CommunityAcquired Pneumonia Immunization Trial in Adults CAPi TA








- Slides: 38

Community-Acquired Pneumonia Immunization Trial in Adults — CAPi. TA M. Bonten 1, M. Bolkenbaas 1, S. Huijts 1, C. Webber 2, S. Gault 2, W. Gruber 3, D. Grobbee 1, 4, for the CAPi. TA study team Julius Center for Health Sciences and Primary Care, UMC Utrecht, the Netherlands 2 Pfizer Vaccine Clinical Research, Maidenhead, UK 3 Pfizer Vaccine Clinical Research, Pearl River, NY, USA 4 Julius Clinical, Zeist, the Netherlands 1

Disclaimer This study was sponsored by Wyeth, which was acquired by Pfizer Inc in October 2009. 2

Background • Effectiveness of pneumococcal polysaccharide vaccines against community-acquired pneumonia (CAP) is uncertain 1, 2 • Pneumococcal conjugate-vaccines are effective in preventing IPD and pneumonia in children, but efficacy in preventing pneumococcal pneumonia or IPD in adults over 65 has not been investigated. 3 • Establishing efficacy of pneumococcal vaccine for preventing pneumonia has been hampered by the lack of a specific and sensitive test for identification of causative S. pneumoniae serotypes. 1. Moberley S, Holden J et al. Cochrane Database of Systematic Reviews 2013, Issue 1. 2. Huss A, Scott P et al. CMAJ 2009; 180: 48 -58 3. Grijalva CG, Pelton SI. Curr Opin Pediatr. 2011 Feb; 23(1): 98 -104. 3

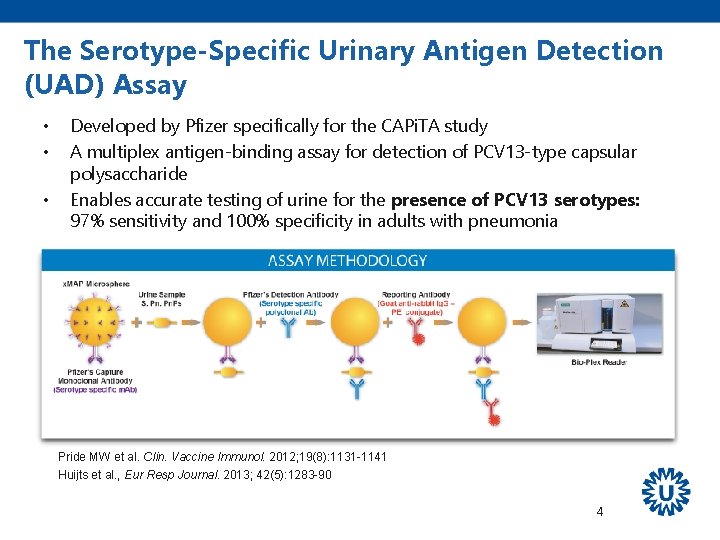
The Serotype-Specific Urinary Antigen Detection (UAD) Assay • • • Developed by Pfizer specifically for the CAPi. TA study A multiplex antigen-binding assay for detection of PCV 13 -type capsular polysaccharide Enables accurate testing of urine for the presence of PCV 13 serotypes: 97% sensitivity and 100% specificity in adults with pneumonia Pride MW et al. Clin. Vaccine Immunol. 2012; 19(8): 1131 -1141 Huijts et al. , Eur Resp Journal. 2013; 42(5): 1283 -90 4

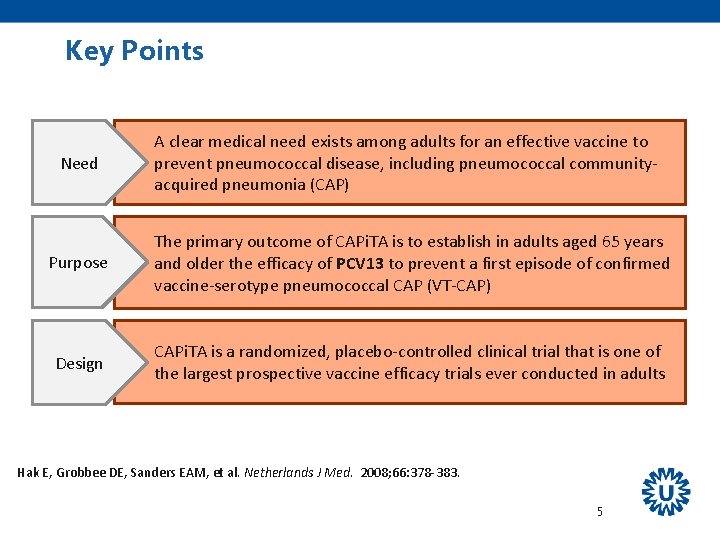
Key Points Need A clear medical need exists among adults for an effective vaccine to prevent pneumococcal disease, including pneumococcal communityacquired pneumonia (CAP) Purpose The primary outcome of CAPi. TA is to establish in adults aged 65 years and older the efficacy of PCV 13 to prevent a first episode of confirmed vaccine-serotype pneumococcal CAP (VT-CAP) Design CAPi. TA is a randomized, placebo-controlled clinical trial that is one of the largest prospective vaccine efficacy trials ever conducted in adults Hak E, Grobbee DE, Sanders EAM, et al. Netherlands J Med. 2008; 66: 378 -383. 5

Why CAPi. TA was Conducted in the Netherlands • The Netherlands does not have a generalized, age-based recommendation for 23 -valent pneumococcal polysaccharide vaccine (PPSV 23) – The Netherlands Health Council recommends that PPSV 23 be provided to only very high-risk individuals, and recommended a placebo-controlled study. – Adults aged 65 years and older are generally PPSV 23 -naïve. • All subjects are linked to a single General Practitioner (GP) – Allowing identification of eligible study candidates and follow-up for relevant endpoints for safety and efficacy • Antibiotic prescription is restricted to physicians, either the GP or in a hospital, facilitating case identification. • The infrastructure of GP-based antibiotic prescription and hospital referral allowed for implementing a standardized approach to detect study subjects hospitalised with CAP in the catchment areas of participating GPs. Hak E, Grobbee DE, Sanders EAM, et al. Netherlands J Med. 2008; 66: 378 -383. 6

