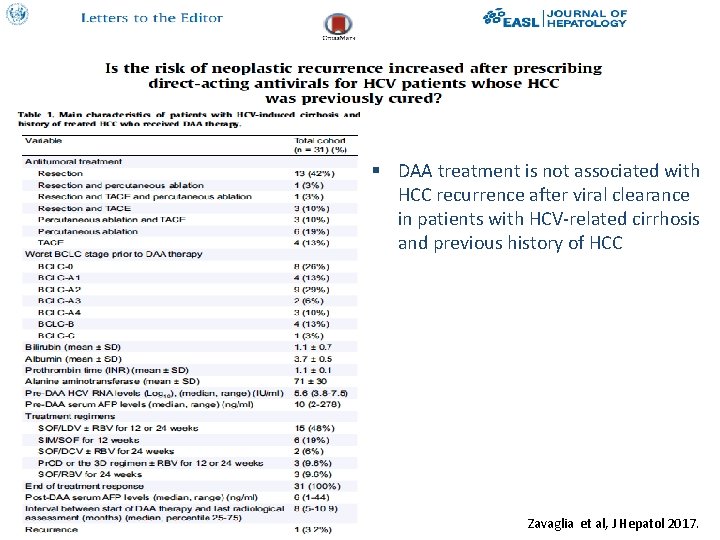
§ DAA treatment is not associated with HCC recurrence after viral clearance in patients with HCV-related cirrhosis and previous history of HCC Zavaglia et al, J Hepatol 2017.

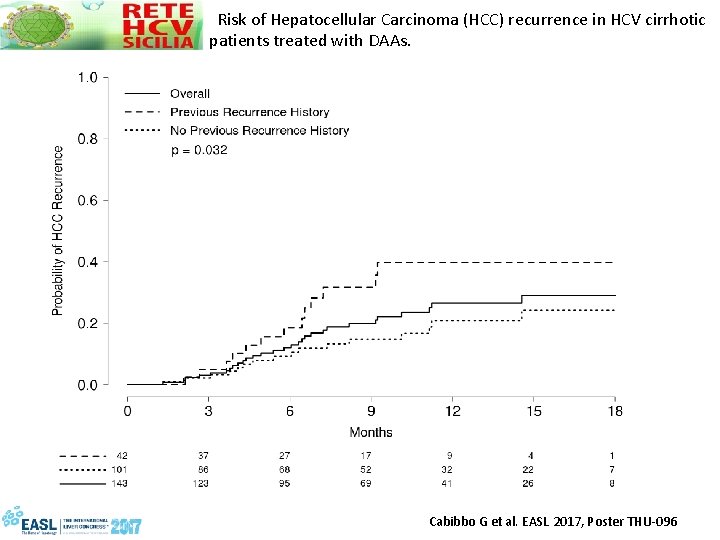
Risk of Hepatocellular Carcinoma (HCC) recurrence in HCV cirrhotic patients treated with DAAs. • 143 patients with HCV cirrhosis C-P A or B and an HCC who achieved complete radiological response after curative treatmen who concluded DAA treatment, from RESIST-HCV, a network started in March 2015 including all 22 Centres in Sicily treating HCV with DAAs • Primary endpoint for analysis was the time to HCC recurrence (days elapsed between initiation of DAA and documented HCC recurrence) Cabibbo G et al. EASL 2017, Poster THU-096

Risk of Hepatocellular Carcinoma (HCC) recurrence in HCV cirrhotic patients treated with DAAs. Cabibbo G et al. EASL 2017, Poster THU-096

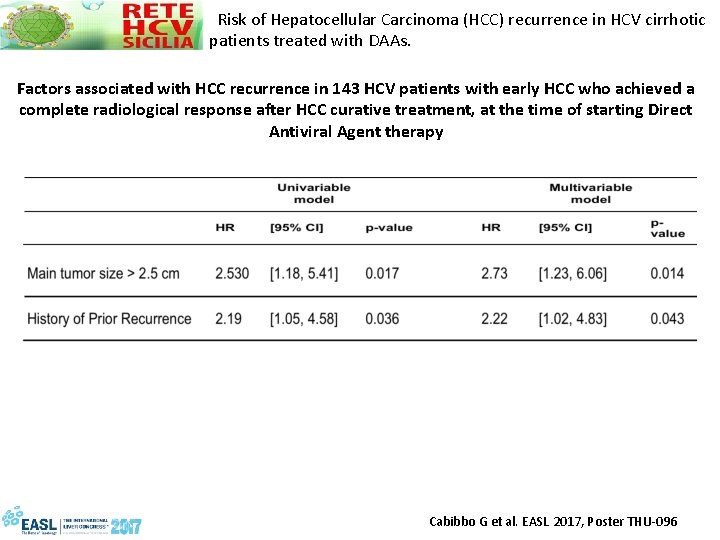
Risk of Hepatocellular Carcinoma (HCC) recurrence in HCV cirrhotic patients treated with DAAs. Factors associated with HCC recurrence in 143 HCV patients with early HCC who achieved a complete radiological response after HCC curative treatment, at the time of starting Direct Antiviral Agent therapy Cabibbo G et al. EASL 2017, Poster THU-096

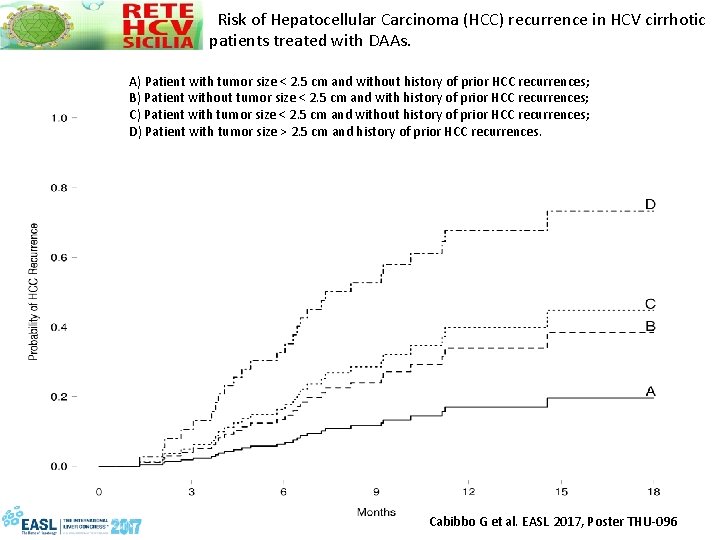
Risk of Hepatocellular Carcinoma (HCC) recurrence in HCV cirrhotic patients treated with DAAs. A) Patient with tumor size < 2. 5 cm and without history of prior HCC recurrences; B) Patient without tumor size < 2. 5 cm and with history of prior HCC recurrences; C) Patient with tumor size < 2. 5 cm and without history of prior HCC recurrences; D) Patient with tumor size > 2. 5 cm and history of prior HCC recurrences. Cabibbo G et al. EASL 2017, Poster THU-096

Tumor recurrence after Interferon-free treatment for hepatitis C in patients with previously treated hepatocellular carcinoma discloses a more aggressive pattern and faster tumor growth María Reig 1*; Zoe Mariño 2*; Christie Perelló 3; Mercedes Iñarrairaegui 4; Andrea Ribeiro 1; Sabela Lens 2; Alba Díaz 5; Ramón Vilana 6; Anna Darnell 6; María Varela 7; Bruno Sangro 4; José Luis Calleja 3; Xavier Forns 2¥ and Jordi Bruix 1¥. Barcelona Clinic Liver Cancer (BCLC) Group. Liver Unit. Hospital Clinic Barcelona. IDIBAPS. University of Barcelona. Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBERehd). Barcelona. Spain. 2 Liver Unit. Hospital Clinic. IDIBAPS. University of Barcelona. CIBERehd. Barcelona. Spain. 3 Liver Unit. Hospital Univesitario Puerta de Hierro. CIBERehd. IDIPHIM. Madrid. Spain 4 Unidad de Hepatología. Clínica Universidad de Navarra. IDISNA. CIBERehd. Pamplona. Spain. 5 Department of Pathology. BCLC group. Hospital Clínic Barcelona. IDIBAPS. University of Barcelona. Spain. 1 * These authors contributed equally as first co-authors. ¥ These authors contributed equally as senior co-authors.

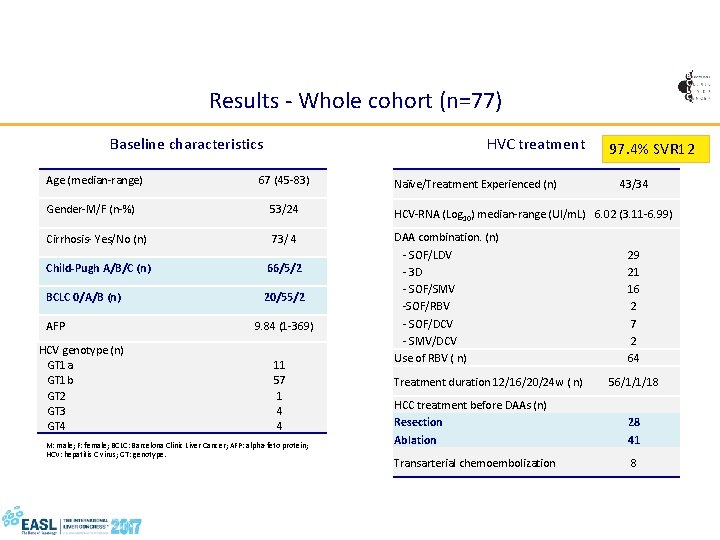
Results - Whole cohort (n=77) HVC treatment Baseline characteristics Age (median-range) 67 (45 -83) Naïve/Treatment Experienced (n) 97. 4% SVR 12 43/34 Gender-M/F (n-%) 53/24 HCV-RNA (Log 10) median-range (UI/m. L) 6. 02 (3. 11 -6. 99) Cirrhosis- Yes/No (n) 73/ 4 Child-Pugh A/B/C (n) 66/5/2 BCLC 0/A/B (n) 20/55/2 DAA combination. (n) - SOF/LDV - 3 D - SOF/SMV -SOF/RBV - SOF/DCV - SMV/DCV Use of RBV ( n) AFP HCV genotype (n) GT 1 a GT 1 b GT 2 GT 3 GT 4 9. 84 (1 -369) 11 57 1 4 4 M: male; F: female; BCLC: Barcelona Clinic Liver Cancer; AFP: alpha-feto protein; HCV: hepatitis C virus; GT: genotype. Treatment duration 12/16/20/24 w ( n) 29 21 16 2 7 2 64 56/1/1/18 HCC treatment before DAAs (n) Resection 28 Ablation 41 PR: pegylated-interferon plus ribavirin; DAA: direct-acting antivirals; KPa: kilopascals; SOF: sofosbuvir; LDV: ledipasvir; 3 D: 3 -drug combination paritaprevir/ritonavir/ombitasvir plus dasabuvir; SMV: simeprevir; DCV: daclatasvir; RBV: ribavirin; w: weeks; Transarterial chemoembolization 8

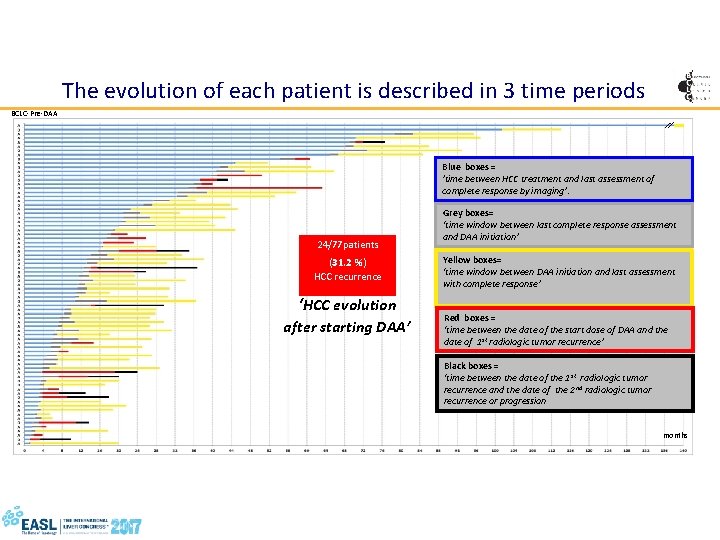
The evolution of each patient is described in 3 time periods BCLC-Pre-DAA Blue boxes = ’time between HCC treatment and last assessment of complete response by imaging’. 24/77 patients (31. 2 %) HCC recurrence ‘HCC evolution after starting DAA’ Grey boxes= ‘time window between last complete response assessment and DAA initiation’ Yellow boxes= ‘time window between DAA initiation and last assessment with complete response’ Red boxes = ‘time between the date of the start dose of DAA and the date of 1 st radiologic tumor recurrence’ Black boxes = ‘time between the date of the 1 st radiologic tumor recurrence and the date of the 2 nd radiologic tumor recurrence or progression months

