CYTOMEGALOVIRUS CMV IN PEDIATRIC SOLID ORGAN TRANSPLANT SOT










- Slides: 62

CYTOMEGALOVIRUS (CMV) IN PEDIATRIC SOLID ORGAN TRANSPLANT (SOT) RECIPIENTS A Pediatric Transplant Infectious Diseases Learning Module

Using the Modules l l l The modules are case-based, with decision points (branches) containing questions ¾ Many questions don’t have right or wrong answers ¾ Click on a response (e. g. a diagnostic test), and you will find out more about it Multiple “action buttons” help you navigate The basic modules are designed to take about 45 minutes to complete You might take longer, especially if you choose to investigate all of the informational links provided Take your time and enjoy!

Navigating the Modules l l l DO NOT use your keyboard arrows or mouse click to advance slides Only use the navigation buttons on each slide – these will keep you from getting lost ¾ If you do get lost, you can hit the “home” button any time to go back Unlabeled button types you might encounter: ¾ Previous slide ¾ Next slide ¾ First slide ¾ More information ¾ Return to decision choices

To Navigate module, please use BACK and NEXT buttons or master navigation slide at the end of the module Top Left corner: correlates to the American Board of Pediatrics, Pediatric ID content reference This slide is NOT part of case presentation. Provided for reference only.

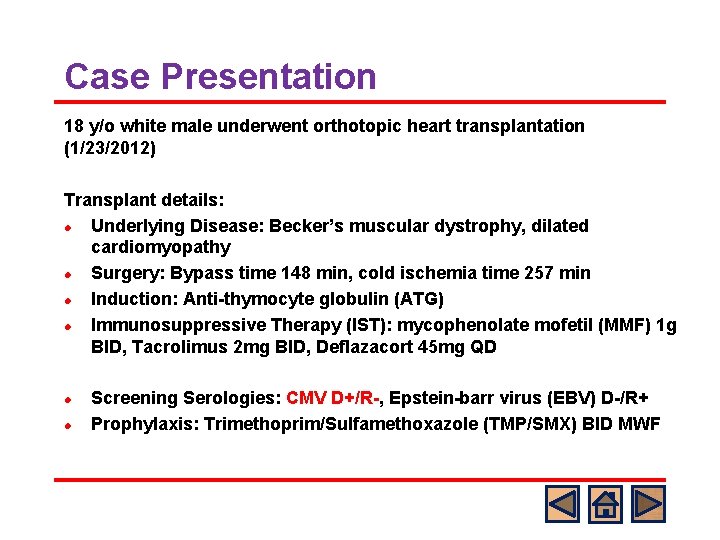
Case Presentation 18 y/o white male underwent orthotopic heart transplantation (1/23/2012) Transplant details: l Underlying Disease: Becker’s muscular dystrophy, dilated cardiomyopathy l Surgery: Bypass time 148 min, cold ischemia time 257 min l Induction: Anti-thymocyte globulin (ATG) l Immunosuppressive Therapy (IST): mycophenolate mofetil (MMF) 1 g BID, Tacrolimus 2 mg BID, Deflazacort 45 mg QD l l Screening Serologies: CMV D+/R-, Epstein-barr virus (EBV) D-/R+ Prophylaxis: Trimethoprim/Sulfamethoxazole (TMP/SMX) BID MWF

Q 1 What would you do to prevent CMV infection and disease? A) B) C) D) Start valganciclovir (val. GCV) 900 mg q day Start val. GCV 900 mg BID Start IV ganciclovir (GCV) 5 mg/kg q 12 Start IV foscarnet

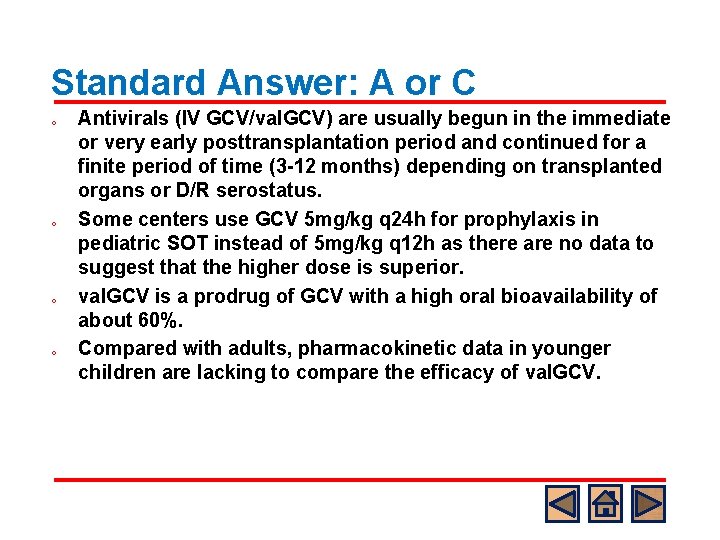
Standard Answer: A or C o o Antivirals (IV GCV/val. GCV) are usually begun in the immediate or very early posttransplantation period and continued for a finite period of time (3 -12 months) depending on transplanted organs or D/R serostatus. Some centers use GCV 5 mg/kg q 24 h for prophylaxis in pediatric SOT instead of 5 mg/kg q 12 h as there are no data to suggest that the higher dose is superior. val. GCV is a prodrug of GCV with a high oral bioavailability of about 60%. Compared with adults, pharmacokinetic data in younger children are lacking to compare the efficacy of val. GCV.

8

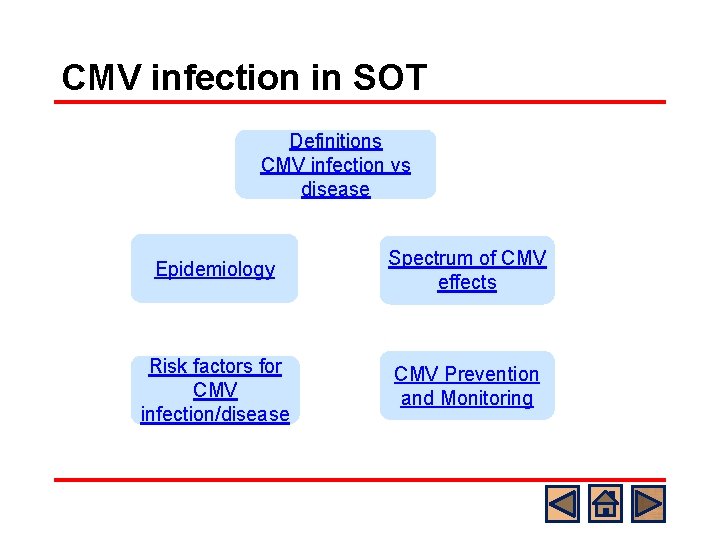
CMV infection in SOT Definitions CMV infection vs disease Epidemiology Spectrum of CMV effects Risk factors for CMV infection/disease CMV Prevention and Monitoring

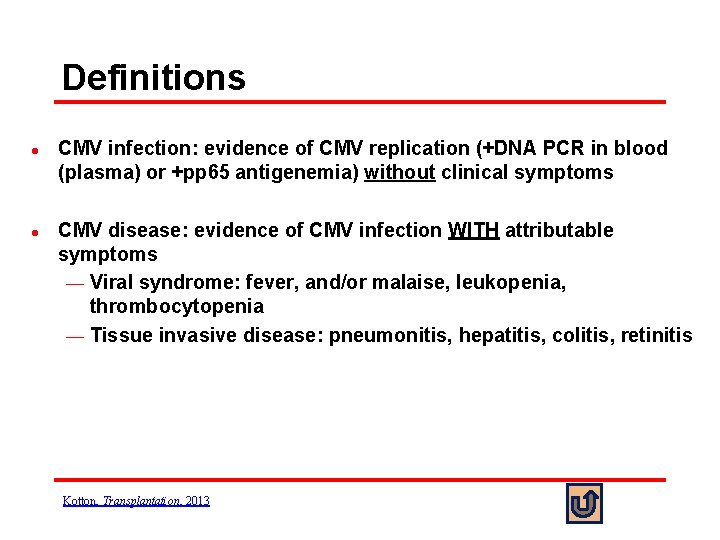
Definitions l l CMV infection: evidence of CMV replication (+DNA PCR in blood (plasma) or +pp 65 antigenemia) without clinical symptoms CMV disease: evidence of CMV infection WITH attributable symptoms ¾ Viral syndrome: fever, and/or malaise, leukopenia, thrombocytopenia ¾ Tissue invasive disease: pneumonitis, hepatitis, colitis, retinitis Kotton, Transplantation, 2013

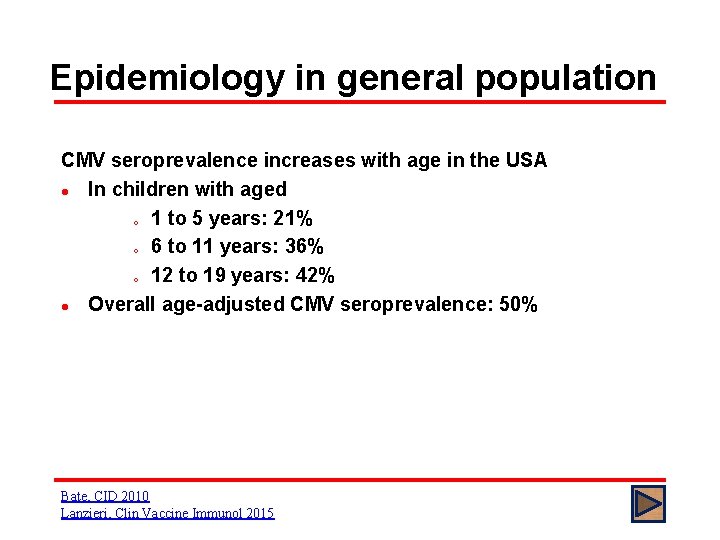
Epidemiology in general population CMV seroprevalence increases with age in the USA l In children with aged o 1 to 5 years: 21% o 6 to 11 years: 36% o 12 to 19 years: 42% l Overall age-adjusted CMV seroprevalence: 50% Bate, CID 2010 Lanzieri, Clin Vaccine Immunol 2015