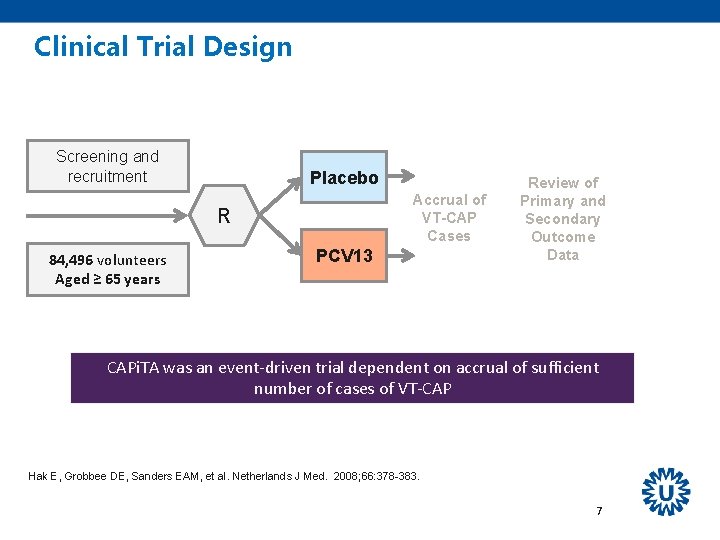
Clinical Trial Design Screening and recruitment Placebo Accrual of VT-CAP Cases R 84, 496 volunteers Aged ≥ 65 years PCV 13 Review of Primary and Secondary Outcome Data CAPi. TA was an event-driven trial dependent on accrual of sufficient number of cases of VT-CAP Hak E, Grobbee DE, Sanders EAM, et al. Netherlands J Med. 2008; 66: 378 -383. 7

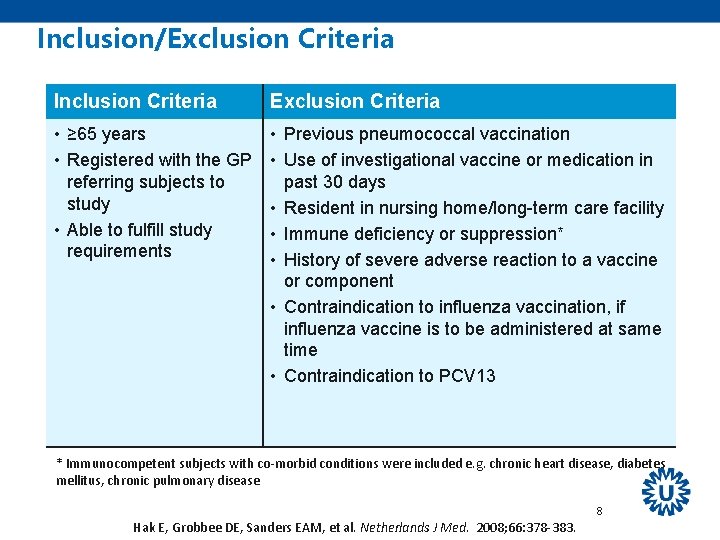
Inclusion/Exclusion Criteria Inclusion Criteria Exclusion Criteria • ≥ 65 years • Registered with the GP referring subjects to study • Able to fulfill study requirements • Previous pneumococcal vaccination • Use of investigational vaccine or medication in past 30 days • Resident in nursing home/long-term care facility • Immune deficiency or suppression* • History of severe adverse reaction to a vaccine or component • Contraindication to influenza vaccination, if influenza vaccine is to be administered at same time • Contraindication to PCV 13 * Immunocompetent subjects with co-morbid conditions were included e. g. chronic heart disease, diabetes mellitus, chronic pulmonary disease 8 Hak E, Grobbee DE, Sanders EAM, et al. Netherlands J Med. 2008; 66: 378 -383.

Primary and Secondary Efficacy Objectives Demonstrate the Efficacy of PCV 13 in the Prevention of a First Episode of: 1. Vaccine-serotype (VT) Pneumococcal CAP 2. VT Non-Bacteremic Non-Invasive (NB/NI) Pneumococcal CAP VT Invasive Pneumococcal Disease (IPD) Analyses populations are per protocol and modified intention -to-treat. Hak E, Grobbee DE, Sanders EAM, et al. Netherlands J Med. 2008; 66: 378 -383. 9

Selected Exploratory Efficacy Objectives Demonstrate the Efficacy of PCV 13 in the Prevention of: • • • First episode of confirmed pneumococcal CAP First episode of confirmed NB/NI pneumococcal CAP First episode of IPD Analyses are per protocol. Hak E, Grobbee DE, Sanders EAM, et al. Netherlands J Med. 2008; 66: 378 -383. 10

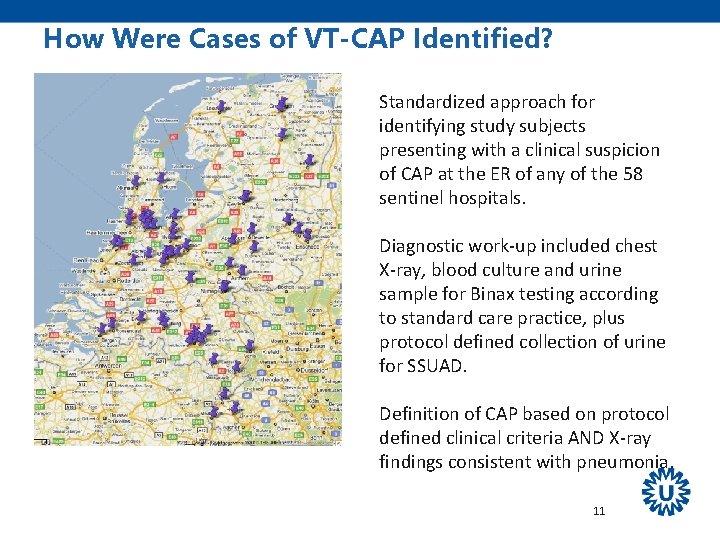
How Were Cases of VT-CAP Identified? Standardized approach for identifying study subjects presenting with a clinical suspicion of CAP at the ER of any of the 58 sentinel hospitals. Diagnostic work-up included chest X-ray, blood culture and urine sample for Binax testing according to standard care practice, plus protocol defined collection of urine for SSUAD. Definition of CAP based on protocol defined clinical criteria AND X-ray findings consistent with pneumonia. 11