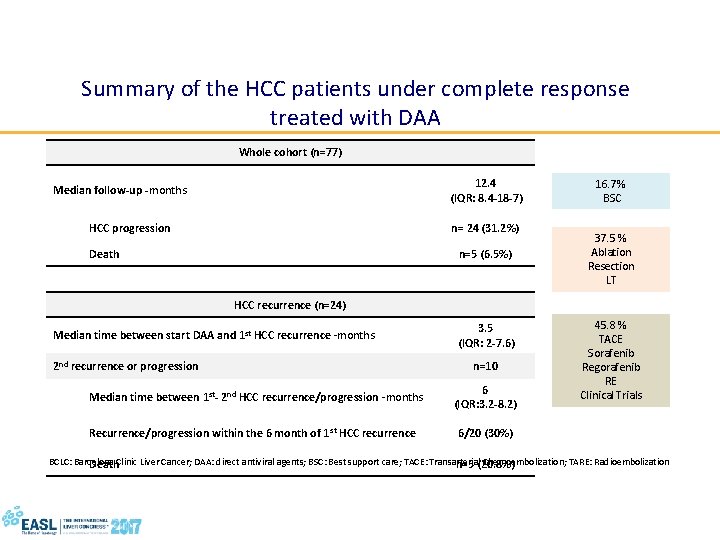
Summary of the HCC patients under complete response treated with DAA Whole cohort (n=77) 12. 4 (IQR: 8. 4 -18 -7) Median follow-up -months HCC progression n= 24 (31. 2%) Death n=5 (6. 5%) 16. 7% BSC 37. 5 % Ablation Resection LT HCC recurrence (n=24) Median time between start DAA and 1 st HCC recurrence -months 2 nd recurrence or progression 3. 5 (IQR: 2 -7. 6) n=10 Median time between 1 st- 2 nd HCC recurrence/progression -months 6 (IQR: 3. 2 -8. 2) Recurrence/progression within the 6 month of 1 st HCC recurrence 6/20 (30%) 45. 8 % TACE Sorafenib Regorafenib RE Clinical Trials BCLC: Barcelona Clinic Liver Cancer; DAA: direct antiviral agents; BSC: Best support care; TACE: Transarterial Chemoembolization; TARE: Radioembolization Death n=5 (20. 8%)

Controversies on HCC recurrence - High heterogeneity of the groups of patients with HCC in terms of clinical features (stage of cirrhosis; morphology of HCC) - Different treatments, from palliative (transarterial chemoembolisation) to potentially curative (ablation and surgery) - Time elapsed between presumed cure of HCC and treatment with DAAs. Calvaruso V, Craxì A. Hepatocellular carcinoma and direct-acting antivirals: A never ending story? Liver International 2017. in press

HCV clearance on DAAs and occurrence of HCC

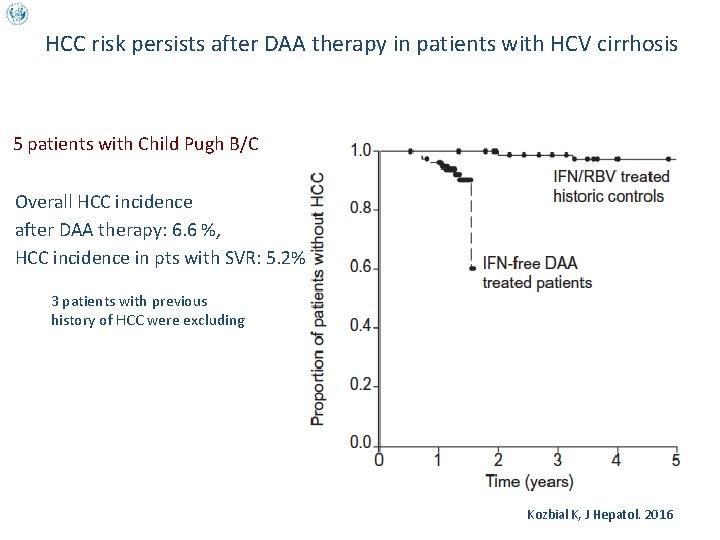
HCC risk persists after DAA therapy in patients with HCV cirrhosis 5 patients with Child Pugh B/C Overall HCC incidence after DAA therapy: 6. 6 %, HCC incidence in pts with SVR: 5. 2% 3 patients with previous history of HCC were excluding Kozbial K, J Hepatol. 2016

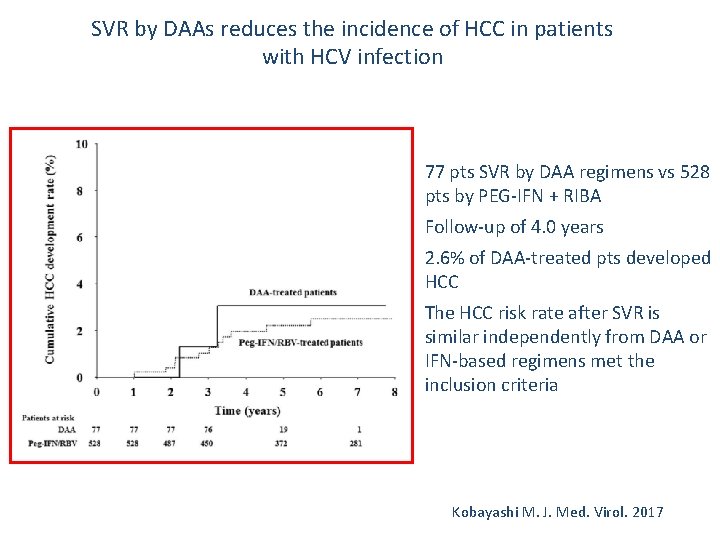
SVR by DAAs reduces the incidence of HCC in patients with HCV infection v v 77 pts SVR by DAA regimens vs 528 pts by PEG-IFN + RIBA Follow-up of 4. 0 years 2. 6% of DAA-treated pts developed HCC The HCC risk rate after SVR is similar independently from DAA or IFN-based regimens met the inclusion criteria Kobayashi M. J. Med. Virol. 2017

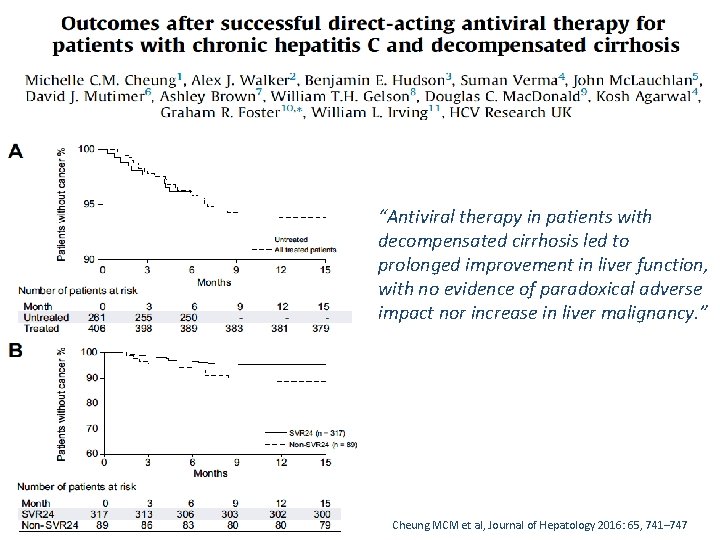
“Antiviral therapy in patients with decompensated cirrhosis led to prolonged improvement in liver function, with no evidence of paradoxical adverse impact nor increase in liver malignancy. ” Cheung MCM et al, Journal of Hepatology 2016: 65, 741– 747

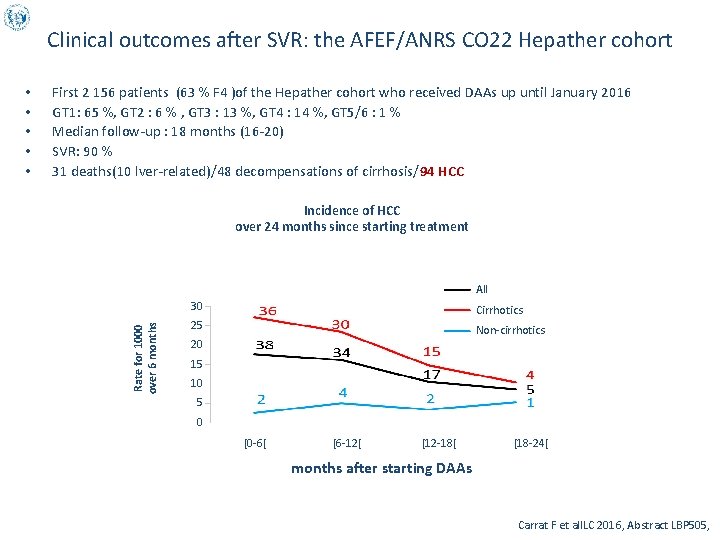
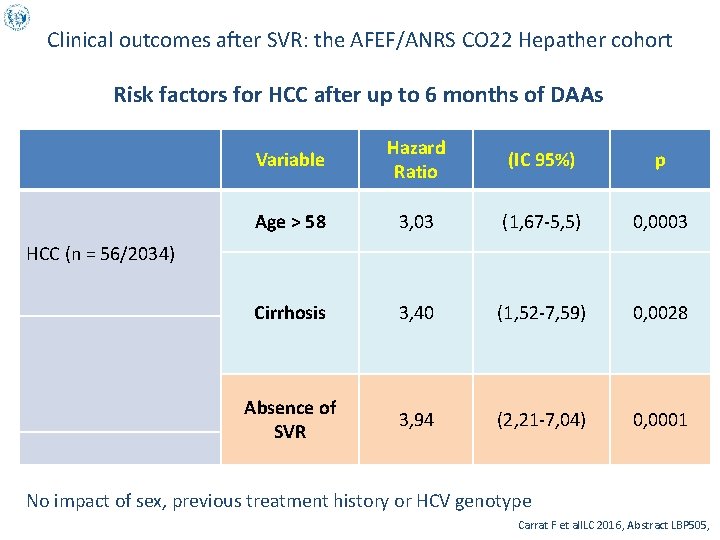
Clinical outcomes after SVR: the AFEF/ANRS CO 22 Hepather cohort First 2 156 patients (63 % F 4 )of the Hepather cohort who received DAAs up until January 2016 GT 1: 65 %, GT 2 : 6 % , GT 3 : 13 %, GT 4 : 14 %, GT 5/6 : 1 % Median follow-up : 18 months (16 -20) SVR: 90 % 31 deaths(10 lver-related)/48 decompensations of cirrhosis/94 HCC Incidence of HCC over 24 months since starting treatment All Rate for 1000 over 6 months • • • 30 Cirrhotics 25 Non-cirrhotics 20 15 10 5 0 [0 -6[ [6 -12[ [12 -18[ [18 -24[ months after starting DAAs Carrat F et al. ILC 2016, Abstract LBP 505,

Clinical outcomes after SVR: the AFEF/ANRS CO 22 Hepather cohort Risk factors for HCC after up to 6 months of DAAs Variable Hazard Ratio (IC 95%) p Age > 58 3, 03 (1, 67 -5, 5) 0, 0003 Cirrhosis 3, 40 (1, 52 -7, 59) 0, 0028 Absence of SVR 3, 94 (2, 21 -7, 04) 0, 0001 HCC (n = 56/2034) No impact of sex, previous treatment history or HCV genotype Carrat F et al. ILC 2016, Abstract LBP 505,