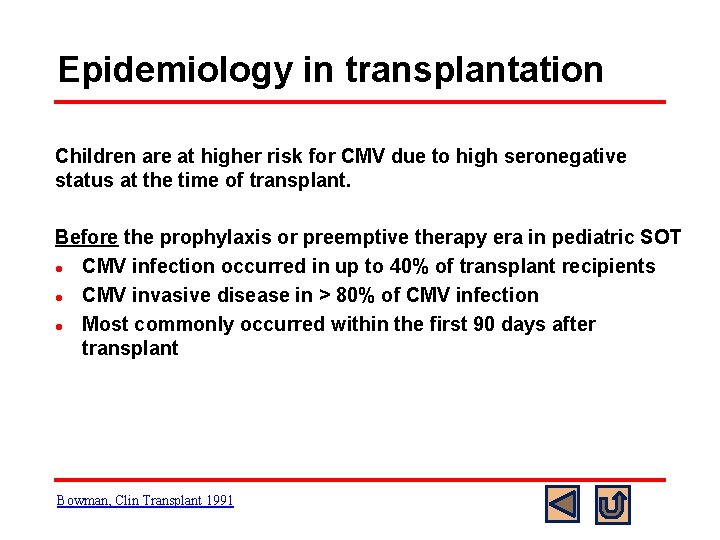
Epidemiology in transplantation Children are at higher risk for CMV due to high seronegative status at the time of transplant. Before the prophylaxis or preemptive therapy era in pediatric SOT l CMV infection occurred in up to 40% of transplant recipients l CMV invasive disease in > 80% of CMV infection l Most commonly occurred within the first 90 days after transplant Bowman, Clin Transplant 1991

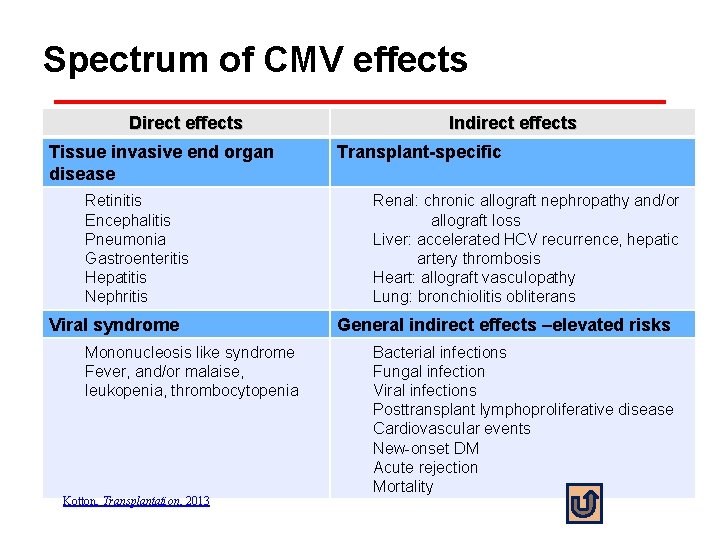
Spectrum of CMV effects Direct effects Tissue invasive end organ disease Retinitis Encephalitis Pneumonia Gastroenteritis Hepatitis Nephritis Viral syndrome Mononucleosis like syndrome Fever, and/or malaise, leukopenia, thrombocytopenia Kotton, Transplantation, 2013 Indirect effects Transplant-specific Renal: chronic allograft nephropathy and/or allograft loss Liver: accelerated HCV recurrence, hepatic artery thrombosis Heart: allograft vasculopathy Lung: bronchiolitis obliterans General indirect effects –elevated risks Bacterial infections Fungal infection Viral infections Posttransplant lymphoproliferative disease Cardiovascular events New-onset DM Acute rejection Mortality

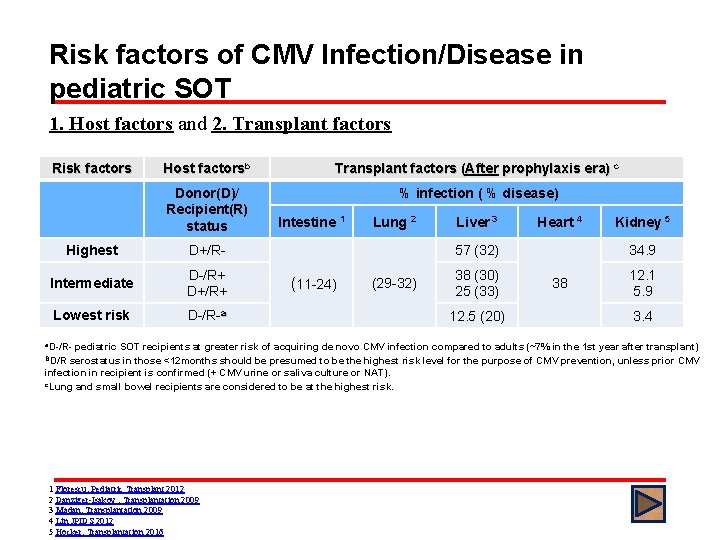
Risk factors of CMV Infection/Disease in pediatric SOT 1. Host factors and 2. Transplant factors Risk factors Host factorsb Transplant factors (After prophylaxis era) c Donor(D)/ Recipient(R) status % infection ( % disease) Highest D+/R- Intermediate D-/R+ D+/R+ Lowest risk D-/R-a Intestine 1 Lung 2 Liver 3 Heart 4 57 (32) (11 -24) (29 -32) 38 (30) 25 (33) 12. 5 (20) Kidney 5 34. 9 38 12. 1 5. 9 3. 4 a. D-/R- pediatric SOT recipients at greater risk of acquiring de novo CMV infection compared to adults (~7% in the 1 st year after transplant) BD/R serostatus in those <12 months should be presumed to be the highest risk level for the purpose of CMV prevention, unless prior CMV infection in recipient is confirmed (+ CMV urine or saliva culture or NAT). c. Lung and small bowel recipients are considered to be at the highest risk. 1 Florescu, Pediatric Transplant 2012 2 Danziger-Isakov , Transplantation 2009 3 Madan, Transplantation 2009 4 Lin JPIDS 2012 5 Hocker, Transplantation 2016

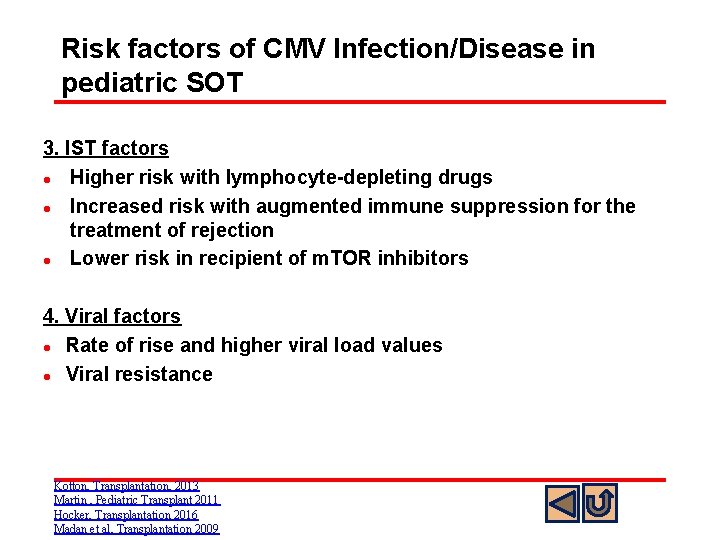
Risk factors of CMV Infection/Disease in pediatric SOT 3. IST factors l Higher risk with lymphocyte-depleting drugs l Increased risk with augmented immune suppression for the treatment of rejection l Lower risk in recipient of m. TOR inhibitors 4. Viral factors l Rate of rise and higher viral load values l Viral resistance Kotton, Transplantation, 2013 Martin , Pediatric Transplant 2011 Hocker, Transplantation 2016 Madan et al, Transplantation 2009

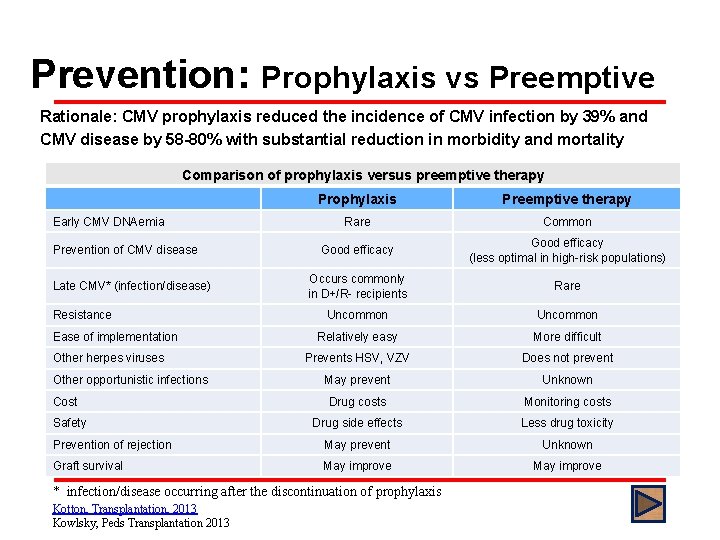
Prevention: Prophylaxis vs Preemptive Rationale: CMV prophylaxis reduced the incidence of CMV infection by 39% and CMV disease by 58 -80% with substantial reduction in morbidity and mortality Comparison of prophylaxis versus preemptive therapy Prophylaxis Preemptive therapy Rare Common Good efficacy (less optimal in high-risk populations) Occurs commonly in D+/R- recipients Rare Uncommon Relatively easy More difficult Prevents HSV, VZV Does not prevent May prevent Unknown Drug costs Monitoring costs Drug side effects Less drug toxicity Prevention of rejection May prevent Unknown Graft survival May improve Early CMV DNAemia Prevention of CMV disease Late CMV* (infection/disease) Resistance Ease of implementation Other herpes viruses Other opportunistic infections Cost Safety * infection/disease occurring after the discontinuation of prophylaxis Kotton, Transplantation, 2013 Kowlsky, Peds Transplantation 2013