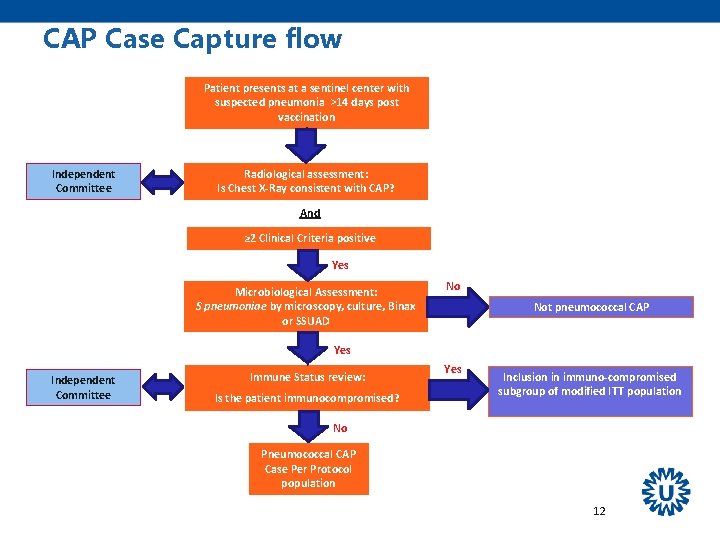
CAP Case Capture flow Patient presents at a sentinel center with suspected pneumonia >14 days post vaccination Independent Committee Radiological assessment: Is Chest X-Ray consistent with CAP? And ≥ 2 Clinical Criteria positive Yes Microbiological Assessment: S pneumoniae by microscopy, culture, Binax or SSUAD No Not pneumococcal CAP Yes Independent Committee Immune Status review: Is the patient immunocompromised? Yes Inclusion in immuno-compromised subgroup of modified ITT population No Pneumococcal CAP Case Per Protocol population 12

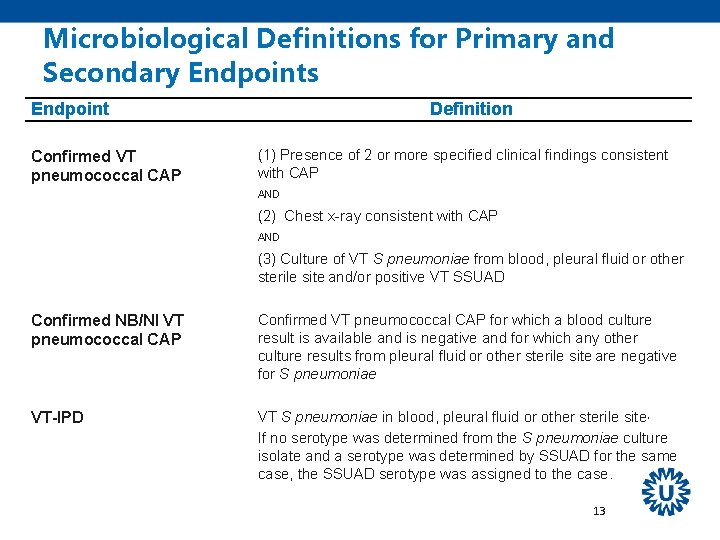
Microbiological Definitions for Primary and Secondary Endpoints Endpoint Confirmed VT pneumococcal CAP Definition (1) Presence of 2 or more specified clinical findings consistent with CAP AND (2) Chest x-ray consistent with CAP AND (3) Culture of VT S pneumoniae from blood, pleural fluid or other sterile site and/or positive VT SSUAD Confirmed NB/NI VT pneumococcal CAP Confirmed VT pneumococcal CAP for which a blood culture result is available and is negative and for which any other culture results from pleural fluid or other sterile site are negative for S pneumoniae VT-IPD VT S pneumoniae in blood, pleural fluid or other sterile site, If no serotype was determined from the S pneumoniae culture isolate and a serotype was determined by SSUAD for the same case, the SSUAD serotype was assigned to the case. 13

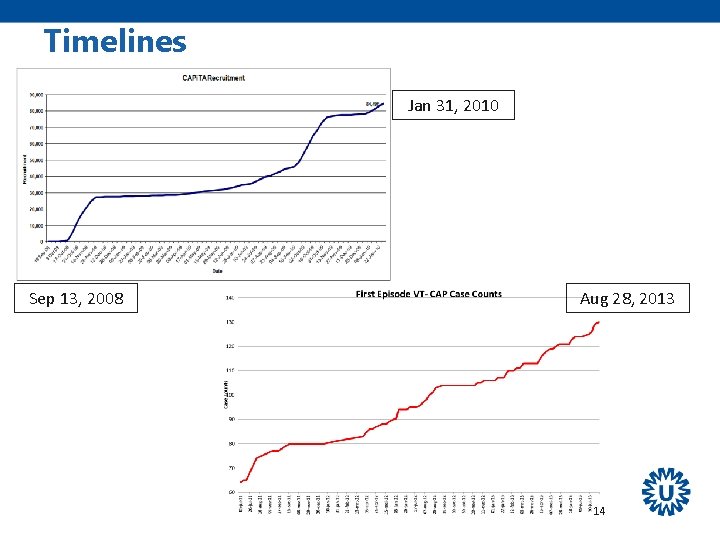
Timelines Jan 31, 2010 Sep 13, 2008 Aug 28, 2013 14

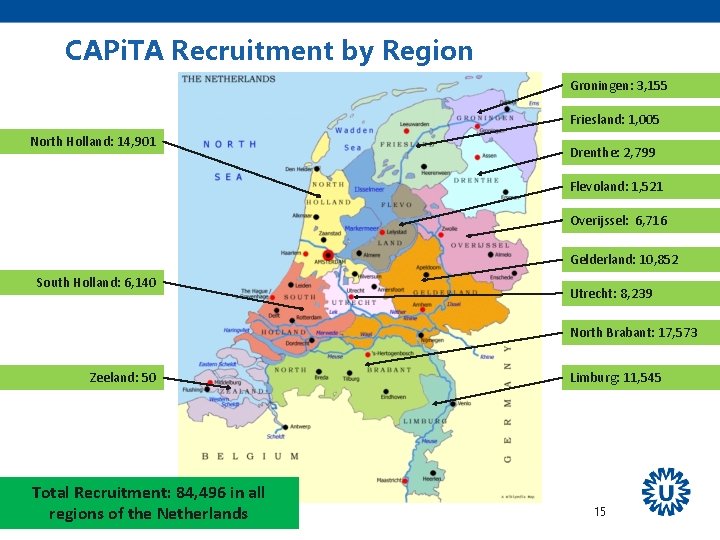
CAPi. TA Recruitment by Region Groningen: 3, 155 Friesland: 1, 005 North Holland: 14, 901 Drenthe: 2, 799 Flevoland: 1, 521 Overijssel: 6, 716 Gelderland: 10, 852 South Holland: 6, 140 Utrecht: 8, 239 North Brabant: 17, 573 Zeeland: 50 Total Recruitment: 84, 496 in all regions of the Netherlands Limburg: 11, 545 15

