Colligative Properties How solutes affect the properties of









- Slides: 16

Colligative Properties How solutes affect the properties of solutions.

Three Major Effects of Solute • Reduces the vapor pressure of the solvent in the solution. • Lowers the freezing point of the solution. • Raises the boiling point of the solution.

What determines colligative properties? • Colligative properties depend on the concentration of the solute particles. • They DO NOT depend on what the solute particles are.

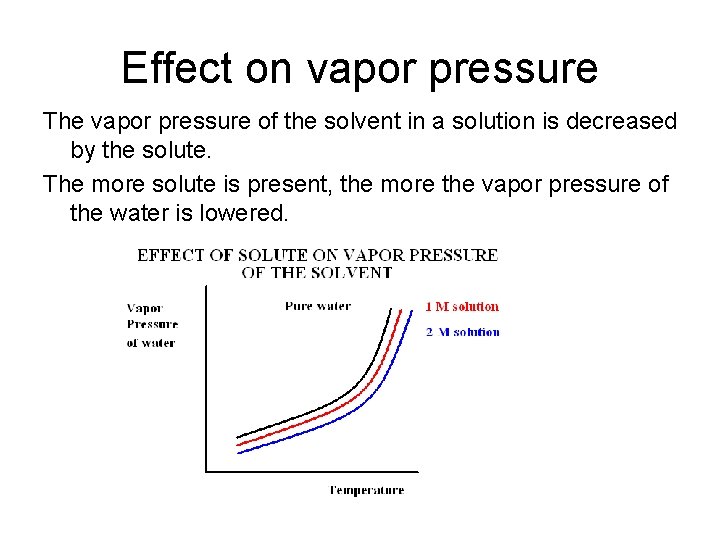
Effect on vapor pressure The vapor pressure of the solvent in a solution is decreased by the solute. The more solute is present, the more the vapor pressure of the water is lowered.

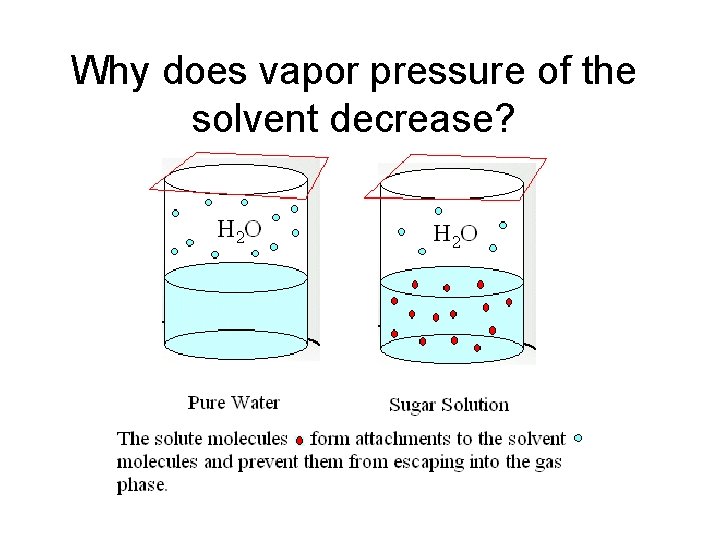
Why does vapor pressure of the solvent decrease?

Effect on Freezing Point The addition of a solute to a solvent causes the freezing point of the solution to decrease.

Car Radiators & antifreeze Antifreeze is added to a car’s cooling system to keep it from freezing and damaging the engine when the temperature falls below freezing.

Definition of Boiling Point The temperature at which the vapor pressure of a liquid becomes equal to the pressure above the liquid.