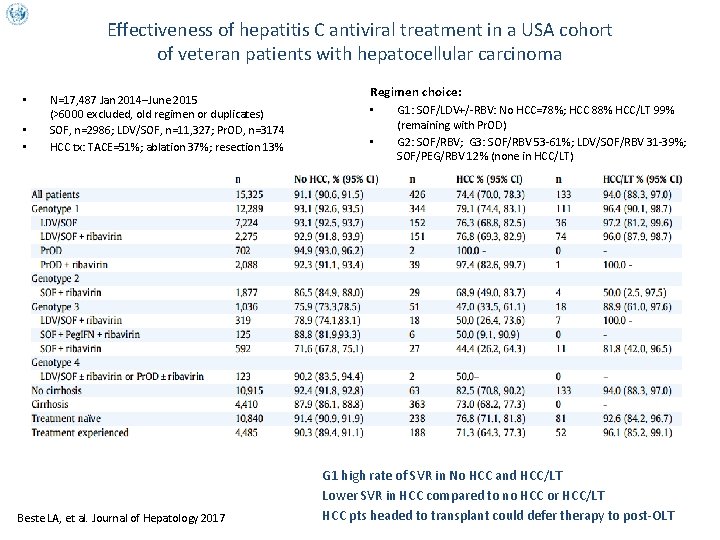
Effectiveness of hepatitis C antiviral treatment in a USA cohort of veteran patients with hepatocellular carcinoma • • • Regimen choice: N=17, 487 Jan 2014–June 2015 (>6000 excluded, old regimen or duplicates) SOF, n=2986; LDV/SOF, n=11, 327; Pr. OD, n=3174 HCC tx: TACE=51%; ablation 37%; resection 13% Beste LA, et al. Journal of Hepatology 2017 • • • G 1: SOF/LDV+/-RBV: No HCC=78%; HCC 88% HCC/LT 99% (remaining with Pr. OD) G 2: SOF/RBV; G 3: SOF/RBV 53 -61%; LDV/SOF/RBV 31 -39%; SOF/PEG/RBV 12% (none in HCC/LT) G 1 high rate of SVR in No HCC and HCC/LT Lower SVR in HCC compared to no HCC or HCC/LT HCC pts headed to transplant could defer therapy to post-OLT

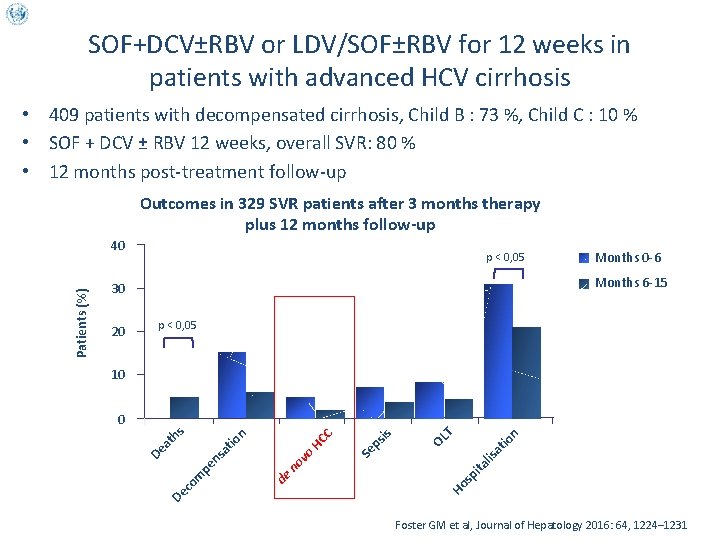
SOF+DCV±RBV or LDV/SOF±RBV for 12 weeks in patients with advanced HCV cirrhosis • 409 patients with decompensated cirrhosis, Child B : 73 %, Child C : 10 % • SOF + DCV ± RBV 12 weeks, overall SVR: 80 % • 12 months post-treatment follow-up Outcomes in 329 SVR patients after 3 months therapy plus 12 months follow-up p < 0, 05 20 Months 0 -6 Months 6 -15 30 p < 0, 05 10 at io n OL T is ps Ho sp ita lis Se HC C de at ns pe co m De no vo io th n s 0 De a Patients (%) 40 Foster GM et al, Journal of Hepatology 2016: 64, 1224– 1231

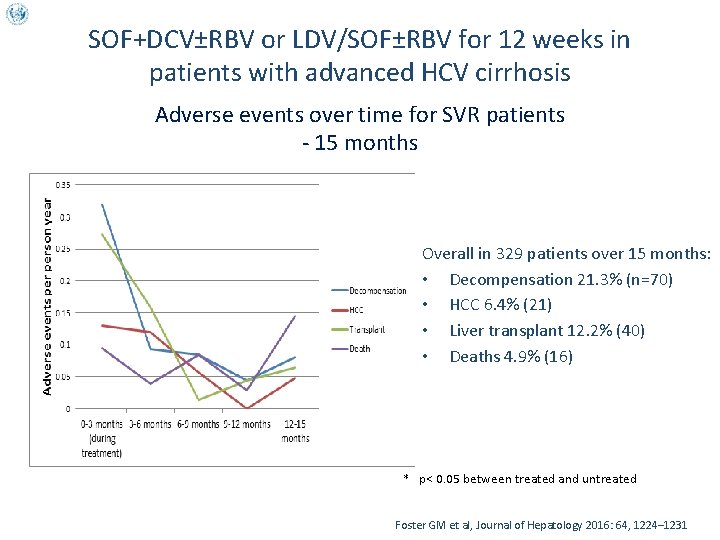
SOF+DCV±RBV or LDV/SOF±RBV for 12 weeks in patients with advanced HCV cirrhosis Adverse events over time for SVR patients - 15 months Overall in 329 patients over 15 months: • Decompensation 21. 3% (n=70) • HCC 6. 4% (21) • Liver transplant 12. 2% (40) • Deaths 4. 9% (16) * p< 0. 05 between treated and untreated Foster GM et al, Journal of Hepatology 2016: 64, 1224– 1231

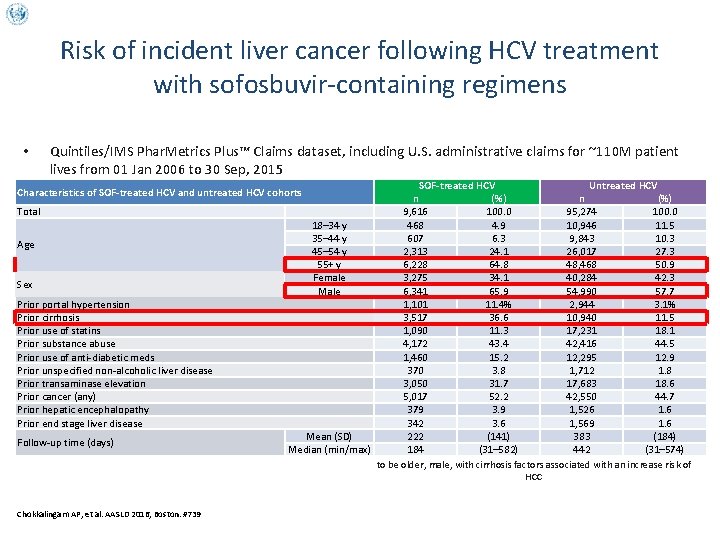
Risk of incident liver cancer following HCV treatment with sofosbuvir-containing regimens • Quintiles/IMS Phar. Metrics Plus™ Claims dataset, including U. S. administrative claims for ~110 M patient lives from 01 Jan 2006 to 30 Sep, 2015 SOF-treated HCV Untreated HCV n (%) 9, 616 100. 0 95, 274 100. 0 18– 34 y 468 4. 9 10, 946 11. 5 35– 44 y 607 6. 3 9, 843 10. 3 45– 54 y 2, 313 24. 1 26, 017 27. 3 55+ y 6, 228 64. 8 48, 468 50. 9 Female 3, 275 34. 1 40, 284 42. 3 Male 6, 341 65. 9 54, 990 57. 7 1, 101 11. 4% 2, 944 3. 1% 3, 517 36. 6 10, 940 11. 5 1, 090 11. 3 17, 231 18. 1 4, 172 43. 4 42, 416 44. 5 1, 460 15. 2 12, 295 12. 9 370 3. 8 1, 712 1. 8 3, 050 31. 7 17, 683 18. 6 5, 017 52. 2 42, 550 44. 7 379 3. 9 1, 526 1. 6 342 3. 6 1, 569 1. 6 Mean (SD) 222 (141) 383 (184) Median (min/max) Compared to untreated HCV patients, patients completing SOF tended 184 (31– 582) 442 (31– 574) to be older, male, with cirrhosis factors associated with an increase risk of HCC Characteristics of SOF-treated HCV and untreated HCV cohorts Total Age Sex Prior portal hypertension Prior cirrhosis Prior use of statins Prior substance abuse Prior use of anti-diabetic meds Prior unspecified non-alcoholic liver disease Prior transaminase elevation Prior cancer (any) Prior hepatic encephalopathy Prior end stage liver disease Follow-up time (days) Chokkalingam AP, et al. AASLD 2016, Boston. #739

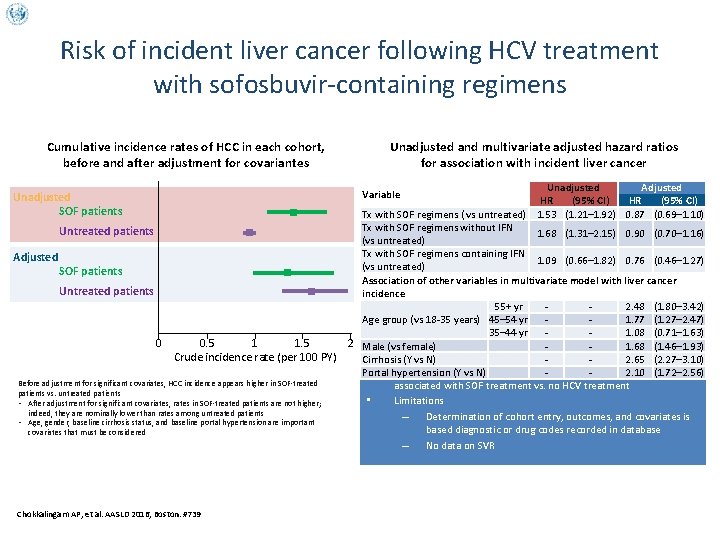
Risk of incident liver cancer following HCV treatment with sofosbuvir-containing regimens Cumulative incidence rates of HCC in each cohort, before and after adjustment for covariantes Unadjusted Adjusted HR (95% CI) Tx with SOF regimens ( vs untreated) 1. 53 (1. 21– 1. 92) 0. 87 (0. 69– 1. 10) Tx with SOF regimens without IFN 1. 68 (1. 31– 2. 15) 0. 90 (0. 70– 1. 16) (vs untreated) Tx with SOF regimens containing IFN 1. 09 (0. 66– 1. 82) 0. 76 (0. 46– 1. 27) (vs untreated) Association of other variables in multivariate model with liver cancer incidence 55+ yr 2. 48 (1. 80– 3. 42) Age group (vs 18 -35 years) 45– 54 yr 1. 77 (1. 27– 2. 47) 35– 44 yr 1. 08 (0. 71– 1. 63) 2 Male (vs female) 1. 68 (1. 46– 1. 93) Cirrhosis (Y vs N) 2. 65 (2. 27– 3. 10) • After adjustment for covariates, no increased risk of incident HCC Portal hypertension (Y vs N) 2. 10 (1. 72– 2. 56) associated with SOF treatment vs. no HCV treatment • Limitations – Determination of cohort entry, outcomes, and covariates is based diagnostic or drug codes recorded in database – No data on SVR Variable Unadjusted SOF patients Untreated patients Adjusted Unadjusted and multivariate adjusted hazard ratios for association with incident liver cancer SOF patients Untreated patients 0 0. 5 1 1. 5 Crude incidence rate (per 100 PY) Before adjustment for significant covariates, HCC incidence appears higher in SOF-treated patients vs. untreated patients • After adjustment for significant covariates, rates in SOF-treated patients are not higher; indeed, they are nominally lower than rates among untreated patients • Age, gender, baseline cirrhosis status, and baseline portal hypertension are important covariates that must be considered Chokkalingam AP, et al. AASLD 2016, Boston. #739


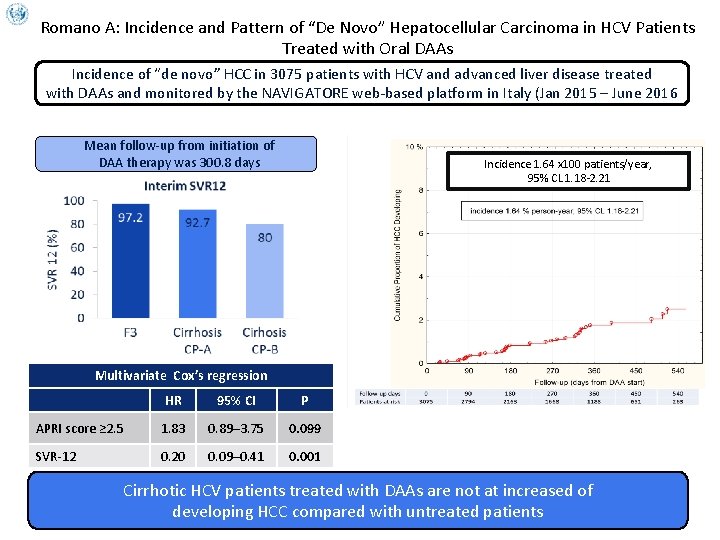
Romano A: Incidence and Pattern of “De Novo” Hepatocellular Carcinoma in HCV Patients Treated with Oral DAAs Incidence of “de novo” HCC in 3075 patients with HCV and advanced liver disease treated with DAAs and monitored by the NAVIGATORE web-based platform in Italy (Jan 2015 – June 2016 Mean follow-up from initiation of DAA therapy was 300. 8 days Incidence 1. 64 x 100 patients/year, 95% CL 1. 18 -2. 21 Multivariate Cox’s regression HR 95% CI P APRI score ≥ 2. 5 1. 83 0. 89– 3. 75 0. 099 SVR-12 0. 20 0. 09– 0. 41 0. 001 Cirrhotic HCV patients treated with DAAs are not at increased of developing HCC compared with untreated patients

