Clinical Perspectives Atypical Antipsychotics Treatment Guidelines and Clinical





























- Slides: 69

Clinical Perspectives: Atypical Antipsychotics, Treatment Guidelines, and Clinical Study Design Dr. Stephen M. Stahl Healthy bodies, healthy lives LAT 501 -15 revised February 2016

Learning Objectives ► To demonstrate that all atypical antipsychotics have numerous binding properties in addition to dopamine D 2 and serotonin 5 HT 2 A antagonism, but no two agents are identical ► To review treatment guidelines and switching strategies from a real world perspective ► To discuss some of the challenges faced by investigational antipsychotics that are navigating the clinical development process 2

Outline 1. Atypical Antipsychotics What Makes An Antipsychotic Atypical? – Receptor Pharmacology – Pines, Dones, and More 2. Comparison of Treatment Guidelines How to Understand Treatment Guidelines – Meta-guidelines – Switching 3. Clinical Study Design Considerations – Placebo Response – Fixed and Flexible Dosing – Rating Scales – Noninferiority 3

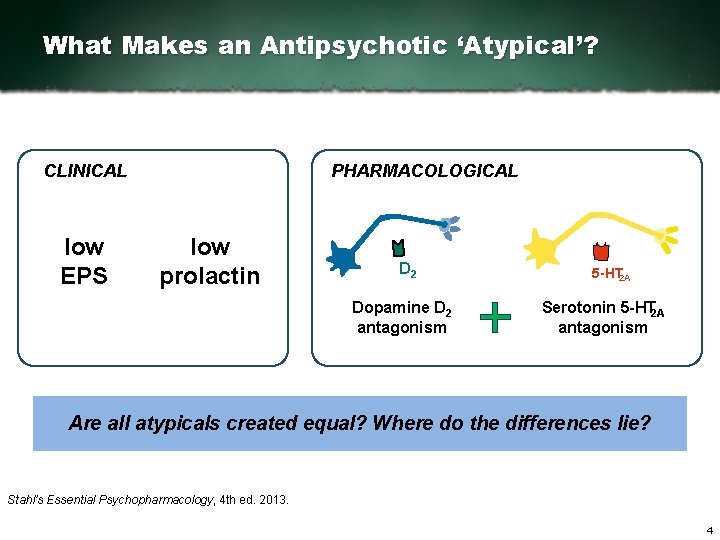
What Makes an Antipsychotic ‘Atypical’? CLINICAL low EPS PHARMACOLOGICAL low prolactin D 2 Dopamine D 2 antagonism 5 HT 2 A Serotonin 5 HT 2 A antagonism Are all atypicals created equal? Where do the differences lie? Stahl’s Essential Psychopharmacology, 4 th ed. 2013. 4

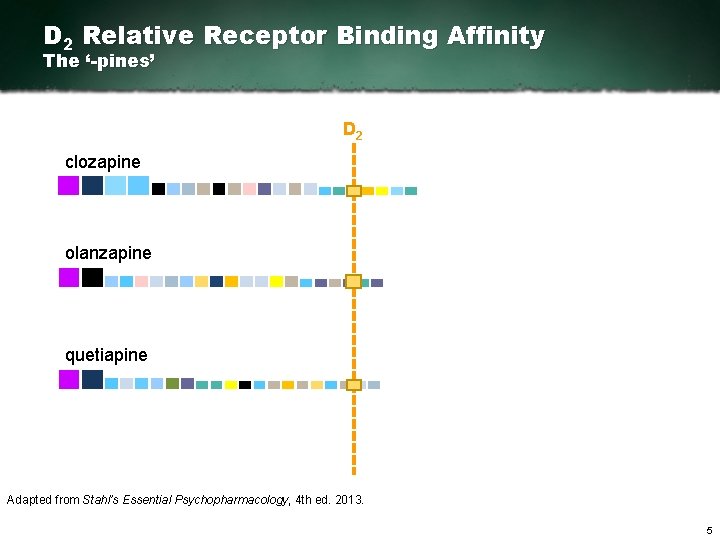
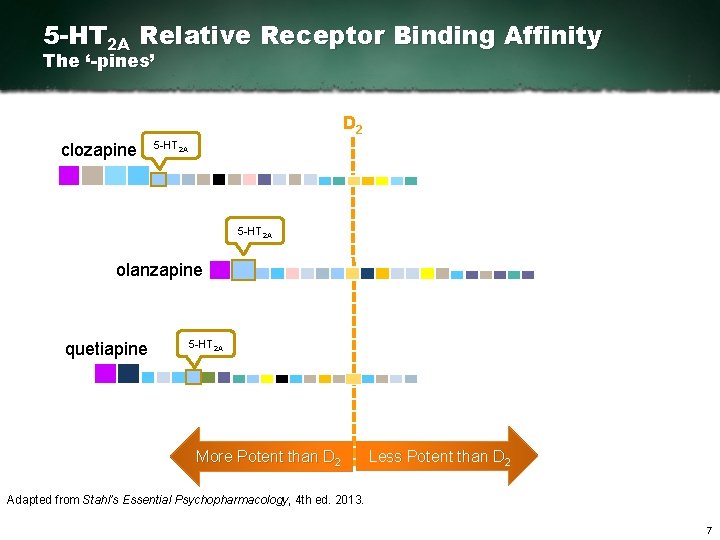
D 2 Relative Receptor Binding Affinity The ‘-pines’ D 2 clozapine olanzapine quetiapine Adapted from Stahl’s Essential Psychopharmacology, 4 th ed. 2013. 5