Baseline Characteristics Characteristic PCV 13 n=42, 237 Placebo n=42, 255 Age (mean, SD) 72. 8 (5. 7) 72. 8 (5. 6) Male (%) 55. 5 56. 3 Female (%) 44. 5 43. 7 Asthma (%) 4. 8 5. 0 Diabetes mellitus: Insulin Use (%) 3. 3 3. 2 Diabetes mellitus: No Insulin Use (%) 9. 1 9. 4 Heart disease (%) 25. 3 25. 4 Liver disease (%) 0. 5 Lung disease (%) 10. 1 10. 3 Splenectomy (%) <0. 1 16

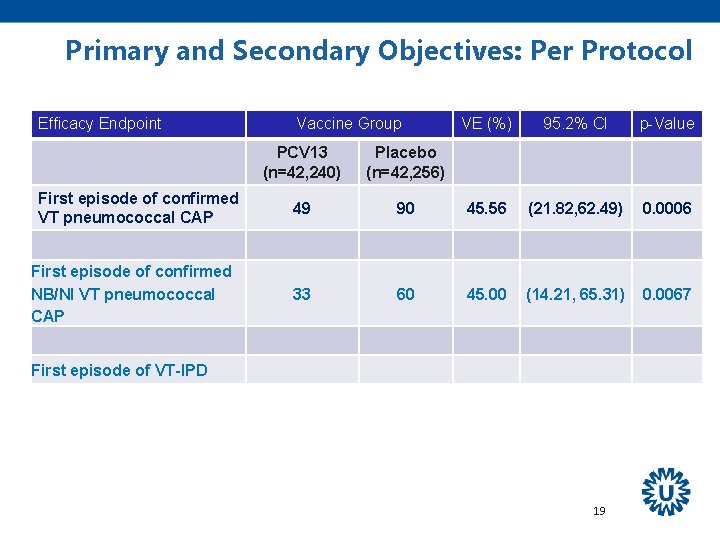
Primary and Secondary Objectives: Per Protocol Efficacy Endpoint Vaccine Group PCV 13 (n=42, 240) VE (%) 95. 2% CI Placebo (n=42, 256) First episode of confirmed VT pneumococcal CAP First episode of confirmed NB/NI VT pneumococcal CAP First episode of VT-IPD 17 p-Value

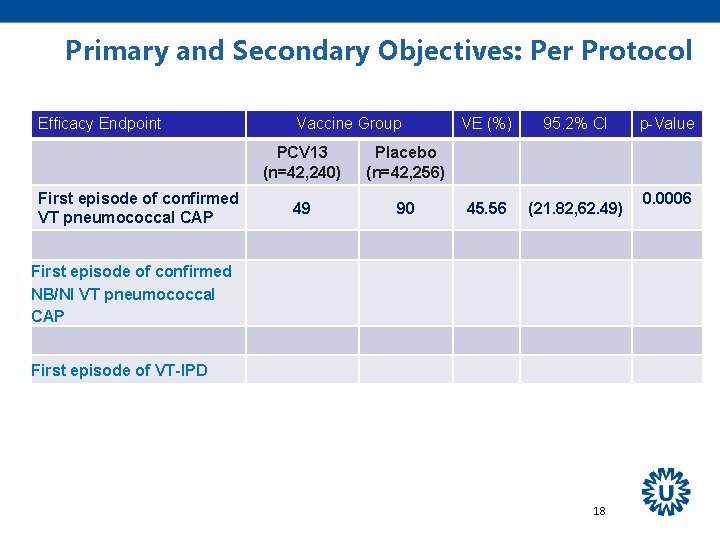
Primary and Secondary Objectives: Per Protocol Efficacy Endpoint First episode of confirmed VT pneumococcal CAP Vaccine Group PCV 13 (n=42, 240) Placebo (n=42, 256) 49 90 VE (%) 95. 2% CI 45. 56 (21. 82, 62. 49) First episode of confirmed NB/NI VT pneumococcal CAP First episode of VT-IPD 18 p-Value 0. 0006

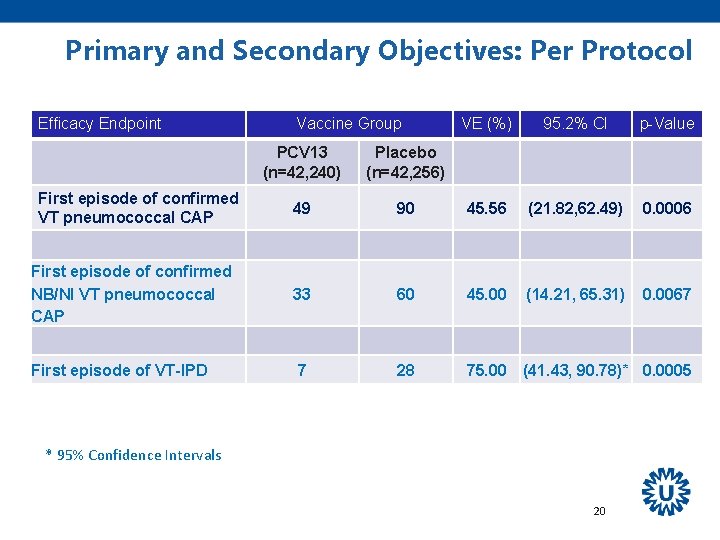
Primary and Secondary Objectives: Per Protocol Efficacy Endpoint First episode of confirmed VT pneumococcal CAP First episode of confirmed NB/NI VT pneumococcal CAP Vaccine Group VE (%) 95. 2% CI p-Value PCV 13 (n=42, 240) Placebo (n=42, 256) 49 90 45. 56 (21. 82, 62. 49) 0. 0006 33 60 45. 00 (14. 21, 65. 31) 0. 0067 First episode of VT-IPD 19

Primary and Secondary Objectives: Per Protocol Efficacy Endpoint Vaccine Group VE (%) 95. 2% CI p-Value PCV 13 (n=42, 240) Placebo (n=42, 256) 49 90 45. 56 (21. 82, 62. 49) 0. 0006 First episode of confirmed NB/NI VT pneumococcal CAP 33 60 45. 00 (14. 21, 65. 31) 0. 0067 First episode of VT-IPD 7 28 75. 00 (41. 43, 90. 78)* 0. 0005 First episode of confirmed VT pneumococcal CAP * 95% Confidence Intervals 20