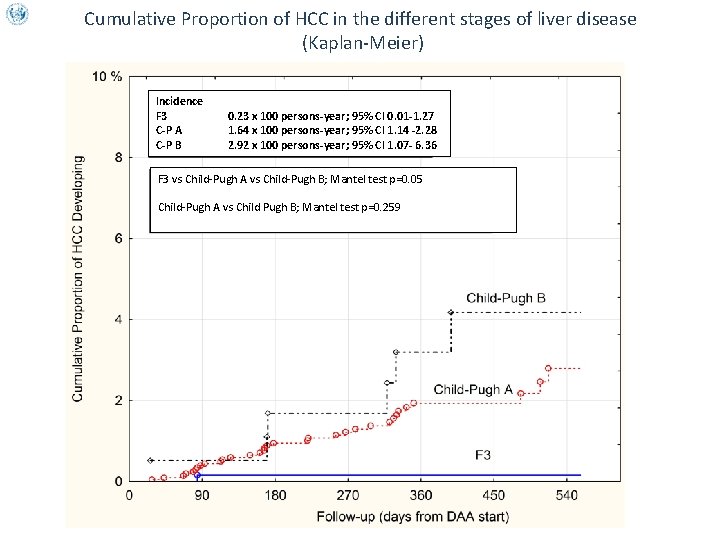
Cumulative Proportion of HCC in the different stages of liver disease (Kaplan-Meier) Incidence F 3 C-P A C-P B 0. 23 x 100 persons-year; 95% CI 0. 01 -1. 27 1. 64 x 100 persons-year; 95% CI 1. 14 -2. 28 2. 92 x 100 persons-year; 95% CI 1. 07 - 6. 36 F 3 vs Child-Pugh A vs Child-Pugh B; Mantel test p=0. 05 Child-Pugh A vs Child Pugh B; Mantel test p=0. 259

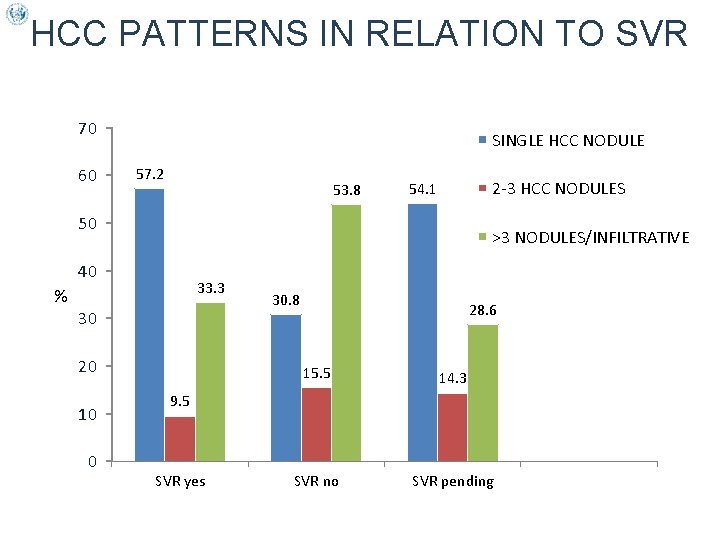
HCC PATTERNS IN RELATION TO SVR 70 60 SINGLE HCC NODULE 57. 2 53. 8 2 -3 HCC NODULES 54. 1 50 >3 NODULES/INFILTRATIVE 40 33. 3 % 30 20 10 0 30. 8 28. 6 15. 5 14. 3 SVR no SVR pending 9. 5 SVR yes

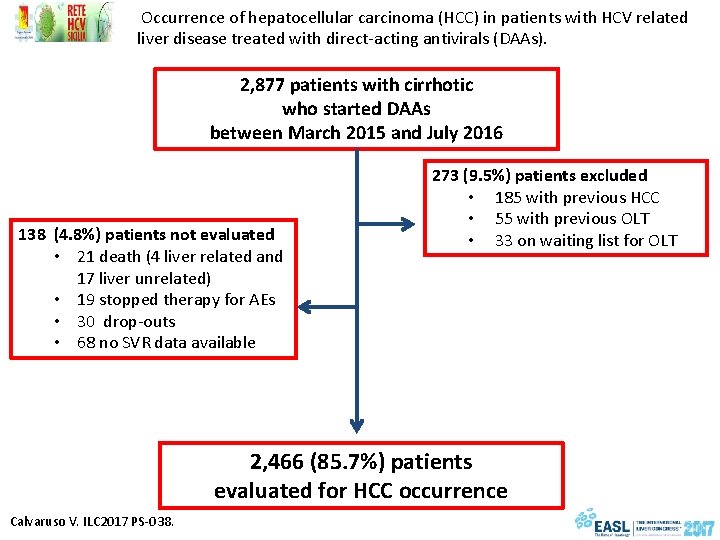
Occurrence of hepatocellular carcinoma (HCC) in patients with HCV related liver disease treated with direct-acting antivirals (DAAs). 2, 877 patients with cirrhotic who started DAAs between March 2015 and July 2016 138 (4. 8%) patients not evaluated • 21 death (4 liver related and 17 liver unrelated) • 19 stopped therapy for AEs • 30 drop-outs • 68 no SVR data available 273 (9. 5%) patients excluded • 185 with previous HCC • 55 with previous OLT • 33 on waiting list for OLT 2, 466 (85. 7%) patients evaluated for HCC occurrence Calvaruso V. ILC 2017 PS-038.

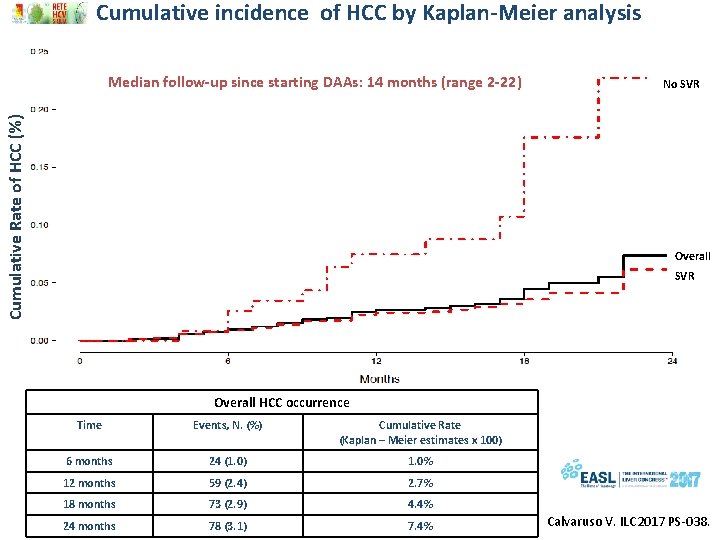
Cumulative incidence of HCC by Kaplan-Meier analysis Cumulative Rate of HCC (%) Median follow-up since starting DAAs: 14 months (range 2 -22) No SVR Overall HCC occurrence Time Events, N. (%) Cumulative Rate (Kaplan – Meier estimates x 100) 6 months 24 (1. 0) 1. 0% 12 months 59 (2. 4) 2. 7% 18 months 73 (2. 9) 4. 4% 24 months 78 (3. 1) 7. 4% Calvaruso V. ILC 2017 PS-038.

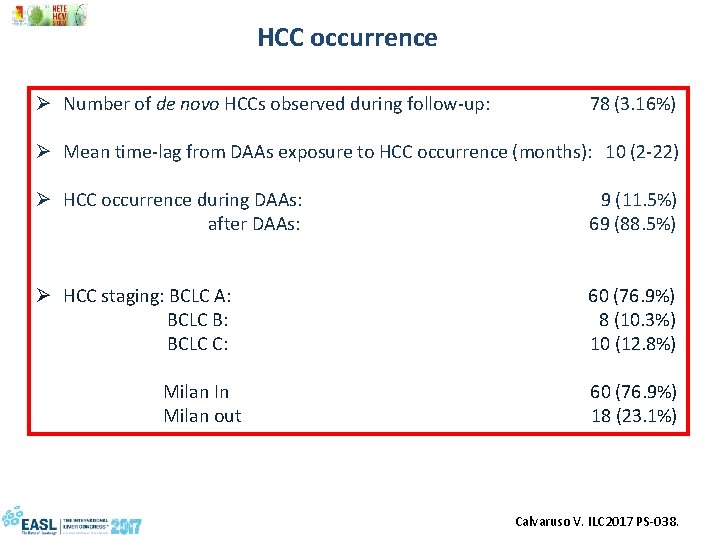
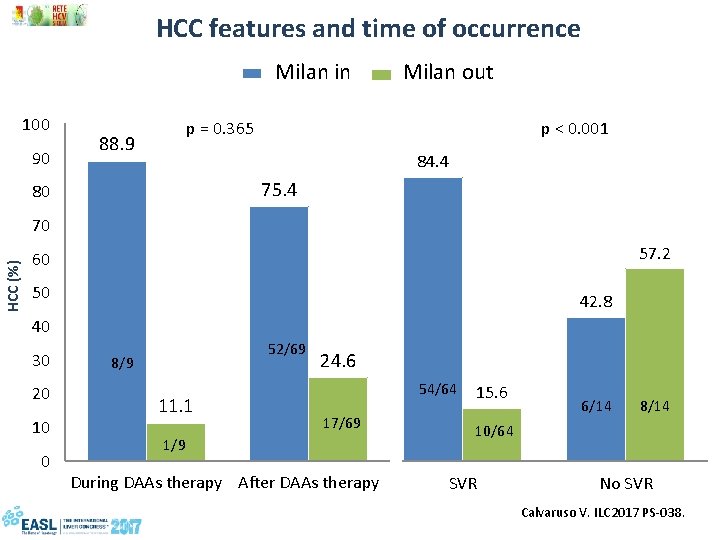
HCC occurrence Ø Number of de novo HCCs observed during follow-up: 78 (3. 16%) Ø Mean time-lag from DAAs exposure to HCC occurrence (months): 10 (2 -22) Ø HCC occurrence during DAAs: 9 (11. 5%) after DAAs: 69 (88. 5%) Ø HCC staging: BCLC A: 60 (76. 9%) BCLC B: 8 (10. 3%) BCLC C: 10 (12. 8%) Milan In 60 (76. 9%) Milan out 18 (23. 1%) Calvaruso V. ILC 2017 PS-038.

HCC features and time of occurrence Milan in Milan out 100 90 88. 9 p = 0. 365 90 75. 4 80 HCC (%) p < 0. 001 80 70 70 60 60 50 50 40 40 30 20 10 0 52/69 8/9 11. 1 1/9 84. 4 57. 2 42. 8 24. 630 20 17/69 10 0 During DAAs therapy After DAAs therapy 54/64 15. 6 6/14 8/14 10/64 SVR No SVR Calvaruso V. ILC 2017 PS-038.

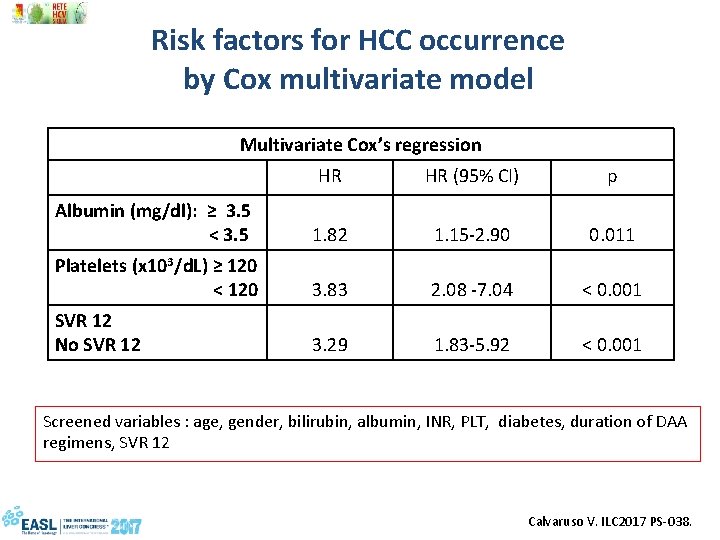
Risk factors for HCC occurrence by Cox multivariate model Multivariate Cox’s regression HR HR (95% CI) p Albumin (mg/dl): ≥ 3. 5 < 3. 5 1. 82 1. 15 -2. 90 0. 011 Platelets (x 103/d. L) ≥ 120 < 120 3. 83 2. 08 -7. 04 < 0. 001 SVR 12 No SVR 12 3. 29 1. 83 -5. 92 < 0. 001 Screened variables : age, gender, bilirubin, albumin, INR, PLT, diabetes, duration of DAA regimens, SVR 12 Calvaruso V. ILC 2017 PS-038.