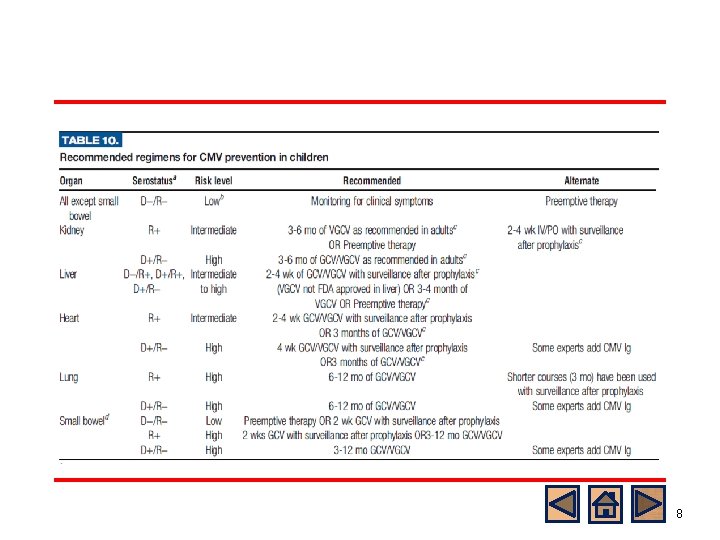
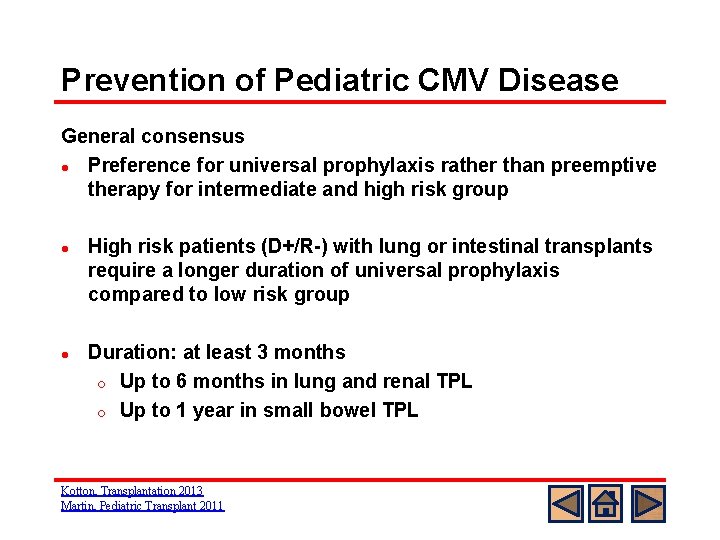
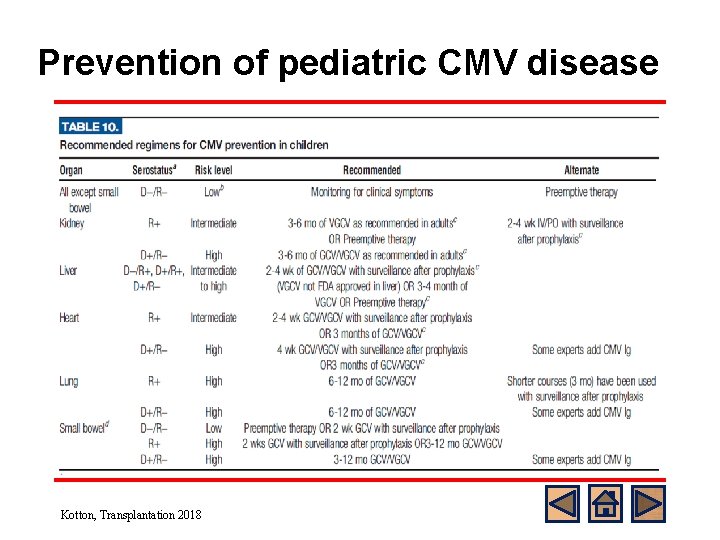
Prevention of Pediatric CMV Disease General consensus l Preference for universal prophylaxis rather than preemptive therapy for intermediate and high risk group l High risk patients (D+/R-) with lung or intestinal transplants require a longer duration of universal prophylaxis compared to low risk group l Duration: at least 3 months o Up to 6 months in lung and renal TPL o Up to 1 year in small bowel TPL Kotton, Transplantation 2013 Martin, Pediatric Transplant 2011

Prevention of pediatric CMV disease Kotton, Transplantation 2018

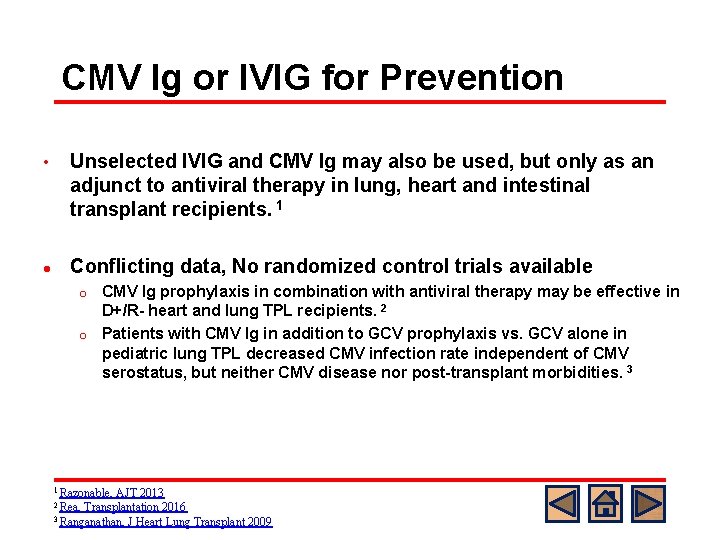
CMV Ig or IVIG for Prevention • l Unselected IVIG and CMV Ig may also be used, but only as an adjunct to antiviral therapy in lung, heart and intestinal transplant recipients. 1 Conflicting data, No randomized control trials available CMV Ig prophylaxis in combination with antiviral therapy may be effective in D+/R- heart and lung TPL recipients. 2 o Patients with CMV Ig in addition to GCV prophylaxis vs. GCV alone in pediatric lung TPL decreased CMV infection rate independent of CMV serostatus, but neither CMV disease nor post-transplant morbidities. 3 o 1 Razonable, AJT 2013 2 Rea, Transplantation 2016 3 Ranganathan, J Heart Lung Transplant 2009

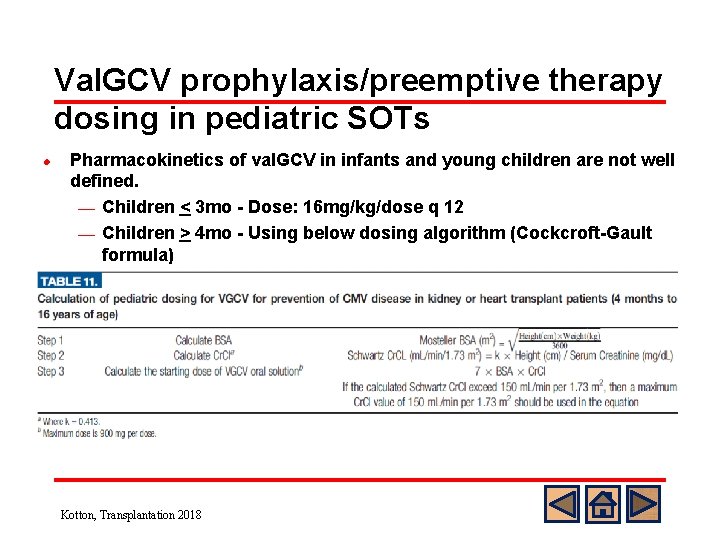
Val. GCV prophylaxis/preemptive therapy dosing in pediatric SOTs l Pharmacokinetics of val. GCV in infants and young children are not well defined. ¾ Children < 3 mo - Dose: 16 mg/kg/dose q 12 ¾ Children > 4 mo - Using below dosing algorithm (Cockcroft-Gault formula) Kotton, Transplantation 2018

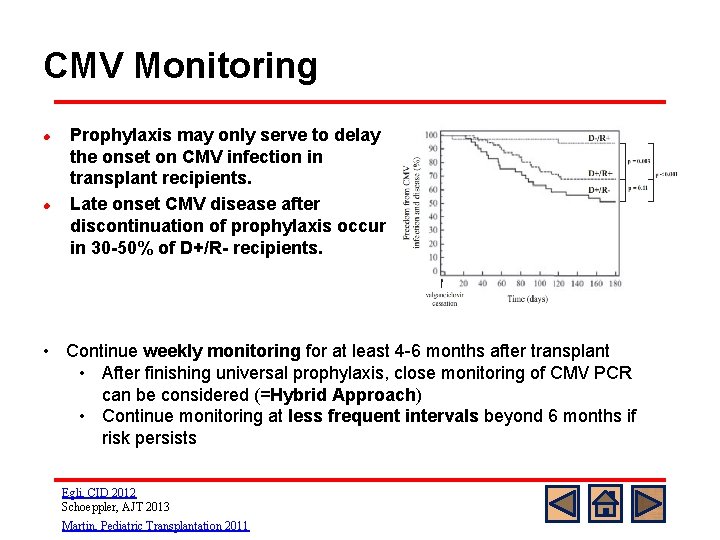
CMV Monitoring l l Prophylaxis may only serve to delay the onset on CMV infection in transplant recipients. Late onset CMV disease after discontinuation of prophylaxis occurs in 30 -50% of D+/R- recipients. • Continue weekly monitoring for at least 4 -6 months after transplant • After finishing universal prophylaxis, close monitoring of CMV PCR can be considered (=Hybrid Approach) • Continue monitoring at less frequent intervals beyond 6 months if risk persists Egli, CID 2012 Schoeppler, AJT 2013 Martin, Pediatric Transplantation 2011

Immunological Monitoring for CMV l Monitoring of CMV-specific T-cell responses can be: Alternative to serology in accurately determining CMV immune status in adults and children with passively transfused or maternal antibodies before TPLs o Adjuvant tool to guide prophylaxis and preemptive therapies after TPLs; personalizing CMV prevention strategies, standard in some adult centers o Useful method to select the patients who are at risk for late-onset CMV disease after prophylaxis or pre-emptive therapy. o Kotton, Transplantation, 2013