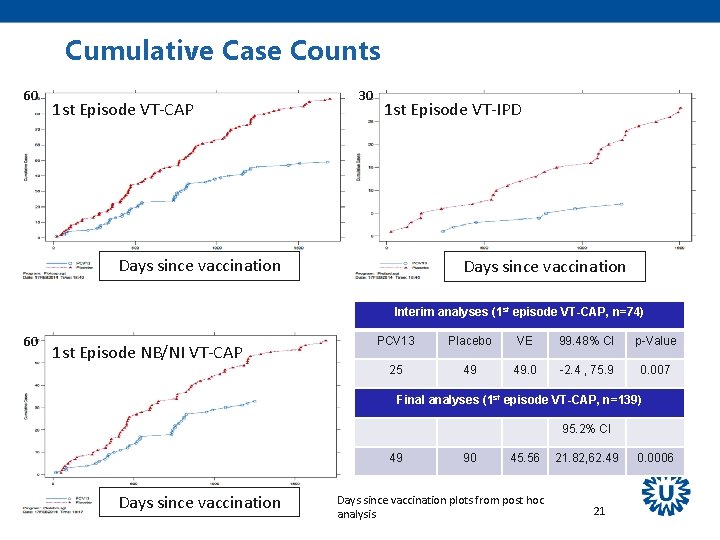
Cumulative Case Counts 60 1 st Episode VT-CAP 30 1 st Episode VT-IPD Days since vaccination Interim analyses (1 st episode VT-CAP, n=74) 60 1 st Episode NB/NI VT-CAP PCV 13 Placebo VE 99. 48% CI p-Value 25 49 49. 0 -2. 4 , 75. 9 0. 007 Final analyses (1 st episode VT-CAP, n=139) 95. 2% CI 49 Days since vaccination 90 45. 56 Days since vaccination plots from post hoc analysis 21. 82, 62. 49 21 0. 0006

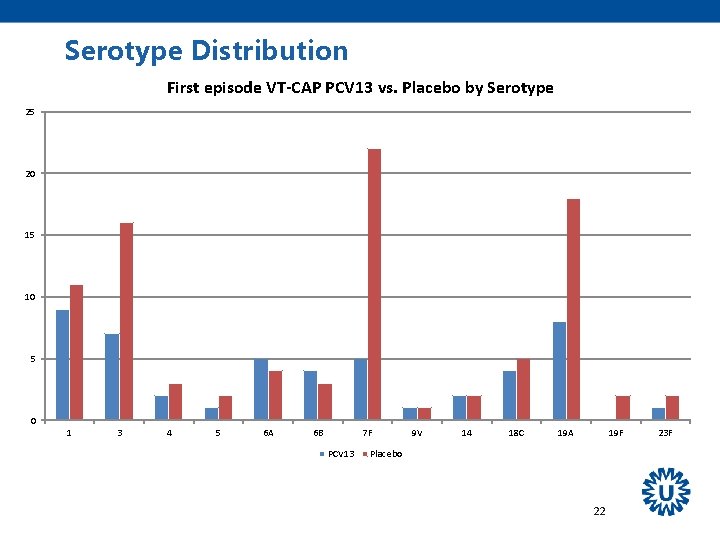
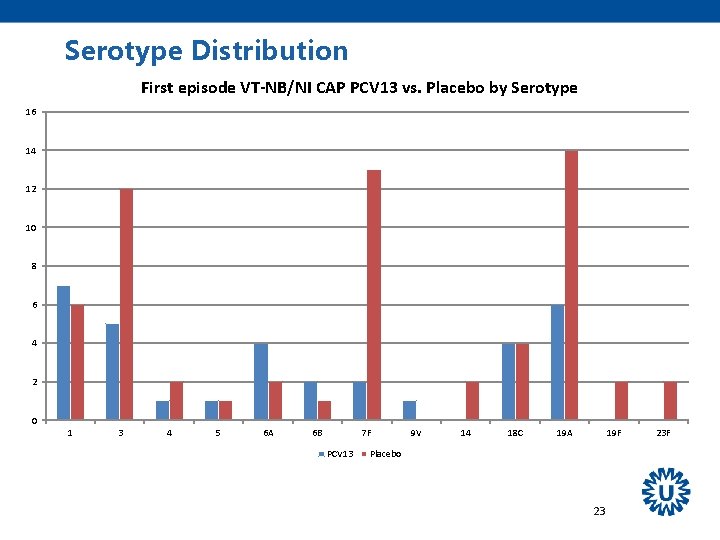
Serotype Distribution First episode VT-CAP PCV 13 vs. Placebo by Serotype 25 20 15 10 5 0 1 3 4 5 6 A 6 B 7 F PCV 13 9 V 14 18 C 19 A 19 F Placebo 22 23 F

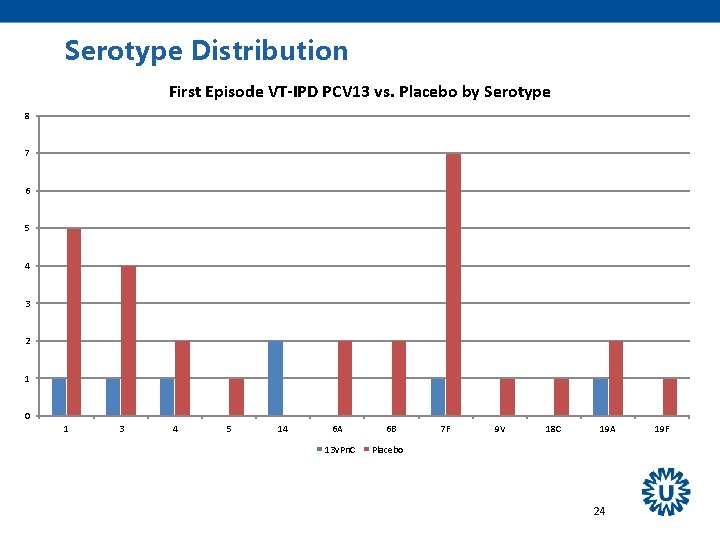
Serotype Distribution First episode VT-NB/NI CAP PCV 13 vs. Placebo by Serotype 16 14 12 10 8 6 4 2 0 1 3 4 5 6 A 6 B 7 F PCV 13 9 V 14 18 C 19 A 19 F Placebo 23 23 F

Serotype Distribution First Episode VT-IPD PCV 13 vs. Placebo by Serotype 8 7 6 5 4 3 2 1 0 1 3 4 5 14 6 A 13 v. Pn. C 6 B 7 F 9 V 18 C 19 A Placebo 24 19 F