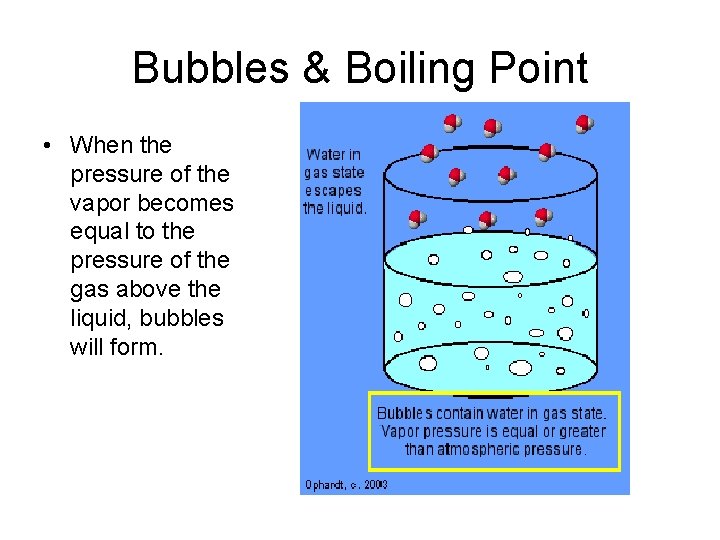
Bubbles & Boiling Point • When the pressure of the vapor becomes equal to the pressure of the gas above the liquid, bubbles will form.

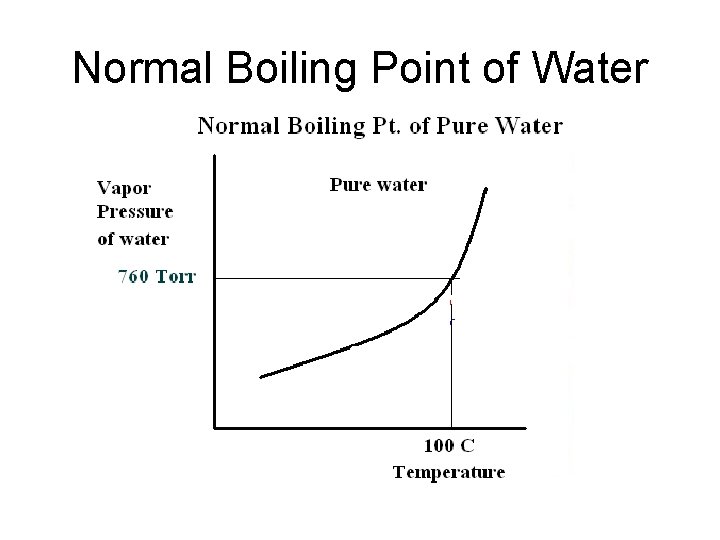
Normal Boiling Point of Water

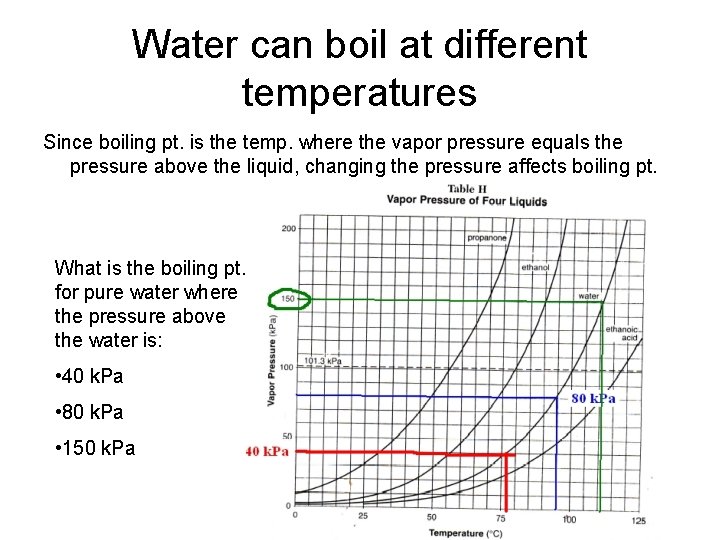
Water can boil at different temperatures Since boiling pt. is the temp. where the vapor pressure equals the pressure above the liquid, changing the pressure affects boiling pt. What is the boiling pt. for pure water where the pressure above the water is: • 40 k. Pa • 80 k. Pa • 150 k. Pa