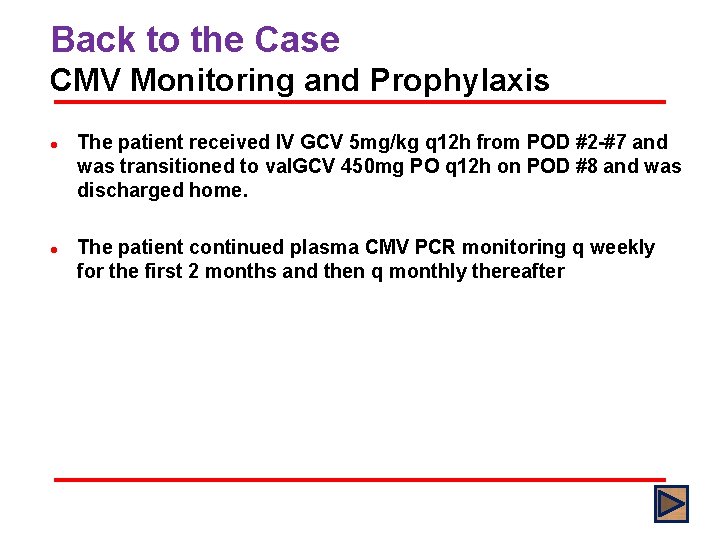
Back to the Case CMV Monitoring and Prophylaxis l l The patient received IV GCV 5 mg/kg q 12 h from POD #2 -#7 and was transitioned to val. GCV 450 mg PO q 12 h on POD #8 and was discharged home. The patient continued plasma CMV PCR monitoring q weekly for the first 2 months and then q monthly thereafter

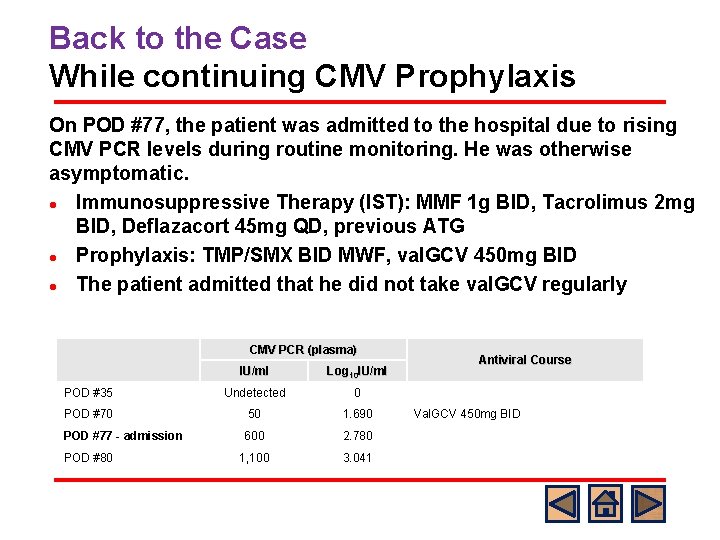
Back to the Case While continuing CMV Prophylaxis On POD #77, the patient was admitted to the hospital due to rising CMV PCR levels during routine monitoring. He was otherwise asymptomatic. l Immunosuppressive Therapy (IST): MMF 1 g BID, Tacrolimus 2 mg BID, Deflazacort 45 mg QD, previous ATG l Prophylaxis: TMP/SMX BID MWF, val. GCV 450 mg BID l The patient admitted that he did not take val. GCV regularly CMV PCR (plasma) IU/ml Log 10 IU/ml POD #35 Undetected 0 POD #70 50 1. 690 POD #77 - admission 600 2. 780 1, 100 3. 041 POD #80 Antiviral Course Val. GCV 450 mg BID

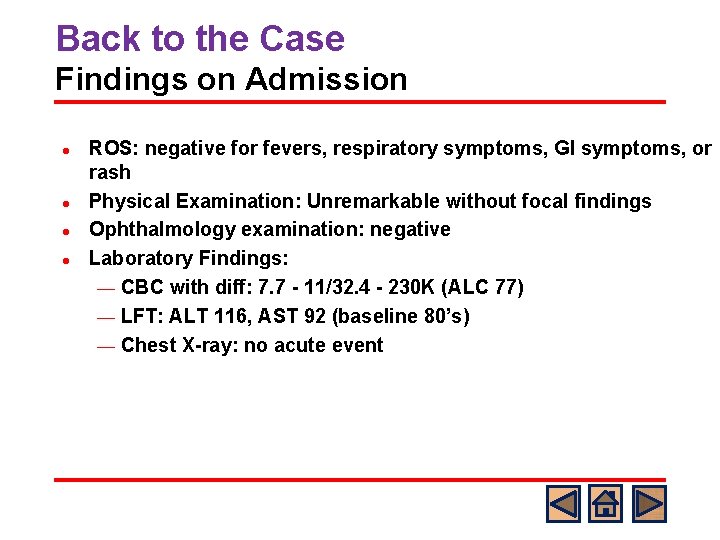
Back to the Case Findings on Admission l l ROS: negative for fevers, respiratory symptoms, GI symptoms, or rash Physical Examination: Unremarkable without focal findings Ophthalmology examination: negative Laboratory Findings: ¾ CBC with diff: 7. 7 - 11/32. 4 - 230 K (ALC 77) ¾ LFT: ALT 116, AST 92 (baseline 80’s) ¾ Chest X-ray: no acute event

What Would You do Next? A) Continue current dose of val. GCV 900 mg q day B) Increase val. GCV dose to 900 mg BID C) Start IV GCV 5 mg/kg q 12 D) Start IV foscarnet

Standard Answer: B or C o o In adult SOT recipients with CMV disease, oral val. GCV shows comparable safety and is not inferior to IV GCV for treatment of CMV disease in SOT recipients regardless of D/R serostatus (VICTOR study; Asberg et al, AJT 2007) Compared with adults, pharmacokinetic data in younger children are lacking to define the dose of val. GCV.

CMV Diagnosis and Treatment Diagnosis CMV disease Treatment CMV disease Secondary prophylaxis

Diagnosis of CMV Disease l Detection of CMV by PCR in blood (plasma) or antigenemia (pp 65) when the patient has attributable symptoms o DNA PCR: WHO International Standard adopted for calibration (results reported IU/ml, not cp/ml) which allows for comparison across laboratories o o l Extremely important and clinically useful No established cutoffs for treatment Definitive diagnosis: CMV detection in tissue o Viral inclusions o Viral antigens by immunohistochemical staining for CMV o Viral cultures Kotton, Transplantation 2013 Martin, Pediatric Transplant 2011

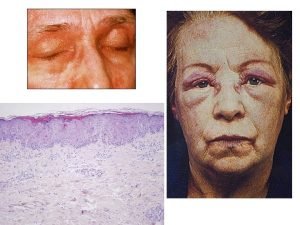
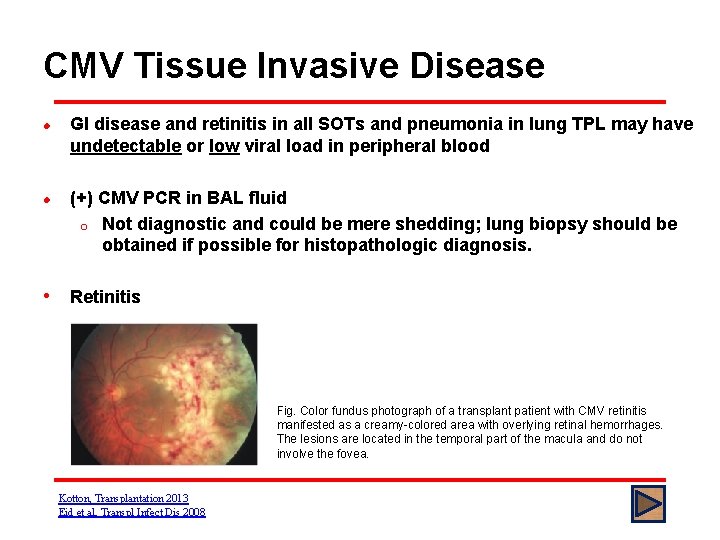
CMV Tissue Invasive Disease l l GI disease and retinitis in all SOTs and pneumonia in lung TPL may have undetectable or low viral load in peripheral blood (+) CMV PCR in BAL fluid o Not diagnostic and could be mere shedding; lung biopsy should be obtained if possible for histopathologic diagnosis. • Retinitis Fig. Color fundus photograph of a transplant patient with CMV retinitis manifested as a creamy-colored area with overlying retinal hemorrhages. The lesions are located in the temporal part of the macula and do not involve the fovea. Kotton, Transplantation 2013 Eid et al, Transpl Infect Dis 2008

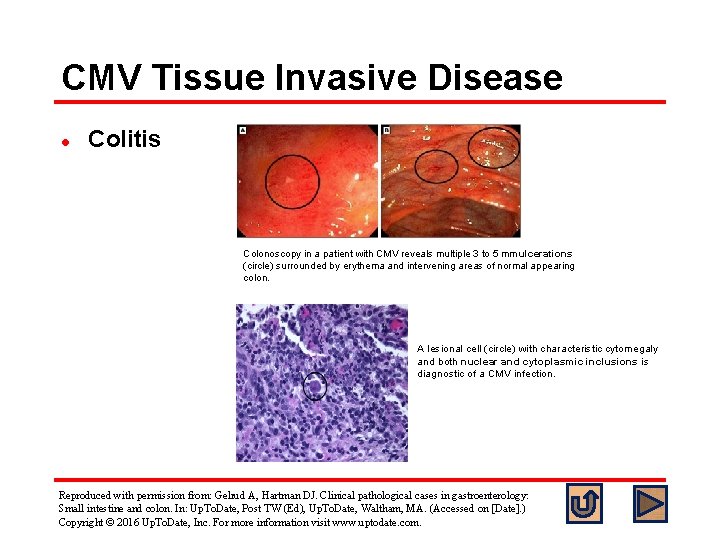
CMV Tissue Invasive Disease l Colitis Colonoscopy in a patient with CMV reveals multiple 3 to 5 mm ulcerations (circle) surrounded by erythema and intervening areas of normal appearing colon. A lesional cell (circle) with characteristic cytomegaly and both nuclear and cytoplasmic inclusions is diagnostic of a CMV infection. Reproduced with permission from: Gelrud A, Hartman DJ. Clinical pathological cases in gastroenterology: Small intestine and colon. In: Up. To. Date, Post TW (Ed), Up. To. Date, Waltham, MA. (Accessed on [Date]. ) Copyright © 2016 Up. To. Date, Inc. For more information visit www. uptodate. com.