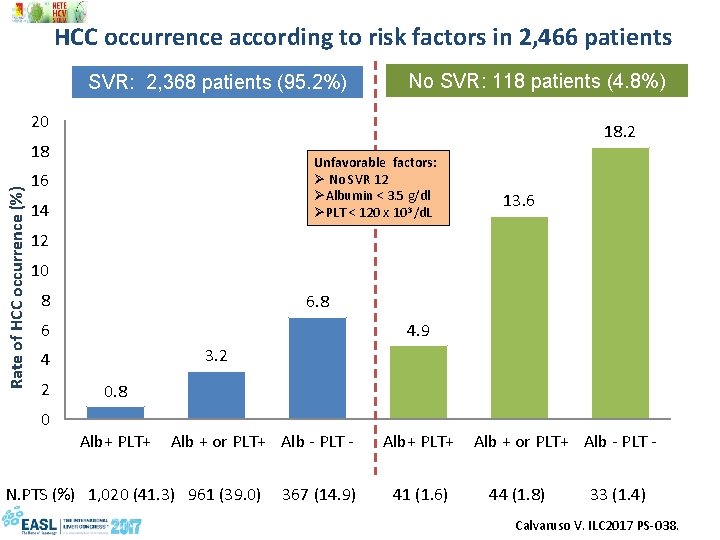
HCC occurrence according to risk factors in 2, 466 patients SVR: 2, 368 patients (95. 2%) No SVR: 118 patients (4. 8%) 20 18. 2 Rate of HCC occurrence (%) 18 Unfavorable factors: Ø No SVR 12 ØAlbumin < 3. 5 g/dl ØPLT < 120 x 103 /d. L 16 14 13. 6 12 10 8 6 4. 9 3. 2 4 2 0 0. 8 Alb+ PLT+ Alb + or PLT+ Alb - PLT - N. PTS (%) 1, 020 (41. 3) 961 (39. 0) 367 (14. 9) 41 (1. 6) 44 (1. 8) 33 (1. 4) Calvaruso V. ILC 2017 PS-038.

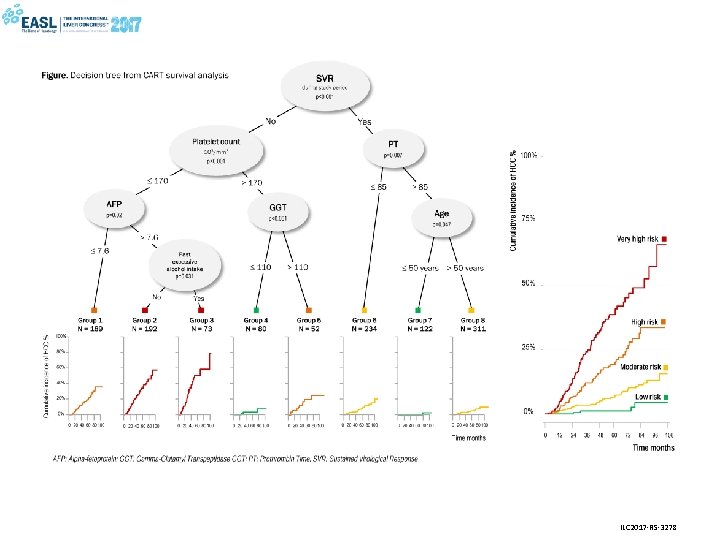
Etienne Audureau, Valérie Bourcier, Richard Layese, Carole Cagnot, Patrick Marcellin, Dominique Guyader, Stanislas Pol, Dominique Larrey, Françoise Roudot-Thoraval, Pierre Nahon and ANRS CO 12 Cir. Vir group IDENTIFYING RESIDUAL RISK OF HCC FOLLOWING HCV ERADICATION IN COMPENSATED CIRRHOSIS: DECISION-TREE AND RANDOM FOREST MODELS DEVELOPED IN THE FRENCH MULTICENTER PROSPECTIVE ANRS CO 12 CIRVIR COHORT

• Aim: To develop new prognostic algorithms for prediction of hepatocellular carcinoma (HCC) risk following HCV eradication in patients with compensated cirrhosis • Study population: 1253 patients from the French prospective multicentre ANRS CO 12 Cir. Vir cohort, of whom 637 (51%) patients achieved a sustained virological response (SVR) and 179 developed HCC (14%) • Methods: Four prognostic models (1) Cox proportional hazards regression, (2) Single decision tree by recursive partitioning (CART), (3) Random survival forest (RSF) and (4) Conditional random forest (CF) combining 1000 decision trees • Main results - Failure to achieve SVR was the most predictive factor of subsequent HCC in the 4 models - Cox modeling found 5 additional independent predictors : age>50 years, past excessive alcohol intake, low platelet count, and increased alpha-fetoprotein (AFP) and gamma-glutamyl transpeptidase (GGT) serum levels. - Decision tree analysis identified 4 classes of increasing risk with varying predictors depending on SVR status (next slide) - Random forest approaches confirmed the importance of the SVR status with more stable predictive performance in crossvalidation • - Conclusions Risk factors for HCC differ according to SVR status In patients with compensated cirrhosis, HCV eradication should be achieved before liver function is impaired Patients who have achieved SVR should be monitored for liver cancer after 50 years of age

ILC 2017 -RS-3278

Innes H, Barclay ST, Hayes PC, Fraser A, Dillon J, Stanley A, Bathgate A, Mc. Donald S, Goldberg D, Valerio H, Fox R, Kennedy N, Bramley P and Hutchinson SJ. THE RISK OF HEPATOCELLULAR CARCINOMA OCCURRENCE FOR CIRRHOTIC PATIENTS WITH A HEPATITIS C SUSTAINED VIRAL RESPONSE, BY TREATMENT REGIMEN: A NATIONWIDE COHORT STUDY.

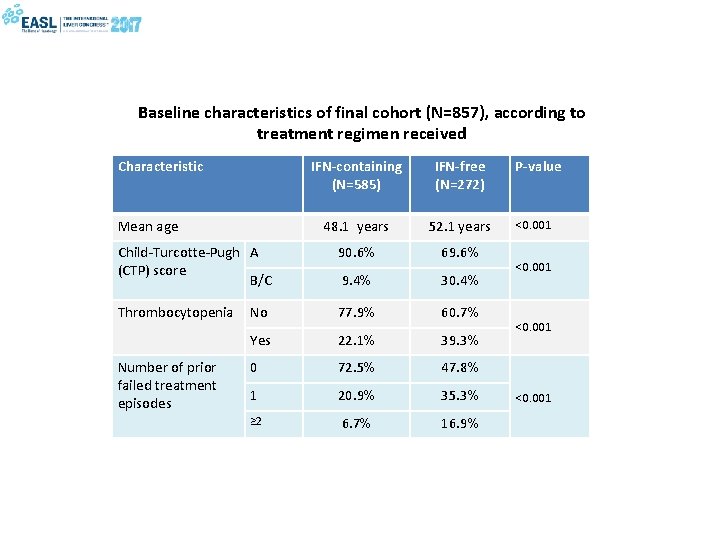
Baseline characteristics of final cohort (N=857), according to treatment regimen received Characteristic IFN-containing (N=585) IFN-free (N=272) 48. 1 years 52. 1 years Child-Turcotte-Pugh A (CTP) score B/C 90. 6% 69. 6% 9. 4% 30. 4% Thrombocytopenia No 77. 9% 60. 7% Yes 22. 1% 39. 3% 0 72. 5% 47. 8% 1 20. 9% 35. 3% ≥ 2 6. 7% 16. 9% Mean age Number of prior failed treatment episodes P-value <0. 001

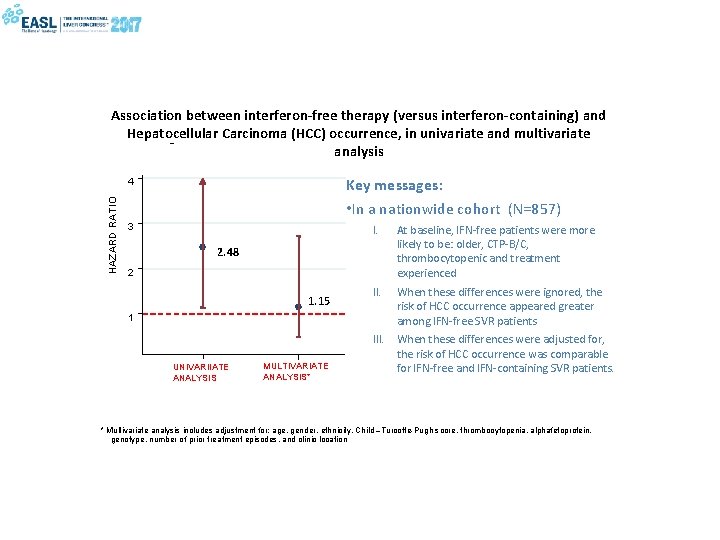
Association between interferon-free therapy (versus interferon-containing) and Hepatocellular Carcinoma (HCC) occurrence, in univariate and multivariate analysis Key messages: • In a nationwide cohort (N=857) HAZARD RATIO 4 3 I. At baseline, IFN-free patients were more likely to be: older, CTP-B/C, thrombocytopenic and treatment experienced II. When these differences were ignored, the risk of HCC occurrence appeared greater among IFN-free SVR patients 2. 48 2 1. 15 1 UNIVARIIATE ANALYSIS MULTIVARIATE ANALYSIS* III. When these differences were adjusted for, the risk of HCC occurrence was comparable for IFN-free and IFN-containing SVR patients. * Multivariate analysis includes adjustment for: age, gender, ethnicity, Child –Turcotte-Pugh score, thrombocytopenia, alphafetoprotein, genotype, number of prior treatment episodes, and clinic location

SUSTAINED VIROLOGIC RESPONSE BY LEDIPASVIR/SOFOSBUVIR REDUCES THE INCIDENCE OF HEPATOCELLULAR CARCINOMA IN JAPANESE PATIENTS WITH HCV GENOTYPE 1 INFECTION. - COMPARISON WITH SIMEPREVIR WITH PEGINTERFERON PLUS RIBAVIRIN Masaaki Korenaga 1, Namiki Izumi 2, Osamu Yokosuka 3, Tetsuo Takehara 4, Naoya Sakamoto 5, Syuhei Nishiguchi 6, Fusao Ikeda 7, Mikio Yanase 8, Hidenori Toyota 9, Takuya Genda 10, Takeji Umemura 11, Hiroshi Yatsuhashi 12, Tatsuya Ide 13, Nobuo Toda 14, Kazushige Nirei 15, Yoshiyuki Ueno 16, Youichi Nishigaki 17, Masao Omata 18, Masashi Mizokami 1 1 Kohnodai Hospital, National Center for Global Health and Medicine, Chiba, 2 Musashino Red Cross Hospital, Tokyo, 3 Chiba University, Chiba, 4 Osaka University, Osaka, 5 Hokkaido University, Sapporo, 6 Hyogo College of Medicine, Hyogo, 7 Okayama University, Okayama, 8 National Center for Global Health and Medicine, Tokyo, 9 Ogaki Municipal Hospital, Gifu, 10 Juntendo University Shizuoka Hospital, Shizuoka, 11 Shinshu University, Nagano, 12 National Hospital Organization Nagasaki Medical Center, Nagasaki, 13 Kurume University, Kurume, 14 Mitsui Memorial Hospital, 15 Nihon University School of Medicine, Tokyo, 16 Yamagata University, Yamagata, 17 Gifu Municipal Hospital, Gifu, 18 Yamanashi Prefectural Hospital