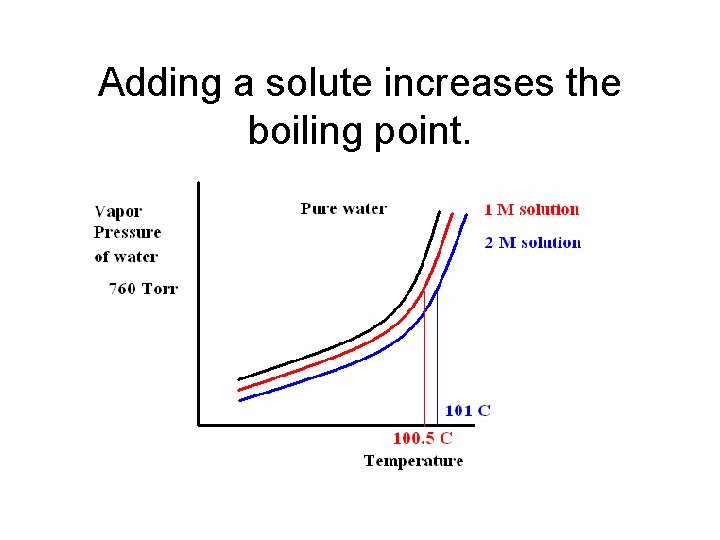
Adding a solute increases the boiling point.

Effect of solute on Boiling Point • The addition of a solute to a solvent raises the boiling point of the solution. During hot weather, the antifreeze in the car radiator system prevents the radiator fluid from boiling over and having the car engine overheat.

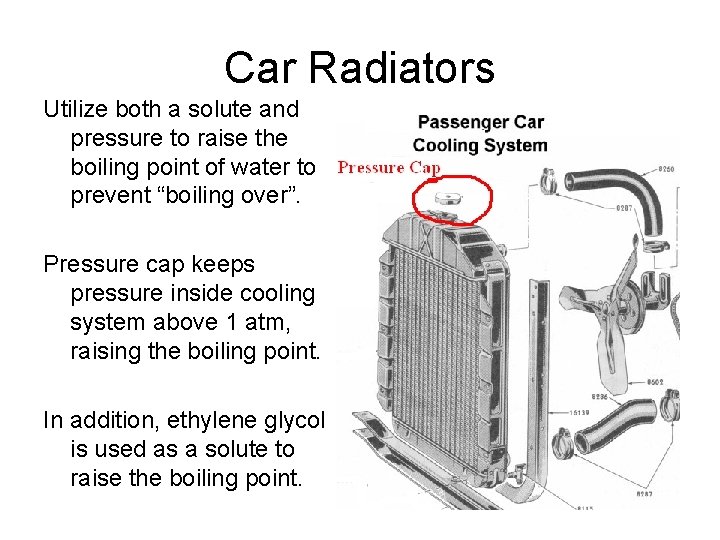
Car Radiators Utilize both a solute and pressure to raise the boiling point of water to prevent “boiling over”. Pressure cap keeps pressure inside cooling system above 1 atm, raising the boiling point. In addition, ethylene glycol is used as a solute to raise the boiling point.

Review 1 Adding a solute to a solvent: • Decreases the vapor pressure of the solvent • Decreases the freezing point of the solution • Increases the Boiling point of the solution • The properties listed above depend on the concentration of the solute particles, not what the particles are.

Review 2 Boiling point is the temperature where the vapor pressure of the liquid equals the pressure above the liquid. At this temperature bubbles begin to form. Normal boiling point is the temperature that the liquid boils when the pressure above the liquid is 1 atm (or 101 k. Pa or 760 Torr) The boiling point of a solvent depends on: • What the solvent is • What the pressure above the solvent is. • The concentration of solute particles dissolved in the solvent.