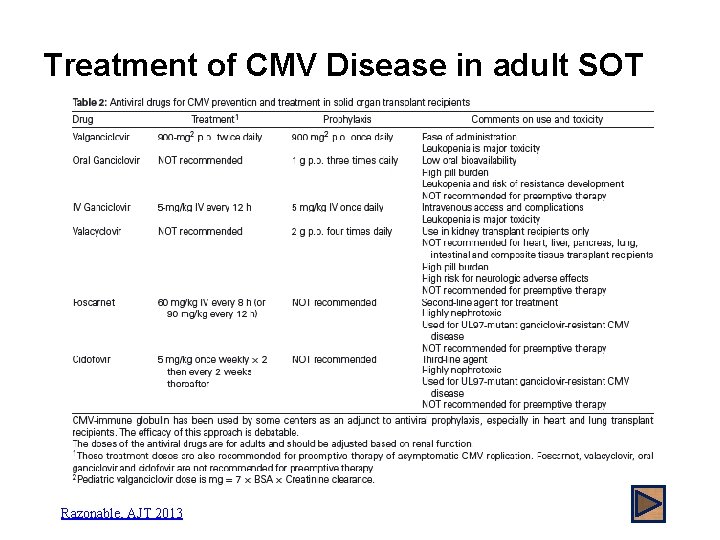
Treatment of CMV Disease in adult SOT Razonable, AJT 2013

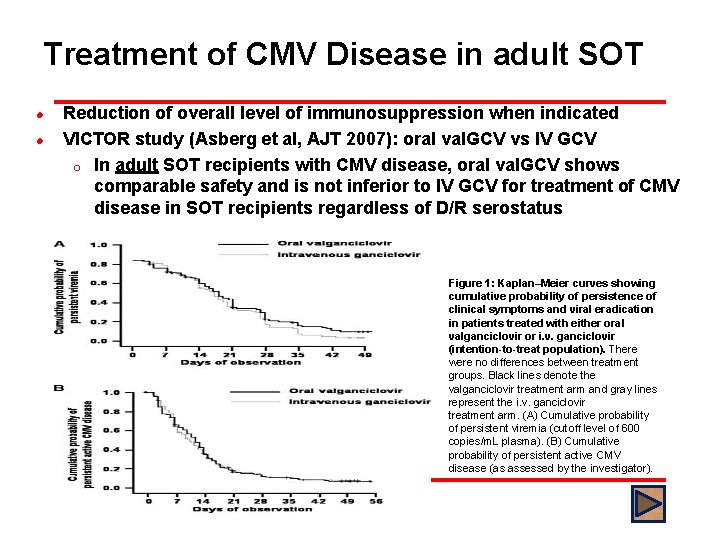
Treatment of CMV Disease in adult SOT l l Reduction of overall level of immunosuppression when indicated VICTOR study (Asberg et al, AJT 2007): oral val. GCV vs IV GCV o In adult SOT recipients with CMV disease, oral val. GCV shows comparable safety and is not inferior to IV GCV for treatment of CMV disease in SOT recipients regardless of D/R serostatus Figure 1: Kaplan–Meier curves showing cumulative probability of persistence of clinical symptoms and viral eradication in patients treated with either oral valganciclovir or i. v. ganciclovir (intention-to-treat population). There were no differences between treatment groups. Black lines denote the valganciclovir treatment arm and gray lines represent the i. v. ganciclovir treatment arm. (A) Cumulative probability of persistent viremia (cutoff level of 600 copies/m. L plasma). (B) Cumulative probability of persistent active CMV disease (as assessed by the investigator).

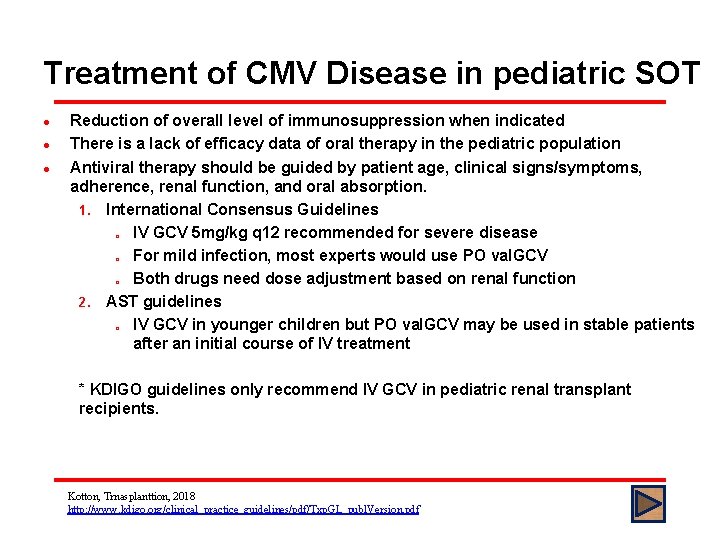
Treatment of CMV Disease in pediatric SOT l l l Reduction of overall level of immunosuppression when indicated There is a lack of efficacy data of oral therapy in the pediatric population Antiviral therapy should be guided by patient age, clinical signs/symptoms, adherence, renal function, and oral absorption. 1. International Consensus Guidelines o IV GCV 5 mg/kg q 12 recommended for severe disease o For mild infection, most experts would use PO val. GCV o Both drugs need dose adjustment based on renal function 2. AST guidelines o IV GCV in younger children but PO val. GCV may be used in stable patients after an initial course of IV treatment * KDIGO guidelines only recommend IV GCV in pediatric renal transplant recipients. Kotton, Trnasplanttion, 2018 http: //www. kdigo. org/clinical_practice_guidelines/pdf/Txp. GL_publ. Version. pdf

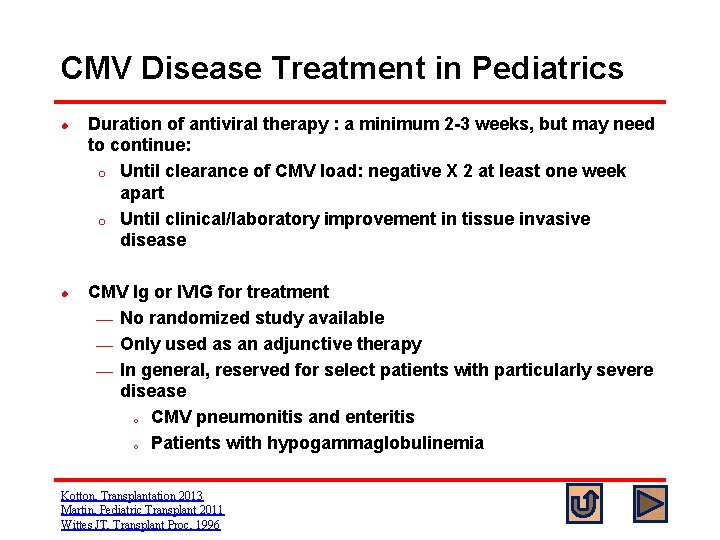
CMV Disease Treatment in Pediatrics l l Duration of antiviral therapy : a minimum 2 -3 weeks, but may need to continue: o Until clearance of CMV load: negative X 2 at least one week apart o Until clinical/laboratory improvement in tissue invasive disease CMV Ig or IVIG for treatment ¾ No randomized study available ¾ Only used as an adjunctive therapy ¾ In general, reserved for select patients with particularly severe disease o CMV pneumonitis and enteritis o Patients with hypogammaglobulinemia Kotton, Transplantation 2013 Martin, Pediatric Transplant 2011 Wittes JT, Transplant Proc, 1996

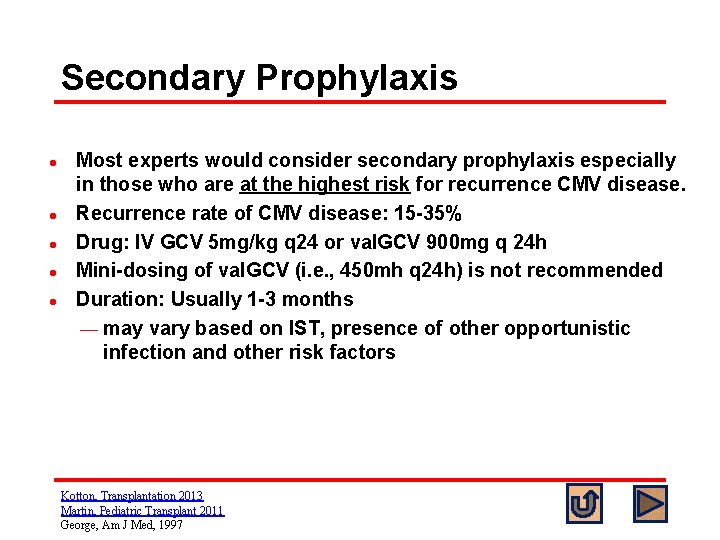
Secondary Prophylaxis l l l Most experts would consider secondary prophylaxis especially in those who are at the highest risk for recurrence CMV disease. Recurrence rate of CMV disease: 15 -35% Drug: IV GCV 5 mg/kg q 24 or val. GCV 900 mg q 24 h Mini-dosing of val. GCV (i. e. , 450 mh q 24 h) is not recommended Duration: Usually 1 -3 months ¾ may vary based on IST, presence of other opportunistic infection and other risk factors Kotton, Transplantation 2013 Martin, Pediatric Transplant 2011 George, Am J Med, 1997

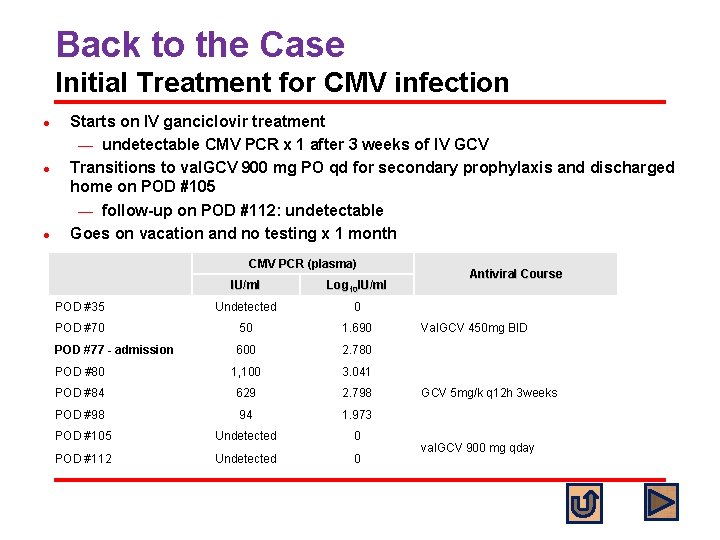
Back to the Case Initial Treatment for CMV infection l l l Starts on IV ganciclovir treatment ¾ undetectable CMV PCR x 1 after 3 weeks of IV GCV Transitions to val. GCV 900 mg PO qd for secondary prophylaxis and discharged home on POD #105 ¾ follow-up on POD #112: undetectable Goes on vacation and no testing x 1 month CMV PCR (plasma) IU/ml Log 10 IU/ml POD #35 Undetected 0 POD #70 50 1. 690 POD #77 - admission 600 2. 780 POD #80 1, 100 3. 041 POD #84 629 2. 798 POD #98 94 1. 973 POD #105 Undetected 0 POD #112 Undetected 0 Antiviral Course Val. GCV 450 mg BID GCV 5 mg/k q 12 h 3 weeks val. GCV 900 mg qday