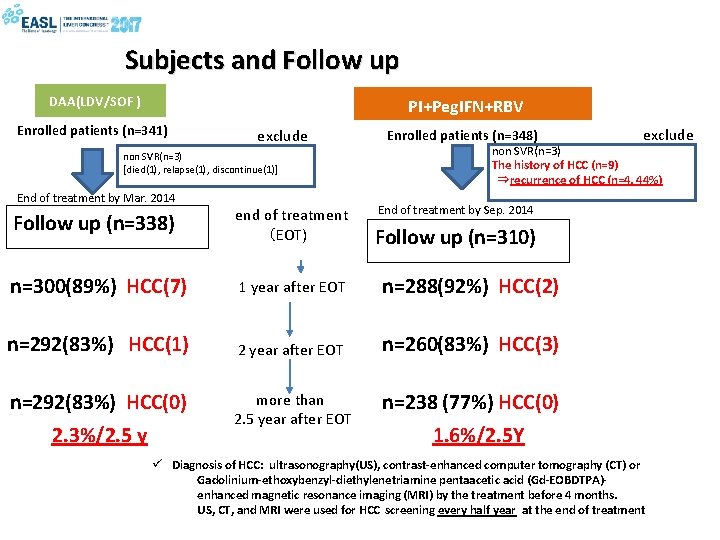
Subjects and Follow up DAA(LDV/SOF ) PI+Peg. IFN+RBV Enrolled patients (n=341) exclude non SVR(n=3) [died(1), relapse(1), discontinue(1)] End of treatment by Mar. 2014 Enrolled patients (n=348) non SVR(n=3) The history of HCC (n=9) ⇒recurrence of HCC (n=4, 44%) End of treatment by Sep. 2014 Follow up (n=338) end of treatment (EOT) n=300(89%) HCC(7) 1 year after EOT n=288(92%) HCC(2) n=292(83%) HCC(1) 2 year after EOT n=260(83%) HCC(3) n=292(83%) HCC(0) more than 2. 5 year after EOT n=238 (77%) HCC(0) 2. 3%/2. 5 y exclude Follow up (n=310) 1. 6%/2. 5 Y ü Diagnosis of HCC: ultrasonography(US), contrast-enhanced computer tomography (CT) or Gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid (Gd-EOBDTPA)enhanced magnetic resonance imaging (MRI) by the treatment before 4 months. US, CT, and MRI were used for HCC screening every half year at the end of treatment

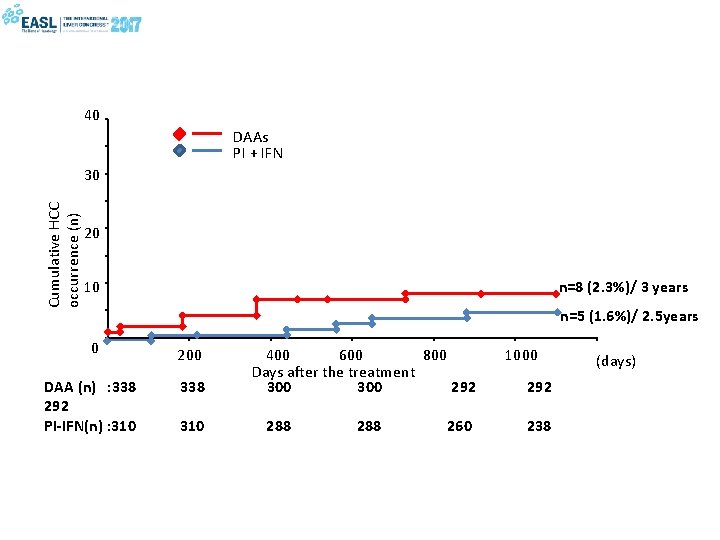
40 DAAs PI + IFN Cumulative HCC occurrence (n) 30 20 n=8 (2. 3%)/ 3 years 10 n=5 (1. 6%)/ 2. 5 years 0 200 DAA (n) : 338 292 PI-IFN(n) : 310 338 310 400 600 800 Days after the treatment 300 292 288 260 1000 292 238 (days)

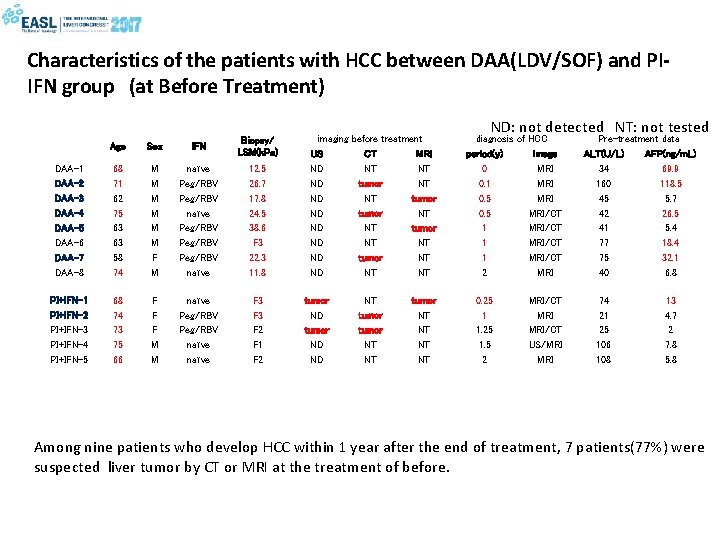
Characteristics of the patients with HCC between DAA(LDV/SOF) and PIIFN group (at Before Treatment) Age Sex IFN Biopsy/ LSM(k. Pa) DAA-1 DAA-2 DAA-3 DAA-4 DAA-5 DAA-6 DAA-7 DAA-8 68 71 62 75 63 63 58 74 M M M F M naïve Peg/RBV Peg/RBV naïve 12. 5 26. 7 17. 8 24. 5 38. 6 F 3 22. 3 11. 8 PI+IFN-1 PI+IFN-2 PI+IFN-3 PI+IFN-4 PI+IFN-5 68 74 73 75 66 F F F M M naïve Peg/RBV naïve F 3 F 2 F 1 F 2 imaging US ND ND tumor ND ND before treatment CT MRI NT NT tumor NT NT tumor NT NT tumor NT NT ND: not detected NT: not tested diagnosis of HCC period(y) image 0 MRI 0. 1 MRI 0. 5 MRI/CT 1 MRI/CT 2 MRI 0. 25 1 1. 25 1. 5 2 MRI/CT US/MRI Pre-treatment data ALT(U/L) AFP(ng/m. L) 34 69. 9 160 118. 5 45 5. 7 42 26. 5 41 5. 4 77 18. 4 75 32. 1 40 6. 8 74 21 25 106 108 13 4. 7 2 7. 8 5. 8 Among nine patients who develop HCC within 1 year after the end of treatment, 7 patients(77%) were suspected liver tumor by CT or MRI at the treatment of before.

No increase in the occurrence rate of HCC in Chinese treated by DAAs compared to IFN after eradication of HCV: a long-term follow-up Dong Ji * 1, 2, 3 , Cheng Wang 3, 4, 5 , Qing Shao 1, 3 , Fan Li 1, 3 , Bing Li 1, 3 , Vanessa Wu 3, 4 , April Wong 4 , Yu D. Wang 3, 4 , Jing Chen 3, 4 , Guo F. Chen 1, 3 , George Lau 3, 4, 6 1 Second Liver Cirrhosis Diagnosis and Treatment Center, 2 Liver Failure Treatment and Research Center, 3 Beijing 302 -Hong Kong Humanity and Health Hepatitis C Diagnosis and Treatment Centre, Beijing 302 Hospital, Beijing, China, 4 Division of Gastroenterology & Hepatology, Humanity & Health Medical Centre, Hong Kong, China, 5 State Key Laboratory of Organ Failure Research, Guangdong Provincial Key Laboratory of Viral Hepatitis Research, Department of Infectious Diseases, Nanfang Hospital, Southern Medical University, Guangzhou, 6 Institute of Translational Hepatology, Beijing 302 Hospital, Beijing, China

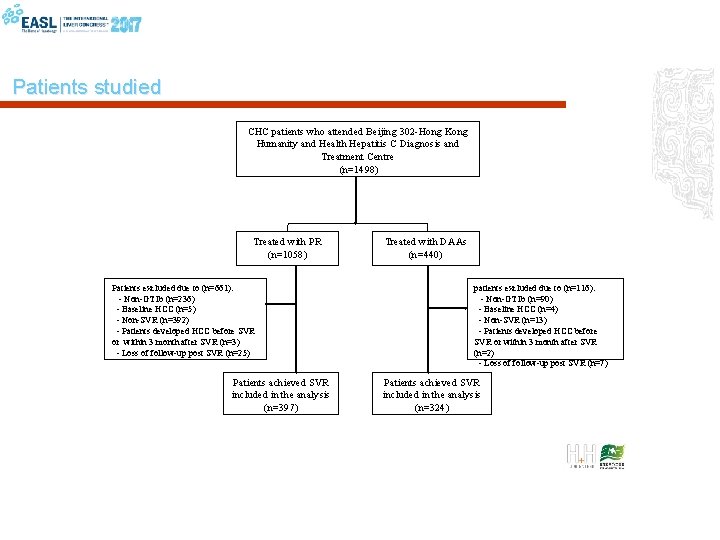
Patients studied CHC patients who attended Beijing 302 -Hong Kong Humanity and Health Hepatitis C Diagnosis and Treatment Centre (n=1498) Treated with PR (n=1058) Patients excluded due to (n=661): - Non-GT 1 b (n=236) - Baseline HCC (n=5) - Non-SVR (n=392) - Patients developed HCC before SVR or within 3 month after SVR (n=3) - Loss of follow-up post SVR (n=25) Patients achieved SVR included in the analysis (n=397) Treated with DAAs (n=440) patients excluded due to (n=116): - Non-GT 1 b (n=90) - Baseline HCC (n=4) - Non-SVR (n=13) - Patients developed HCC before SVR or within 3 month after SVR (n=2) - Loss of follow-up post SVR (n=7) Patients achieved SVR included in the analysis (n=324)

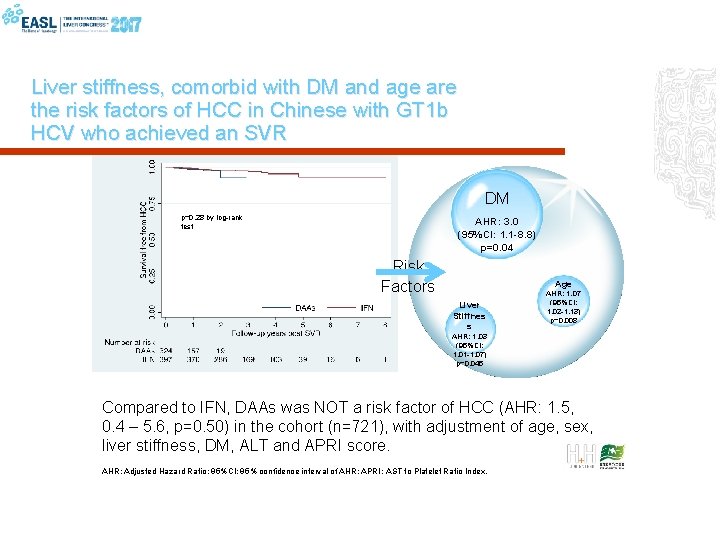
Liver stiffness, comorbid with DM and age are the risk factors of HCC in Chinese with GT 1 b HCV who achieved an SVR DM p=0. 28 by log-rank test AHR: 3. 0 (95%CI: 1. 1 -8. 8) p=0. 04 Risk Factors Age Liver Stiffnes s AHR: 1. 07 (95%CI: 1. 02 -1. 13) p=0. 008 AHR: 1. 03 (95%CI: 1. 01 -1. 07) p=0. 045 Compared to IFN, DAAs was NOT a risk factor of HCC (AHR: 1. 5, 0. 4 – 5. 6, p=0. 50) in the cohort (n=721), with adjustment of age, sex, liver stiffness, DM, ALT and APRI score. AHR: Adjusted Hazard Ratio; 95%CI: 95% confidence interval of AHR; APRI: AST to Platelet Ratio Index.