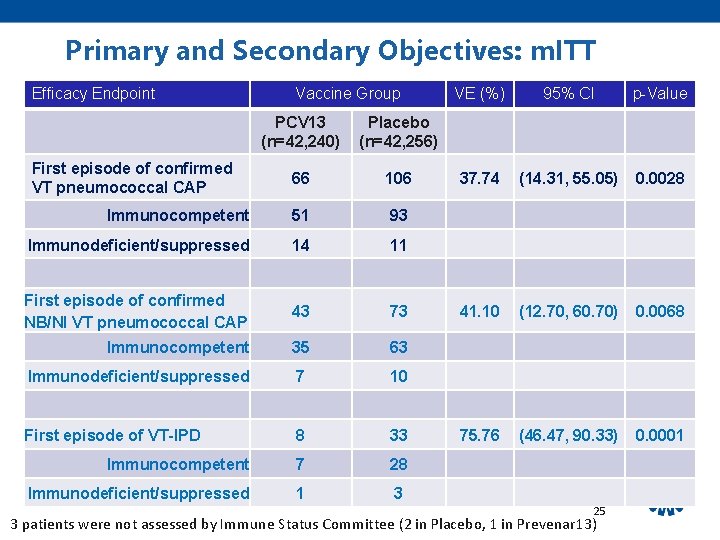
Primary and Secondary Objectives: m. ITT Efficacy Endpoint Vaccine Group PCV 13 (n=42, 240) Placebo (n=42, 256) 66 106 Immunocompetent 51 93 Immunodeficient/suppressed 14 11 First episode of confirmed NB/NI VT pneumococcal CAP 43 73 Immunocompetent 35 63 Immunodeficient/suppressed 7 10 First episode of VT-IPD 8 33 Immunocompetent 7 28 Immunodeficient/suppressed 1 3 First episode of confirmed VT pneumococcal CAP VE (%) 95% CI p-Value 37. 74 (14. 31, 55. 05) 0. 0028 41. 10 (12. 70, 60. 70) 0. 0068 75. 76 (46. 47, 90. 33) 0. 0001 25 3 patients were not assessed by Immune Status Committee (2 in Placebo, 1 in Prevenar 13)

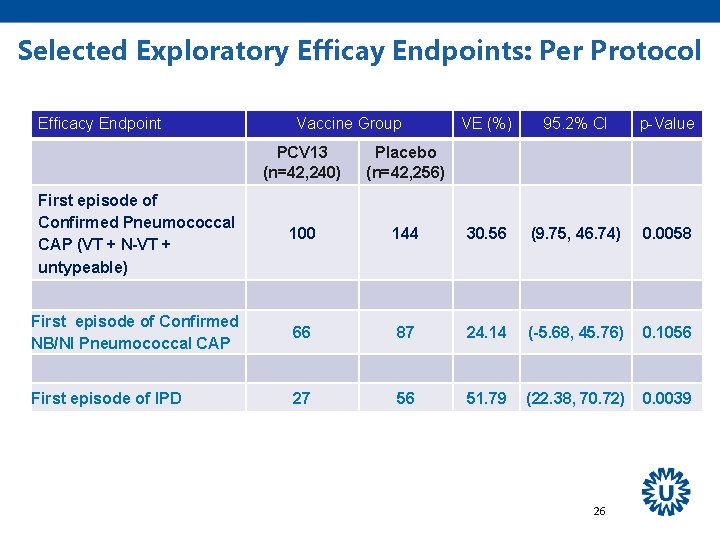
Selected Exploratory Efficay Endpoints: Per Protocol Efficacy Endpoint Vaccine Group VE (%) 95. 2% CI p-Value PCV 13 (n=42, 240) Placebo (n=42, 256) First episode of Confirmed Pneumococcal CAP (VT + N-VT + untypeable) 100 144 30. 56 (9. 75, 46. 74) 0. 0058 First episode of Confirmed NB/NI Pneumococcal CAP 66 87 24. 14 (-5. 68, 45. 76) 0. 1056 First episode of IPD 27 56 51. 79 (22. 38, 70. 72) 0. 0039 26

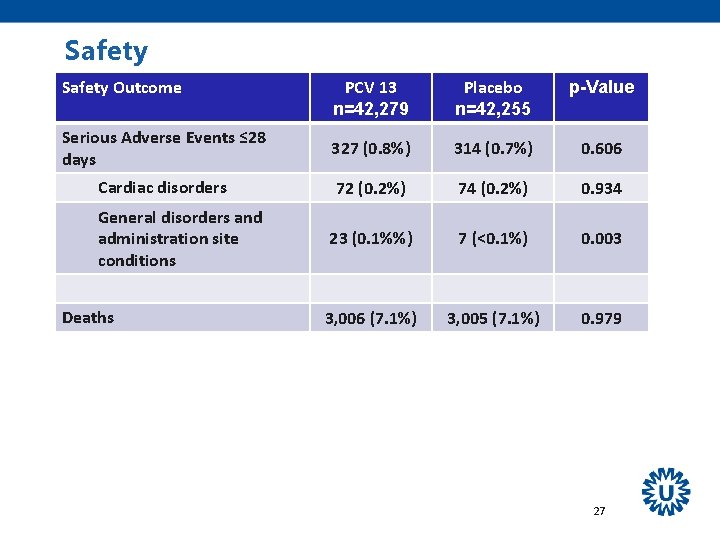
Safety Outcome PCV 13 n=42, 279 Placebo n=42, 255 p-Value Serious Adverse Events ≤ 28 days 327 (0. 8%) 314 (0. 7%) 0. 606 Cardiac disorders 72 (0. 2%) 74 (0. 2%) 0. 934 23 (0. 1%%) 7 (<0. 1%) 0. 003 3, 006 (7. 1%) 3, 005 (7. 1%) 0. 979 General disorders and administration site conditions Deaths 27

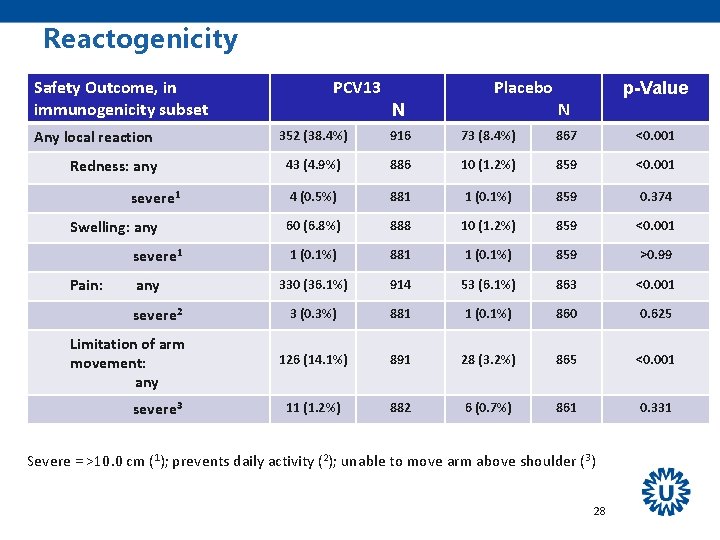
Reactogenicity Safety Outcome, in immunogenicity subset Any local reaction Redness: any severe 1 Swelling: any severe 1 Pain: any severe 2 Limitation of arm movement: any severe 3 PCV 13 Placebo N p-Value N 352 (38. 4%) 916 73 (8. 4%) 867 <0. 001 43 (4. 9%) 886 10 (1. 2%) 859 <0. 001 4 (0. 5%) 881 1 (0. 1%) 859 0. 374 60 (6. 8%) 888 10 (1. 2%) 859 <0. 001 1 (0. 1%) 881 1 (0. 1%) 859 >0. 99 330 (36. 1%) 914 53 (6. 1%) 863 <0. 001 3 (0. 3%) 881 1 (0. 1%) 860 0. 625 126 (14. 1%) 891 28 (3. 2%) 865 <0. 001 11 (1. 2%) 882 6 (0. 7%) 861 0. 331 Severe = >10. 0 cm (1); prevents daily activity (2); unable to move arm above shoulder (3) 28