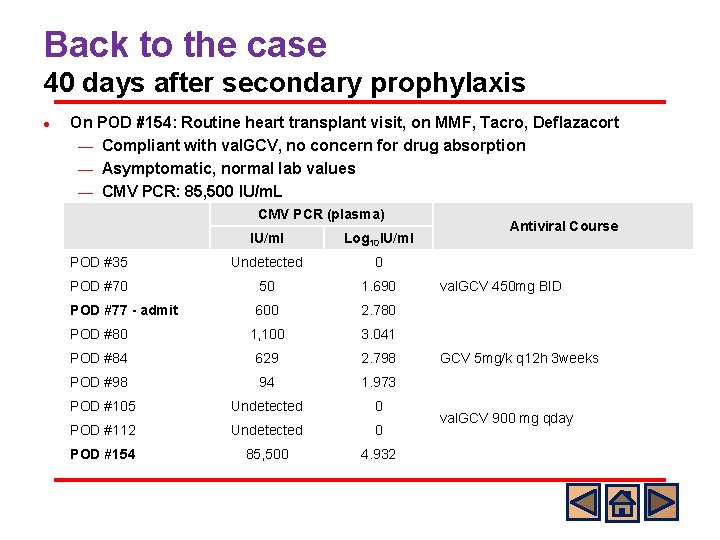
Back to the case 40 days after secondary prophylaxis l On POD #154: Routine heart transplant visit, on MMF, Tacro, Deflazacort ¾ Compliant with val. GCV, no concern for drug absorption ¾ Asymptomatic, normal lab values ¾ CMV PCR: 85, 500 IU/m. L CMV PCR (plasma) IU/ml Log 10 IU/ml POD #35 Undetected 0 POD #70 50 1. 690 POD #77 - admit 600 2. 780 POD #80 1, 100 3. 041 POD #84 629 2. 798 POD #98 94 1. 973 POD #105 Undetected 0 POD #112 Undetected 0 POD #154 85, 500 4. 932 Antiviral Course val. GCV 450 mg BID GCV 5 mg/k q 12 h 3 weeks val. GCV 900 mg qday

What would you do next? A) B) C) D) Start IV GCV 5 mg/kg q 12 Start IV GCV 10 mg/kg q 12 Start IV foscarnet Send CMV resistance testing

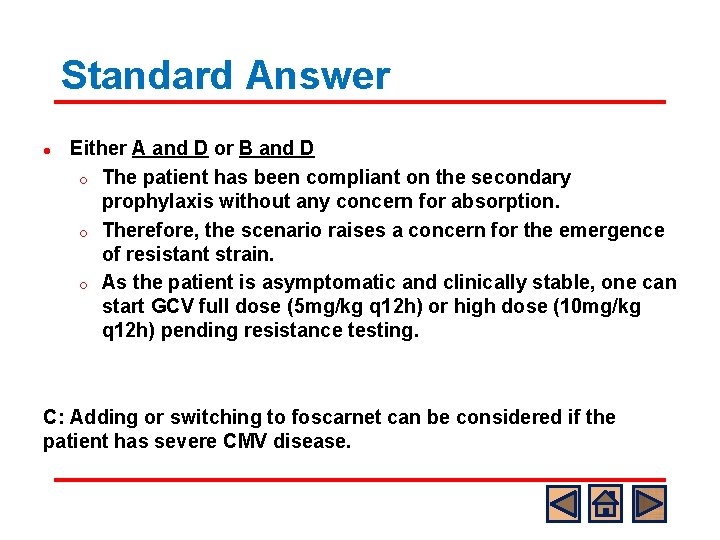
Standard Answer l Either A and D or B and D o The patient has been compliant on the secondary prophylaxis without any concern for absorption. o Therefore, the scenario raises a concern for the emergence of resistant strain. o As the patient is asymptomatic and clinically stable, one can start GCV full dose (5 mg/kg q 12 h) or high dose (10 mg/kg q 12 h) pending resistance testing. C: Adding or switching to foscarnet can be considered if the patient has severe CMV disease.

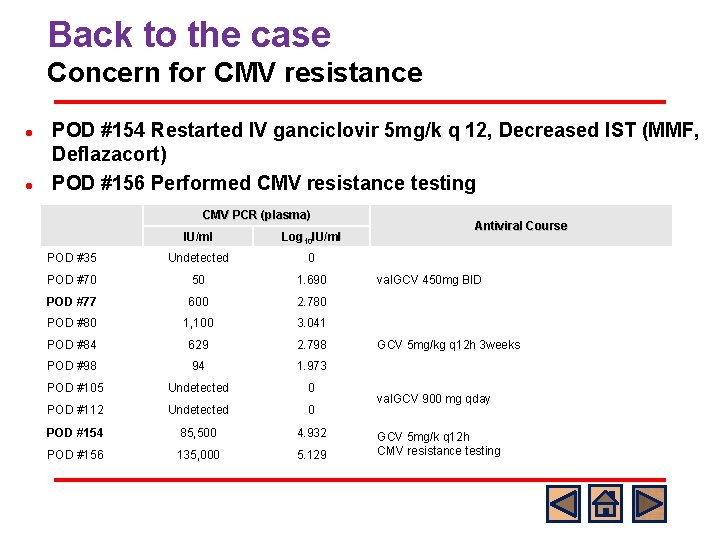
Back to the case Concern for CMV resistance l l POD #154 Restarted IV ganciclovir 5 mg/k q 12, Decreased IST (MMF, Deflazacort) POD #156 Performed CMV resistance testing CMV PCR (plasma) IU/ml Log 10 IU/ml POD #35 Undetected 0 POD #70 50 1. 690 POD #77 600 2. 780 POD #80 1, 100 3. 041 POD #84 629 2. 798 POD #98 94 1. 973 POD #105 Undetected 0 POD #112 Undetected 0 POD #154 85, 500 4. 932 POD #156 135, 000 5. 129 Antiviral Course val. GCV 450 mg BID GCV 5 mg/kg q 12 h 3 weeks val. GCV 900 mg qday GCV 5 mg/k q 12 h CMV resistance testing

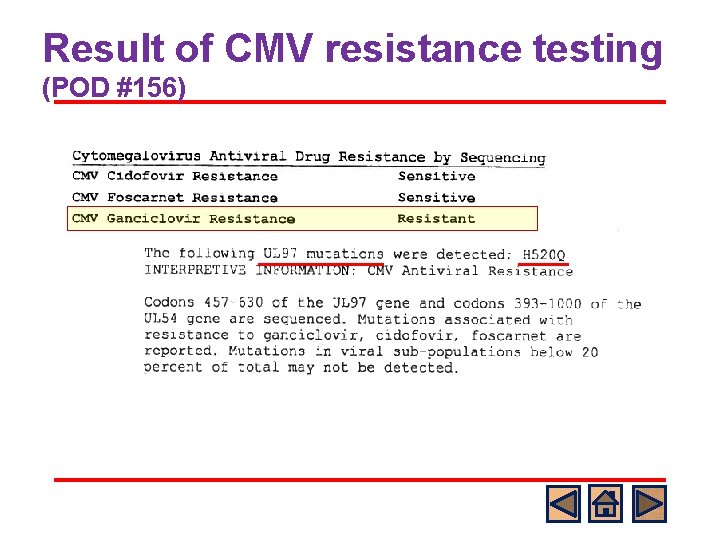
Result of CMV resistance testing (POD #156)

What would you do next? A) Increase IV GCV to high dose B) Switch to Foscarnet C) Continue IV GCV and start Foscarnet D) Start Cidofovir

Standard Answer l B o o o UL 97 mutations which confer GCV resistance appear first in patients receiving prolonged antiviral therapy with GCV and val. GCV. Ganciclovir requires phosphorylation by the phosphotransferase encoded by CMV UL 97 mutations do not affect foscarnet (or cidofovir) susceptibility, as these drugs do not require phosphorylation by the UL 97 gene product. D: Cidofovir is not affected by UL 97 mutations but there is little information on the efficacy of cidofovir as salvage therapy in SOT.