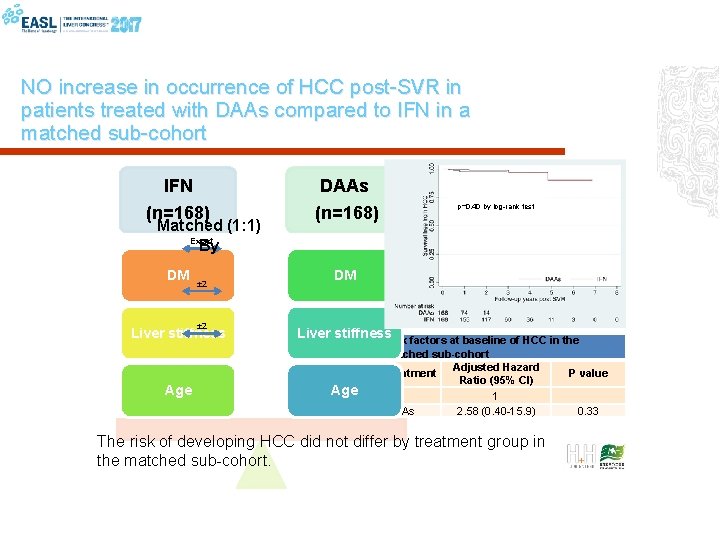
NO increase in occurrence of HCC post-SVR in patients treated with DAAs compared to IFN in a matched sub-cohort IFN DAAs (n=168) DM DM Matched (1: 1) Exact By ± 2 Liver stiffness Age p=0. 40 by log-rank test Liver stiffness Risk factors at baseline of HCC in the Age matched sub-cohort Adjusted Hazard Treatment Ratio (95% CI) IFN 1 DAAs 2. 58 (0. 40 -15. 9) The risk of developing HCC did not differ by treatment group in the matched sub-cohort. P value 0. 33

No evidence for higher risk of hepatocellular carcinoma occurrence or recurrence following direct-acting antiviral HCV therapy: A systematic review, meta-analyses, and meta-regression Waziry R 1, Hajarizadeh B 1, Grebely J 1, Amin J 2, Law M 1, Danta M 3, George J 4, Dore GJ 1 1 The Kirby Institute, UNSW Sydney, Australia, 2 Faculty of Medicine and Health Sciences, Macquarie University, Sydney, Australia, 3 St Vincent’s Clinical School, UNSW Sydney, Australia 4 Storr Liver Unit, Westmead Millennium Institute and Westmead Hospital, University of Sydney, Australia.

Background • Risk of hepatocellular carcinoma development following SVR with IFN in persons with advanced liver disease Morgan et al 2013. Annals of Internal Medicine, 158: 329 -337

Study aims 1. Compare risk of HCC occurrence following SVR with DAA vs IFN based therapy in patients with HCV-related cirrhosis 2. Compare risk of HCC recurrence following SVR with DAA vs IFN based therapy in patients who received curative HCC treatment

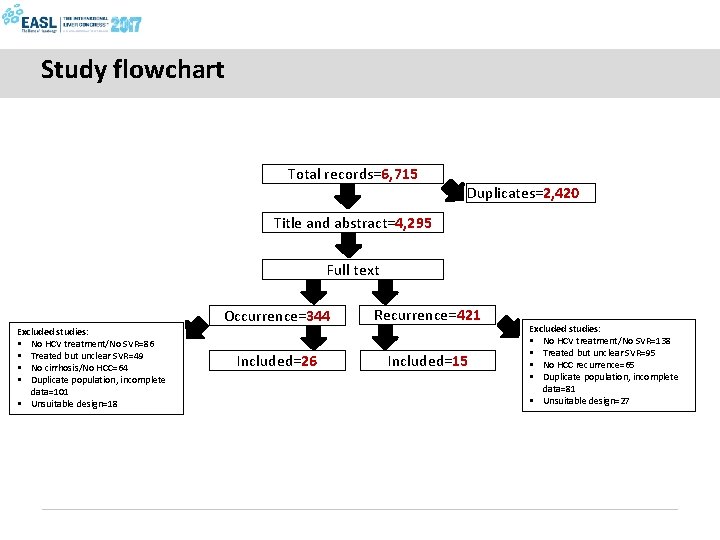
Study flowchart Total records=6, 715 Duplicates=2, 420 Title and abstract=4, 295 Full text Excluded studies: § No HCV treatment/No SVR=86 § Treated but unclear SVR=49 § No cirrhosis/No HCC=64 § Duplicate population, incomplete data=101 § Unsuitable design=18 Occurrence=344 Recurrence=421 Included=26 Included=15 Excluded studies: § No HCV treatment/No SVR=138 § Treated but unclear SVR=95 § No HCC recurrence=65 § Duplicate population, incomplete data=81 § Unsuitable design=27

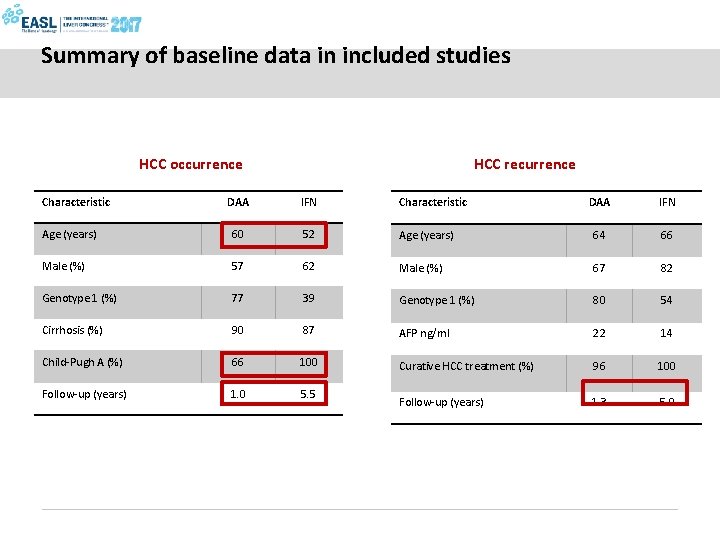
Summary of baseline data in included studies HCC occurrence HCC recurrence DAA IFN Characteristic DAA IFN Age (years) 60 52 Age (years) 64 66 Male (%) 57 62 Male (%) 67 82 Genotype 1 (%) 77 39 Genotype 1 (%) 80 54 Cirrhosis (%) 90 87 AFP ng/ml 22 14 Child-Pugh A (%) 66 100 Curative HCC treatment (%) 96 100 Follow-up (years) 1. 0 5. 5 Follow-up (years) 1. 3 5. 0 Characteristic

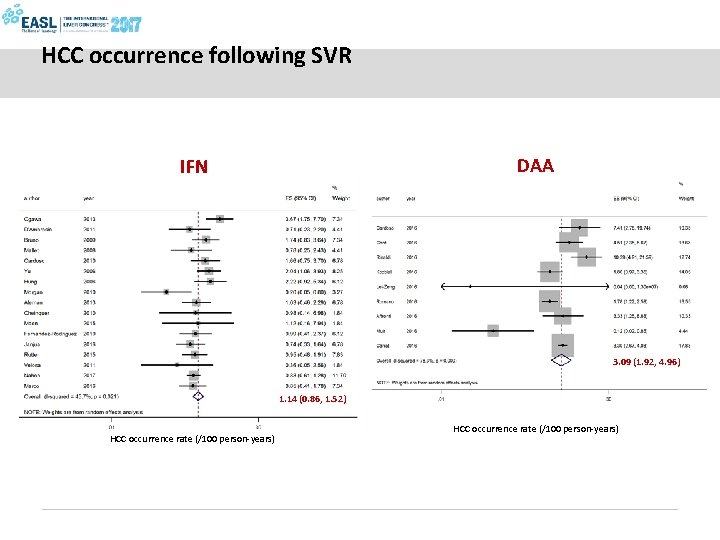
HCC occurrence following SVR DAA IFN 3. 09 (1. 92, 4. 96) 1. 14 (0. 86, 1. 52) HCC occurrence rate (/100 person-years)

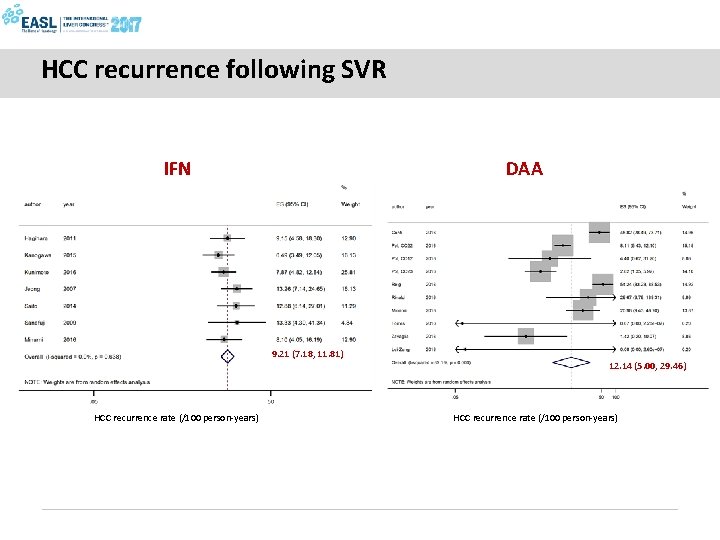
HCC recurrence following SVR IFN DAA 9. 21 (7. 18, 11. 81) 12. 14 (5. 00, 29. 46) HCC recurrence rate (/100 person-years)

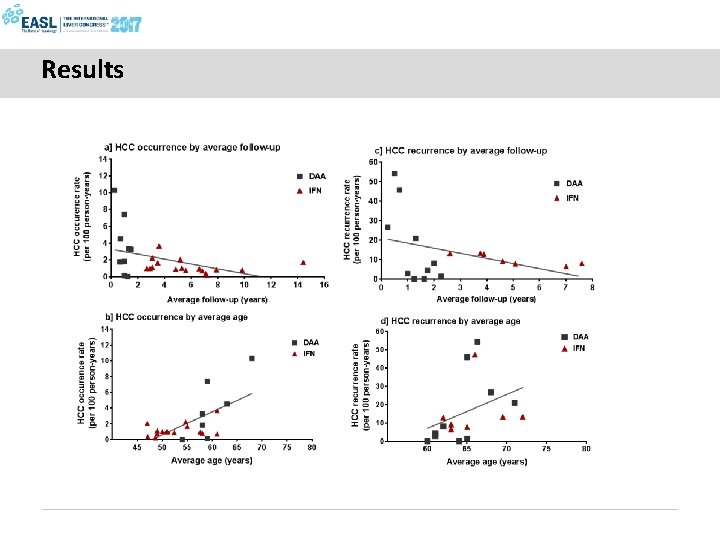
Results

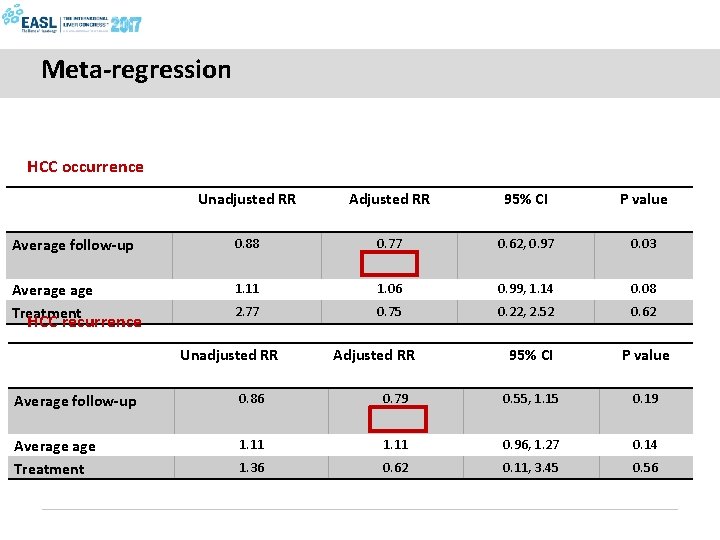
Meta-regression HCC occurrence Unadjusted RR Adjusted RR 95% CI P value Average follow-up 0. 88 0. 77 0. 62, 0. 97 0. 03 Average Treatment 1. 11 1. 06 0. 99, 1. 14 0. 08 2. 77 0. 75 0. 22, 2. 52 0. 62 HCC recurrence Unadjusted RR Adjusted RR 95% CI P value Average follow-up 0. 86 0. 79 0. 55, 1. 15 0. 19 Average Treatment 1. 11 0. 96, 1. 27 0. 14 1. 36 0. 62 0. 11, 3. 45 0. 56

Conclusions § No evidence for differential HCC occurrence or recurrence risk following SVR from DAA and IFN-based regimens § Higher incidence following DAA therapy can be explained by shorter duration follow-up (cohort effect), and older age (higher baseline risk)

A different behaviour of HCC after HCV clearance on DAAs?