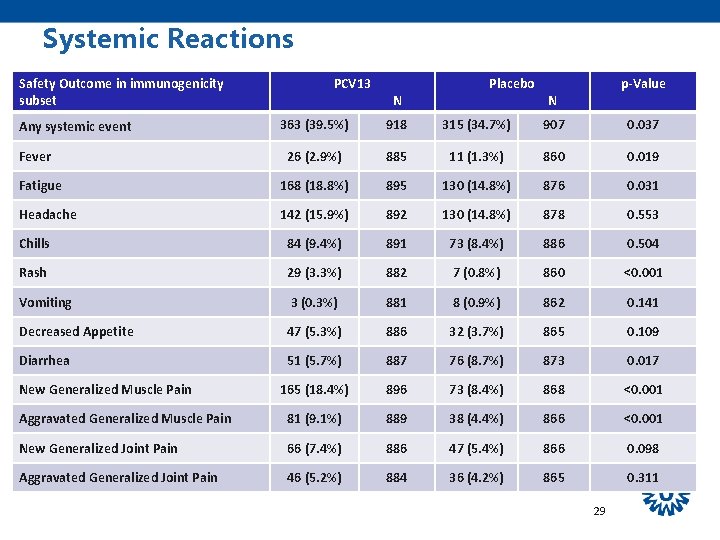
Systemic Reactions Safety Outcome in immunogenicity subset PCV 13 N Placebo p-Value N 363 (39. 5%) 918 315 (34. 7%) 907 0. 037 26 (2. 9%) 885 11 (1. 3%) 860 0. 019 Fatigue 168 (18. 8%) 895 130 (14. 8%) 876 0. 031 Headache 142 (15. 9%) 892 130 (14. 8%) 878 0. 553 Chills 84 (9. 4%) 891 73 (8. 4%) 886 0. 504 Rash 29 (3. 3%) 882 7 (0. 8%) 860 <0. 001 Vomiting 3 (0. 3%) 881 8 (0. 9%) 862 0. 141 Decreased Appetite 47 (5. 3%) 886 32 (3. 7%) 865 0. 109 Diarrhea 51 (5. 7%) 887 76 (8. 7%) 873 0. 017 165 (18. 4%) 896 73 (8. 4%) 868 <0. 001 Aggravated Generalized Muscle Pain 81 (9. 1%) 889 38 (4. 4%) 866 <0. 001 New Generalized Joint Pain 66 (7. 4%) 886 47 (5. 4%) 866 0. 098 Aggravated Generalized Joint Pain 46 (5. 2%) 884 36 (4. 2%) 865 0. 311 Any systemic event Fever New Generalized Muscle Pain 29

Conclusions Vaccine efficacy of PCV 13 was: ü 45. 56% (95. 2% CI 21. 82%-62. 49%; p=0. 0006) for preventing the first episode of VT-CAP ü 45. 00% (95. 2% CI 14. 21%-65. 31%; p=0. 0067) for preventing the first episode of NB/NI VT-CAP ü 75. 00% (95% CI 41. 43%-90. 78%; p=0. 0005) for preventing the first episode of VT-IPD Safety profile was satisfactory and consistent with prior adult experience 30

Other Findings All 13 serotypes were circulating - PCV 7 NIP in children started in 2006, - PCV 10 introduced in March 2011 Most prominent effects on serotypes 3, 7 F, 19 A Effectiveness seemed to remain stable over the period of observation (mean 3. 97 years). 31

Acknowledgements • • • Julius Clinical 2, 260 GPs Sub-investigators and research nurses in 58 sentinel hospitals Drs. Vos, Lely, van Leersum, Coche (Adjudication Committee) Drs. Van Paasen, Ellerbroek, Cohen Tervaert, Dam (Immune Status Committee) Drs. Springer, Lughtmeier, van der Schuur, Lamers (Mortality Assessment Committee) Dr. Van de Ende (National Reference Center, AMC) Dr. Coenjaerts (Dept of Med Microbiology UMCU) Linnaeusinstituut , Spaarneziekenhuis Haarlem (Substudy) • • Dr. M. Peeters, Tilburg (June 6 th, 2010) Dr. R. Veenhoven, Haarlem (October 6 th, 2013) • • • 32

Backups

Subset Analysis Immunogenicity and Nasopharyngeal Carriage Blood Sample Nasopharyngeal Swab P P • Subset of study subjects – n=2000 (1000 each from PCV 13 and placebo groups) • Immunogenicity • Nasopharyngeal Carriage – Pneumococcal carriage of PCV 13 and non-PCV 13 serotypes plus other organisms Hak E, Grobbee DE, Sanders EAM, et al. Netherlands J Med. 2008; 66: 378 -383 Data on File. CAPi. TA Study Protocol 6115 A 1 -3006 -NL 34