CMV Resistance When to suspect resistant CMV? CMV Resistance Testing Management of CMV resistance

When Should I Suspect CMV Resistance? 1. 2. 3. 4. 5. Prolonged antiviral pre-treatment ¾ Occurrence of resistance mutations without prior antiviral drug exposure is very rare ¾ > 6 weeks cumulative antiviral therapy, median 5 mo Persistently elevated or rising viral load despite > 2 weeks of full dose therapy ¾ It is not uncommon to see increase in viral loads during the 1 st 2 weeks of therapy (CMV half life is 3 -8 days) Suboptimal drug dose/concentrations High initial and ongoing CMV replication Previous high risk recipient (CMV D+/R-) ¾ Incidence of resistance is 5 -12% ¾ Highest in lung transplant Kotton, Transplantation, 2013

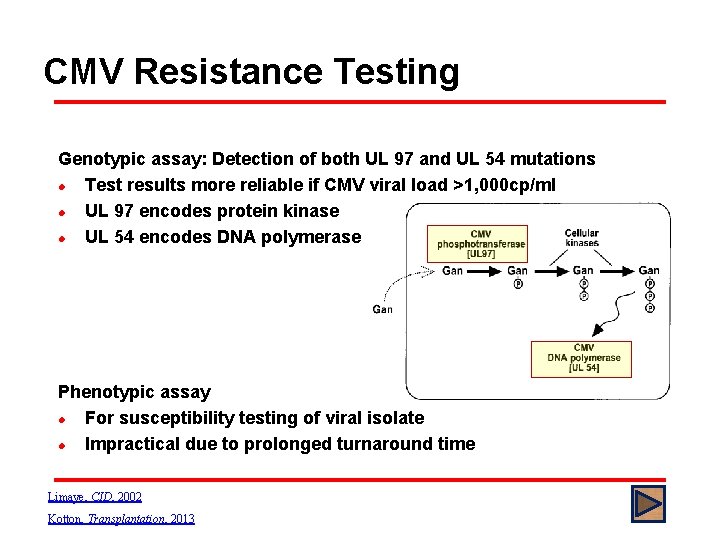
CMV Resistance Testing Genotypic assay: Detection of both UL 97 and UL 54 mutations l Test results more reliable if CMV viral load >1, 000 cp/ml l UL 97 encodes protein kinase l UL 54 encodes DNA polymerase Phenotypic assay l For susceptibility testing of viral isolate l Impractical due to prolonged turnaround time Limaye, CID, 2002 Kotton, Transplantation, 2013

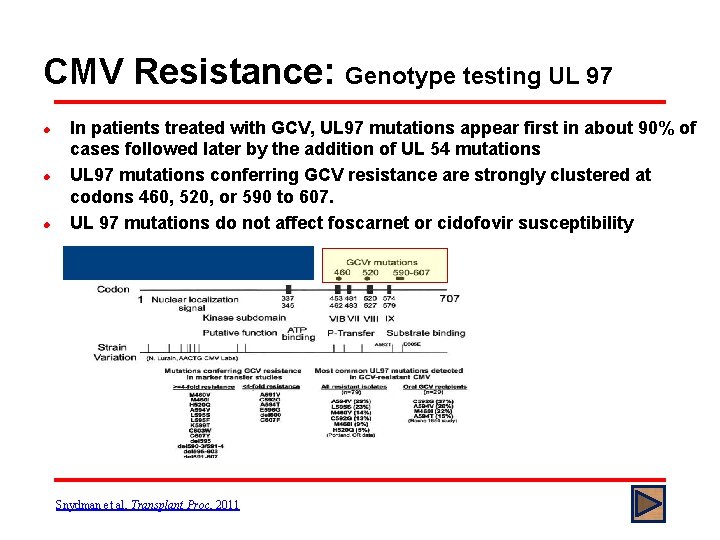
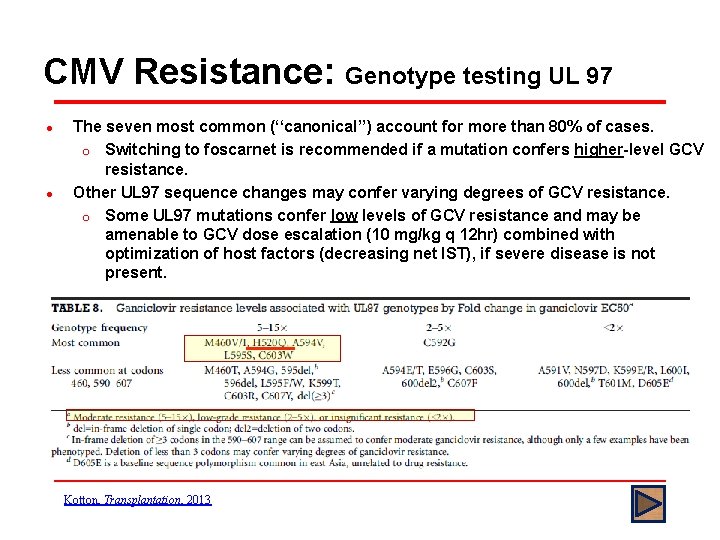
CMV Resistance: Genotype testing UL 97 l l l In patients treated with GCV, UL 97 mutations appear first in about 90% of cases followed later by the addition of UL 54 mutations UL 97 mutations conferring GCV resistance are strongly clustered at codons 460, 520, or 590 to 607. UL 97 mutations do not affect foscarnet or cidofovir susceptibility Snydman et al, Transplant Proc, 2011

CMV Resistance: Genotype testing UL 97 l l The seven most common (‘‘canonical’’) account for more than 80% of cases. o Switching to foscarnet is recommended if a mutation confers higher-level GCV resistance. Other UL 97 sequence changes may confer varying degrees of GCV resistance. o Some UL 97 mutations confer low levels of GCV resistance and may be amenable to GCV dose escalation (10 mg/kg q 12 hr) combined with optimization of host factors (decreasing net IST), if severe disease is not present. Kotton, Transplantation, 2013

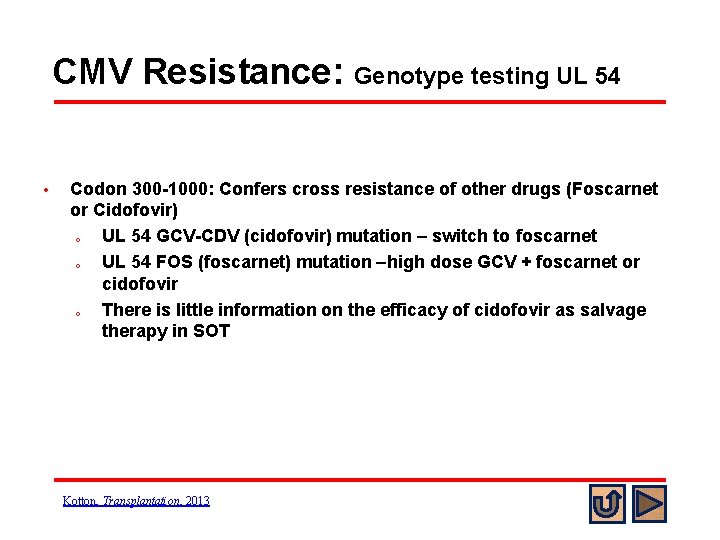
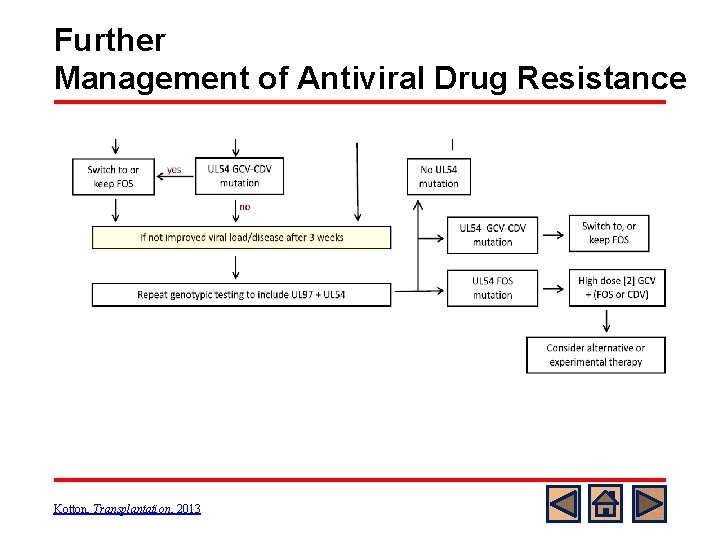
CMV Resistance: Genotype testing UL 54 • Codon 300 -1000: Confers cross resistance of other drugs (Foscarnet or Cidofovir) o UL 54 GCV-CDV (cidofovir) mutation – switch to foscarnet o UL 54 FOS (foscarnet) mutation –high dose GCV + foscarnet or cidofovir o There is little information on the efficacy of cidofovir as salvage therapy in SOT Kotton, Transplantation, 2013

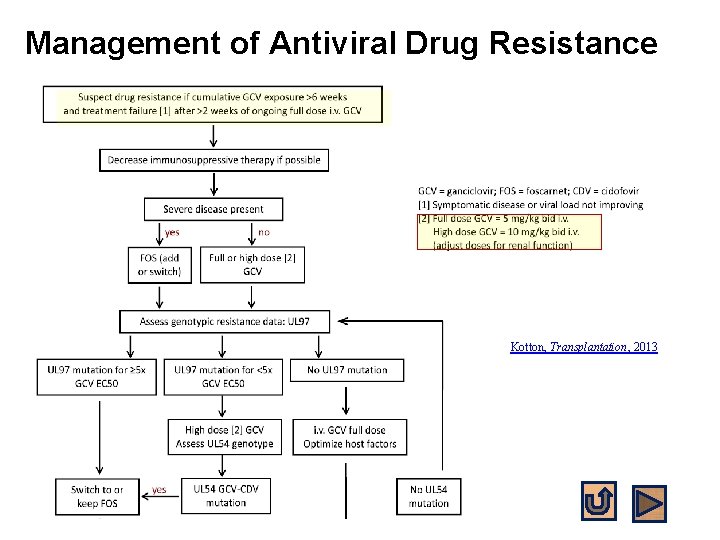
Management of Antiviral Drug Resistance Kotton, Transplantation, 2013