Persisting drivers of post-SVR carcinogenesis? SVR HCV Tumor initiation/promotionsupporting niche • • Fibrosis? Somatic DNA aberrations? Epigenetic imprinting? …

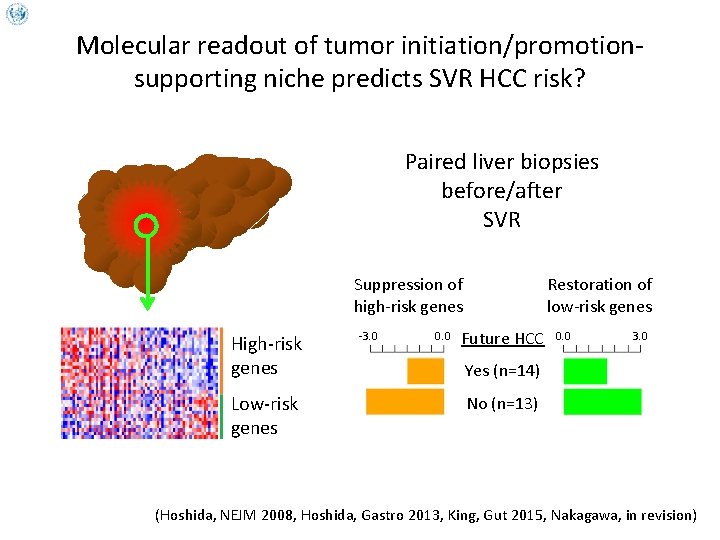
Molecular readout of tumor initiation/promotionsupporting niche predicts SVR HCC risk? Paired liver biopsies before/after SVR Suppression of high-risk genes High-risk genes Low-risk genes -3. 0 0. 0 Restoration of low-risk genes Future HCC 0. 0 3. 0 Yes (n=14) No (n=13) (Hoshida, NEJM 2008, Hoshida, Gastro 2013, King, Gut 2015, Nakagawa, in revision)

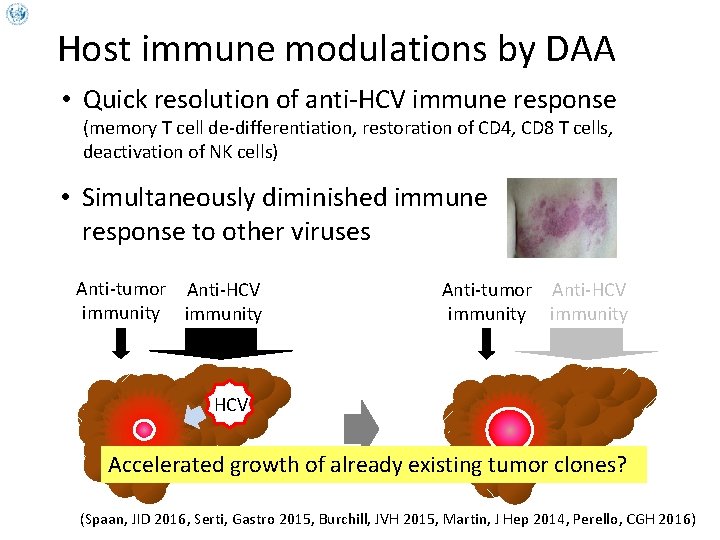
Host immune modulations by DAA • Quick resolution of anti-HCV immune response (memory T cell de-differentiation, restoration of CD 4, CD 8 T cells, deactivation of NK cells) • Simultaneously diminished immune response to other viruses Anti-tumor Anti-HCV immunity HCV Accelerated growth of already existing tumor clones? (Spaan, JID 2016, Serti, Gastro 2015, Burchill, JVH 2015, Martin, J Hep 2014, Perello, CGH 2016)

#LB-21, Tanaka: Genome-Wide Association Study Identifies a TLL 1 Variant Associated with Development of Hepatocellular Carcinoma after Eradication of HCV GWAS of 456 Japanese patients who achieved SVR by IFN-based therapy and either developed HCC at ≥ 1 yr after EOT (n=123) or did not develop HCC for ≥ 5 yr after EOT (n=333) Replication analysis was carried out on 79 candidate SNPs in 130 HCC and 356 non-HCC patients SNP rs 17047200, located within the intron of TLL 1 on chromosome 4, showed a strong association with HCC (OR=2. 37, p=2. 66 × 10 -8) Tll 1/TLL 1 m. RNA increased in liver tissues in rodent models and chronic HCV patients according to the progression of hepatic fibrosis Cumulative incidence of HCC up to 10 yr after EOT was significantly higher in patients with rs 17047200 AT/TT vs patients with AA in the GWAS (p=0. 009) vs patients in the replication cohort (p<0. 001) vs patients in the combined cohort (p<0. 001) Independent risk factors for HCC in multivariate analysis: • rs 17047200 AT/TT (HR=1. 86, p=0. 002) • • • Male gender Older age Lower platelet count Lower albumin level Higher post-treatment α-fetoprotein level TLL 1 may contribute to HCC development mainly via hepatic fibrogenesis, and genetic testing for the TLL 1 SNP could be useful for implementing personalized surveillance of HCC after SVR

Frank Jühling* 1, 2, Simonetta Bandiera 1, 2, Nourdine Hamdane 1, 2, Christine Thumann 1, 2, Sarah C. Durand 1, 2, Hussein El Saghire 1, 2, Irwin Davidson 3, 4, 5, François Habersetzer 1, 6, Patrick Pessaux 1, 6, Nabeel Bardeesy 7, Christian Schmidl 8, 9, 10, Christoph Bock 8, 9, 10, Yujin Hoshida 11, Mirjam B. Zeisel 1, 2, Thomas F. Baumert 1, 2, 6 1 INSERM U 1110, 2 Institut de Recherche sur les Maladies Virales et Hépatiques, Université de Strasbourg, 3 INSERM, 4 Université de Strasbourg, 5 CNRS, 6 Institut Hospitalo-Universitaire, Nouvel Hôpital Civil, Strasbourg, France, 7 Massachussetts General Hospital, Harvard Medical School, Boston, United States, 8 Ce. MM Research Center for Molecular Medicine, Austrian Academy of Sciences, 9 Department of Laboratory Medicine, Medical University of Vienna, Austria, 10 Max Planck Institute for Informatics, Saarbrücken, Germany, 11 Division of Liver Diseases, Department of Medicine, Liver Cancer Program, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai, New York City, United States HEPATITIS C VIRUS-INDUCED EPIGENETIC CHANGES PERSIST POST CURE

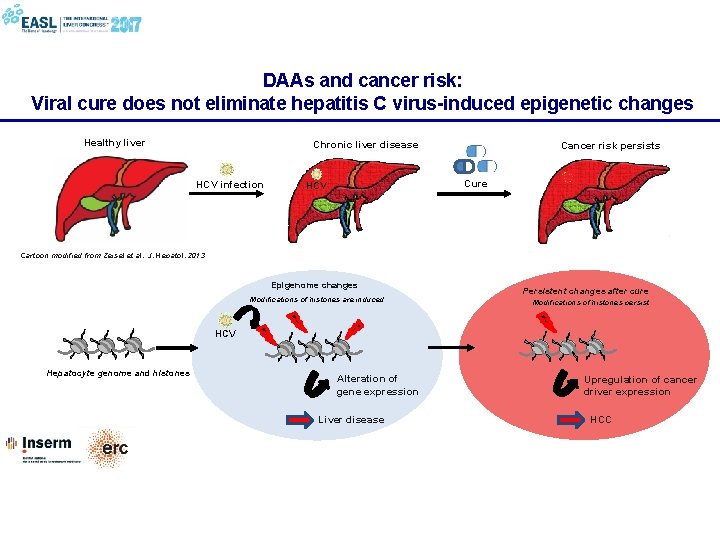
DAAs and cancer risk: Viral cure does not eliminate hepatitis C virus-induced epigenetic changes Healthy liver Chronic liver disease HCV infection Cancer risk persists Cure HCV Cartoon modified from Zeisel et al. , J. Hepatol. 2013 Epigenome changes Modifications of histones are induced Persistent changes after cure Modifications of histones persist HCV Hepatocyte genome and histones Alteration of gene expression Liver disease Upregulation of cancer driver expression HCC

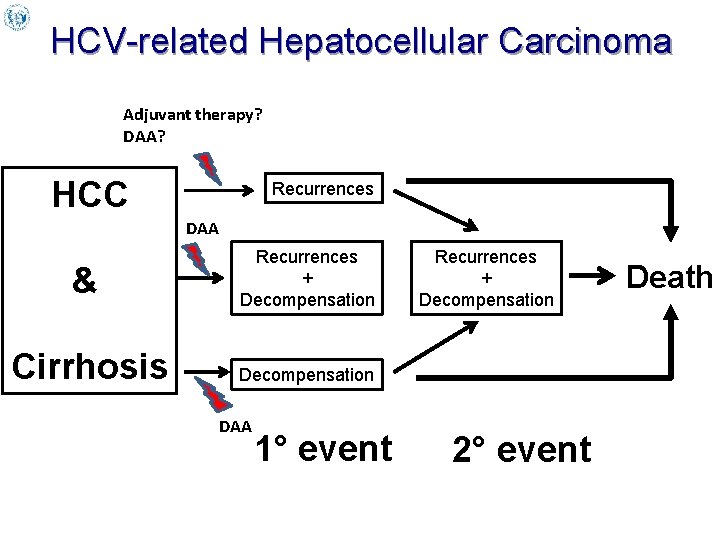
HCV-related Hepatocellular Carcinoma Adjuvant therapy? DAA? HCC Recurrences DAA & Recurrences + Decompensation Cirrhosis Decompensation DAA 1° event Recurrences + Decompensation 2° event Death

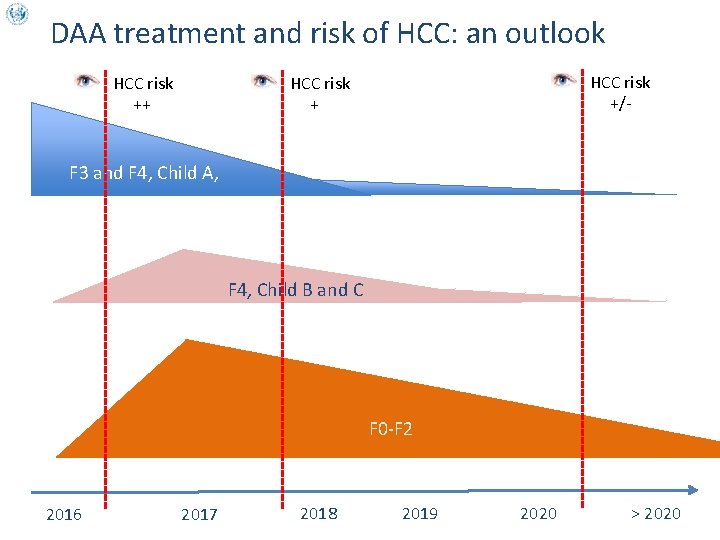
DAA treatment and risk of HCC: an outlook HCC risk ++ HCC risk +/- HCC risk + F 3 and F 4, Child A, F 4, Child B and C F 0 -F 2 2016 2017 2018 2019 2020 > 2020

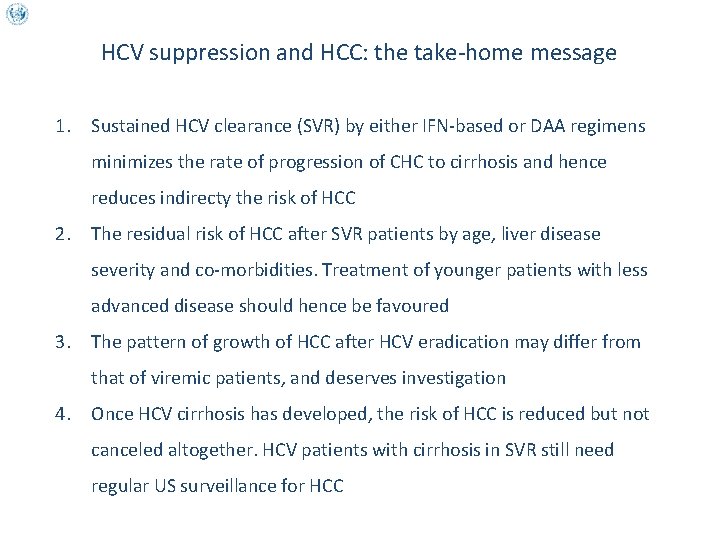
HCV suppression and HCC: the take-home message 1. Sustained HCV clearance (SVR) by either IFN-based or DAA regimens minimizes the rate of progression of CHC to cirrhosis and hence reduces indirecty the risk of HCC 2. The residual risk of HCC after SVR patients by age, liver disease severity and co-morbidities. Treatment of younger patients with less advanced disease should hence be favoured 3. The pattern of growth of HCC after HCV eradication may differ from that of viremic patients, and deserves investigation 4. Once HCV cirrhosis has developed, the risk of HCC is reduced but not canceled altogether. HCV patients with cirrhosis in SVR still need regular US surveillance for HCC