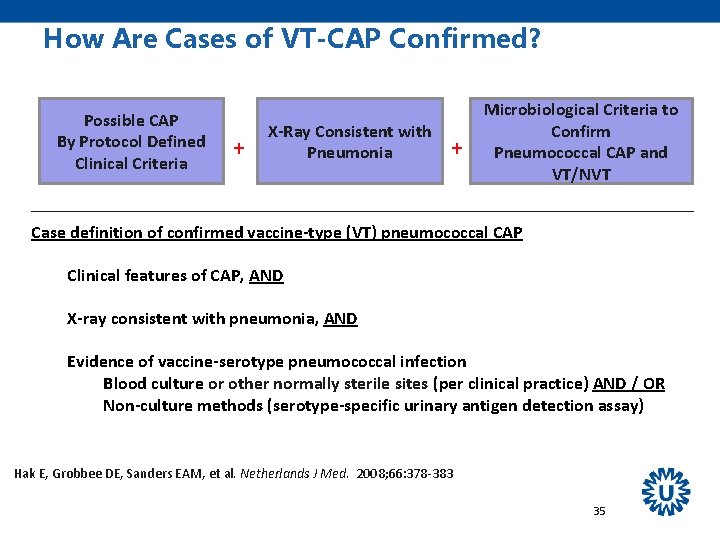
How Are Cases of VT-CAP Confirmed? Possible CAP By Protocol Defined Clinical Criteria + X-Ray Consistent with Pneumonia + Microbiological Criteria to Confirm Pneumococcal CAP and VT/NVT Case definition of confirmed vaccine-type (VT) pneumococcal CAP Clinical features of CAP, AND X-ray consistent with pneumonia, AND Evidence of vaccine-serotype pneumococcal infection Blood culture or other normally sterile sites (per clinical practice) AND / OR Non-culture methods (serotype-specific urinary antigen detection assay) Hak E, Grobbee DE, Sanders EAM, et al. Netherlands J Med. 2008; 66: 378 -383 35

Analysis Populations 36

Patient Selection • General Practitioner selects eligibles >65 jaar, not meeting exclusion criteria “Eligible candidate” receives invitation letter + study information on behalf of their own GP “Eligible candidate” return card if interested in study participation • • • “Eligible candidates” are invited by study team to come to vaccination facility “Eligible candidates” receive detailed study information by study physician Informed consent is obtained Demographic data are collected and presence of exclusion criteria are checked Study participant receives pneumococcal vaccination (or placebo) • GP reports Serious Adverse Events occurring within the first 28 days after vaccination GP reports death and loss to follow-up until end of study • • • 37

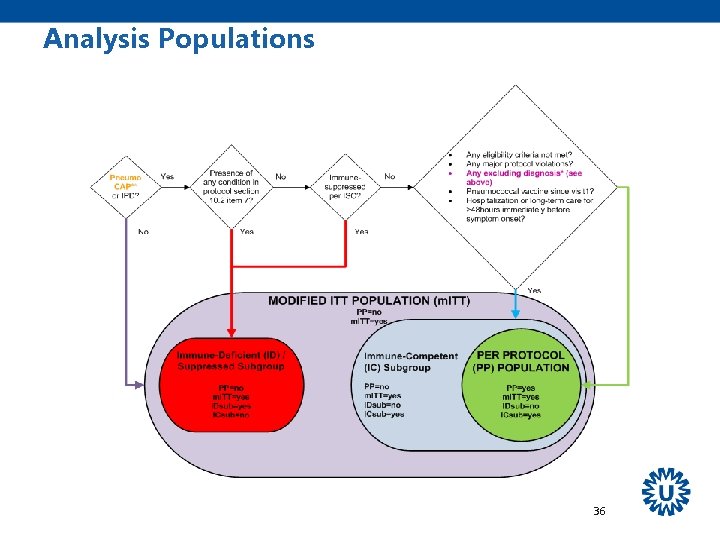
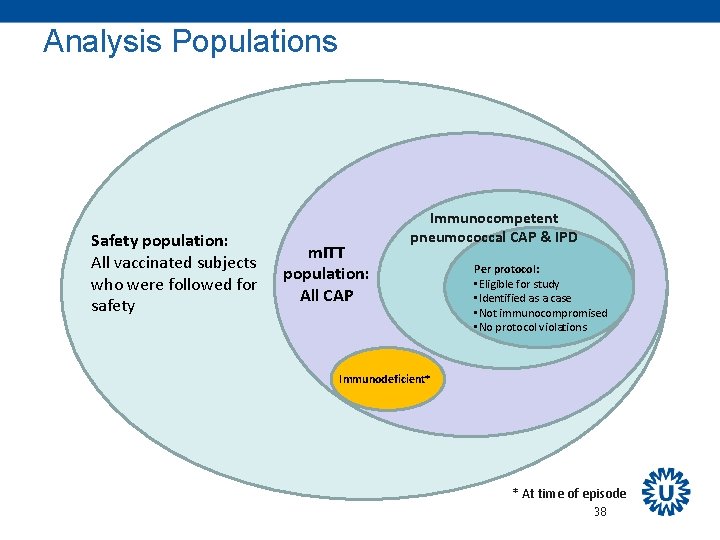
Analysis Populations Safety population: All vaccinated subjects who were followed for safety m. ITT population: All CAP Immunocompetent pneumococcal CAP & IPD Per protocol: • Eligible for study • Identified as a case • Not immunocompromised • No protocol violations Immunodeficient* * At time of episode 38
 Coherent accelerator processor interface
Coherent accelerator processor interface Atom yarıçapı nasıl artar
Atom yarıçapı nasıl artar Capi mode
Capi mode Capi system
Capi system Capi survey
Capi survey Hepatojuguler reflü nedir
Hepatojuguler reflü nedir Capi export
Capi export Capi papi
Capi papi Büyük ve küçük çapı bilinen oval çizmek
Büyük ve küçük çapı bilinen oval çizmek 2010/7 sayılı genelge tkgm
2010/7 sayılı genelge tkgm Ranvier boğum sayısı
Ranvier boğum sayısı Atom çapı nasıl artar
Atom çapı nasıl artar Codice deontologico infermieri capi
Codice deontologico infermieri capi 1 liranın çapı
1 liranın çapı Nursing care plan for pneumonia
Nursing care plan for pneumonia Hepatizatie rosie
Hepatizatie rosie Difference between typical and atypical pneumonia
Difference between typical and atypical pneumonia Evolução de enfermagem paciente com pneumonia
Evolução de enfermagem paciente com pneumonia Legionella pneumonia
Legionella pneumonia Bronchopneumonia
Bronchopneumonia Pneumonia
Pneumonia Pneumonia lung sound
Pneumonia lung sound Aspiration pneumonia treatment
Aspiration pneumonia treatment Pathophysiology of pneumonia
Pathophysiology of pneumonia Lobær pneumoni
Lobær pneumoni Pneumonia
Pneumonia Pneumonia lobaris
Pneumonia lobaris Loss of defence
Loss of defence Blood concept map answer sheet
Blood concept map answer sheet Legionella pneumonia
Legionella pneumonia Pneumonia
Pneumonia Pathophysiology of pneumonia
Pathophysiology of pneumonia Reflexos normoativos
Reflexos normoativos Suprasternal notch
Suprasternal notch Iatrogeic
Iatrogeic Pneumonia lobar
Pneumonia lobar Gross lungs
Gross lungs Pneumonia
Pneumonia Bronchiolitis
Bronchiolitis