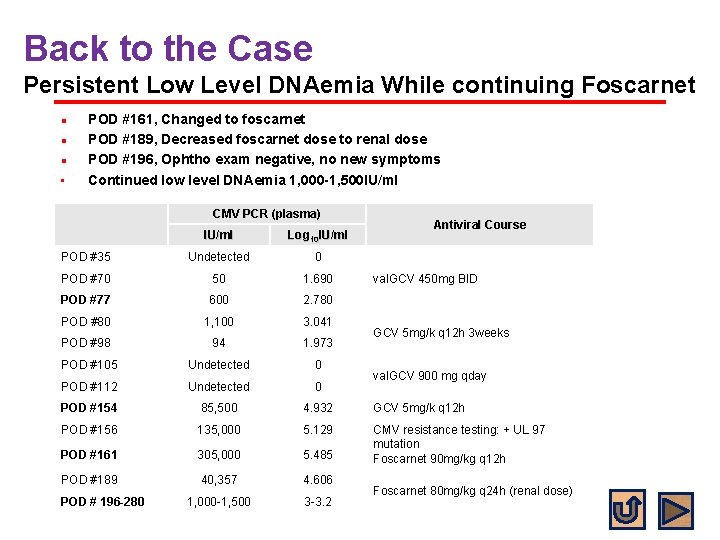
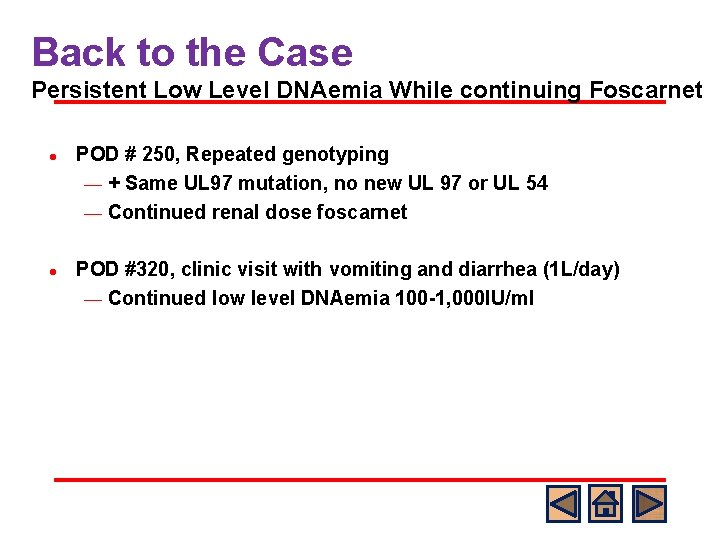
Back to the Case Persistent Low Level DNAemia While continuing Foscarnet l l l • POD #161, Changed to foscarnet POD #189, Decreased foscarnet dose to renal dose POD #196, Ophtho exam negative, no new symptoms Continued low level DNAemia 1, 000 -1, 500 IU/ml CMV PCR (plasma) Antiviral Course IU/ml Log 10 IU/ml POD #35 Undetected 0 POD #70 50 1. 690 POD #77 600 2. 780 POD #80 1, 100 3. 041 POD #98 94 1. 973 POD #105 Undetected 0 POD #112 Undetected 0 POD #154 85, 500 4. 932 GCV 5 mg/k q 12 h POD #156 135, 000 5. 129 POD #161 305, 000 5. 485 CMV resistance testing: + UL 97 mutation Foscarnet 90 mg/kg q 12 h POD #189 40, 357 4. 606 1, 000 -1, 500 3 -3. 2 POD # 196 -280 val. GCV 450 mg BID GCV 5 mg/k q 12 h 3 weeks val. GCV 900 mg qday Foscarnet 80 mg/kg q 24 h (renal dose)

What would you do next? A) Repeat genotyping including UL 97 and UL 54 B) Switch to IV GCV 10 mg/kg q 12 h C) Start cidofovir D) Consider alternative or experimental therapy

Standard Answer A l As we were not able to clear DNAemia after 3 months of foscarnet therapy, repeat genotyping including UL 97 and UL 54 was obtained.

Further Management of Antiviral Drug Resistance Kotton, Transplantation, 2013

Back to the Case Persistent Low Level DNAemia While continuing Foscarnet l l POD # 250, Repeated genotyping ¾ + Same UL 97 mutation, no new UL 97 or UL 54 ¾ Continued renal dose foscarnet POD #320, clinic visit with vomiting and diarrhea (1 L/day) ¾ Continued low level DNAemia 100 -1, 000 IU/ml

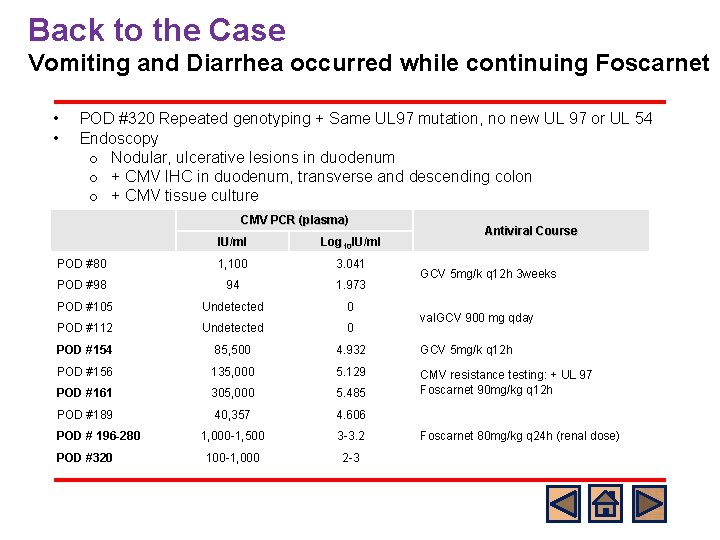
Back to the Case Vomiting and Diarrhea occurred while continuing Foscarnet • • POD #320 Repeated genotyping + Same UL 97 mutation, no new UL 97 or UL 54 Endoscopy o Nodular, ulcerative lesions in duodenum o + CMV IHC in duodenum, transverse and descending colon o + CMV tissue culture CMV PCR (plasma) Antiviral Course IU/ml Log 10 IU/ml POD #80 1, 100 3. 041 POD #98 94 1. 973 POD #105 Undetected 0 POD #112 Undetected 0 POD #154 85, 500 4. 932 GCV 5 mg/k q 12 h POD #156 135, 000 5. 129 POD #161 305, 000 5. 485 CMV resistance testing: + UL 97 Foscarnet 90 mg/kg q 12 h POD #189 40, 357 4. 606 1, 000 -1, 500 3 -3. 2 100 -1, 000 2 -3 POD # 196 -280 POD #320 GCV 5 mg/k q 12 h 3 weeks val. GCV 900 mg qday Foscarnet 80 mg/kg q 24 h (renal dose)

Back to the Case Experimental drugs l l Switched to maribavir (under study protocol) X 3 months which cleared CMV DNAemia and resolved enterocolitis. The patient has been doing well without recurrence of disease

Experimental CMV Antiviral Agents l l l Maribavir: CMV UL 97 kinase inhibitor ¾ Side Effects: Dose-dependent taste disturbance, nausea, vomiting Letermovir: CMV UL 56 terminase inhibitor ¾ No hematologic or renal side effects seen to date Donor-derived CMV-specific T cells

Summary l l l l CMV disease risk is highest when primary CMV infection occurs in an SOT recipient with no preexisting CMV-specific immunity (D+/R-). Other risk factors include transplant type, net immunosuppression status. Children are at higher risk for CMV due to high seronegative status at the time of transplant. Universal CMV prophylaxis or pre-emptive therapy reduce the incidence of CMV infection and CMV disease with substantial reduction in morbidity and mortality. PCR in blood or antigenemia should be used for monitoring of CMV infection. Definitive diagnosis requires CMV detection in tissue. Antiviral therapy should be guided by patient age, clinical signs/symptoms, adherence, renal function, and oral absorption. Understanding resistance mechanisms are important for successful therapy.

This module was developed by: l Eunkyung Song, MD, Nationwide Children’s Hospital, Columbus, OH l Marc Foca, MD, Columbia University, New York City, NY Original Version Date: 10/24/2016 Revised on: 02/10/2020 Part of an educational effort through the PIDS Transplant Infectious Disease working group and the AST ID Community of Practice

Feedback on the Modules l l l PLEASE help to provide us with feedback on the content of these modules! Let us know what you learned and what we can do better Your feedback will help us to improve this work and design future modules l l For any questions or concerns, please contact Tanvi Sharma tanvi. sharma@childrens. harvard. edu
 Cytomegalovirus
Cytomegalovirus Sot storm tracker
Sot storm tracker Cách trị sốt thương hàn
Cách trị sốt thương hàn Sit and reach
Sit and reach Trùng kiết lị
Trùng kiết lị Sot
Sot Substantiivi harjoituksia
Substantiivi harjoituksia Kiểm định bỏ sót biến
Kiểm định bỏ sót biến Orbit
Orbit Sot and reach
Sot and reach Cmv
Cmv Best viruses
Best viruses Gonorrhea ophthalmia neonatorum
Gonorrhea ophthalmia neonatorum Cmv retinitis
Cmv retinitis Cmv klierkoorts
Cmv klierkoorts Cmv microcephaly
Cmv microcephaly Cmv
Cmv Pitting edema
Pitting edema Cmv
Cmv Keratomalasi
Keratomalasi Apa saja organ penyusun sistem gerak manusia
Apa saja organ penyusun sistem gerak manusia Organ and organ system
Organ and organ system Cell tissue organ organ system organism
Cell tissue organ organ system organism Tractus respiratorius
Tractus respiratorius Cell to tissue to organ to organ system to organism
Cell to tissue to organ to organ system to organism Spleen solid organ
Spleen solid organ Leukemoid reaction
Leukemoid reaction Neograft hair transplant in miami
Neograft hair transplant in miami Transplant de ficat costuri
Transplant de ficat costuri Face transplant
Face transplant Slk transplant
Slk transplant Types of transplantation
Types of transplantation Heterotopic liver transplant meaning
Heterotopic liver transplant meaning Kidney donor transplant
Kidney donor transplant National transplant registry malaysia
National transplant registry malaysia Diet after bone marrow transplant
Diet after bone marrow transplant Eliza ann grier
Eliza ann grier Allogeneic stem cell transplant
Allogeneic stem cell transplant Face transplant
Face transplant Kidney donor transplant
Kidney donor transplant Allogeneic stem cell transplant
Allogeneic stem cell transplant Embryo transplant gcse
Embryo transplant gcse Unos transplant management forum
Unos transplant management forum Disadvantage of kidney transplant
Disadvantage of kidney transplant Example of crystalline solid
Example of crystalline solid Crystalline or amorphous
Crystalline or amorphous When a solid completely penetrates another solid
When a solid completely penetrates another solid Crystalline solids
Crystalline solids Interpenetration of surfaces
Interpenetration of surfaces What is solid solution example
What is solid solution example Crystalline solid and amorphous solid
Crystalline solid and amorphous solid Evaporation separation example
Evaporation separation example Covalent network solid vs molecular solid
Covalent network solid vs molecular solid Law of constancy of interfacial angle
Law of constancy of interfacial angle For adult
For adult Pediatric epinephrine dose chart
Pediatric epinephrine dose chart Erratic in tagalog
Erratic in tagalog Nci pediatric oncology branch
Nci pediatric oncology branch Hospital environment for sick child definition
Hospital environment for sick child definition 4 2 1 fluid rule
4 2 1 fluid rule Buserline
Buserline Jumpstart triage
Jumpstart triage Pyelonephritis child treatment
Pyelonephritis child treatment