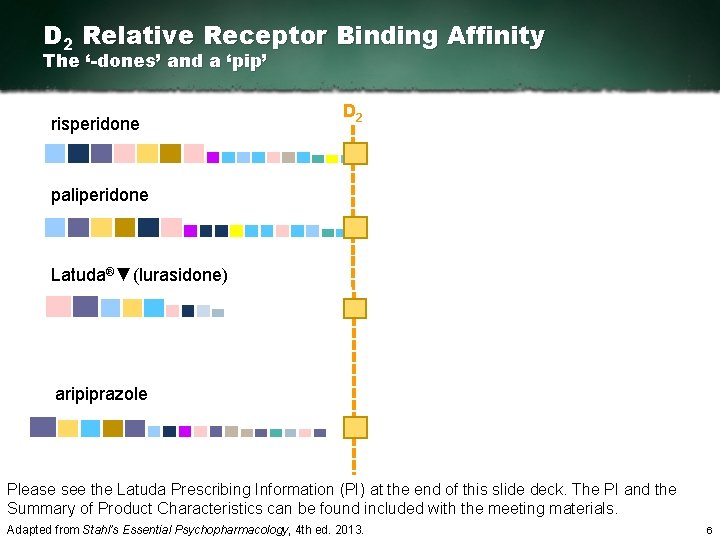
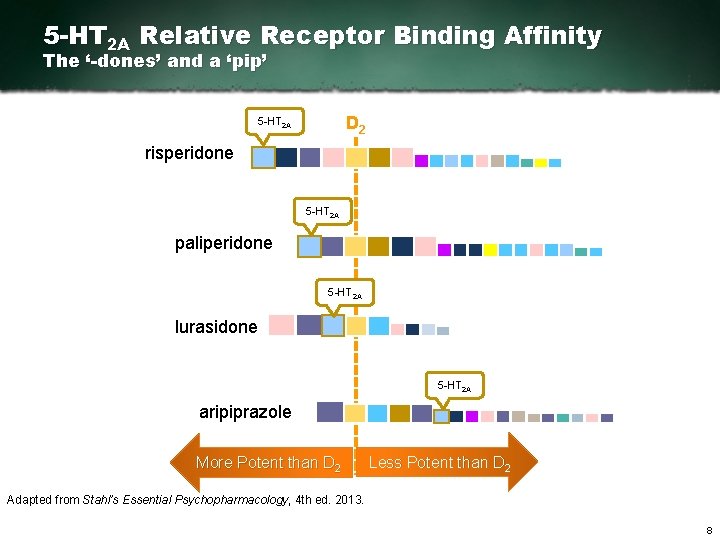
D 2 Relative Receptor Binding Affinity The ‘-dones’ and a ‘pip’ risperidone D 2 paliperidone Latuda®▼(lurasidone) aripiprazole Please see the Latuda Prescribing Information (PI) at the end of this slide deck. The PI and the Summary of Product Characteristics can be found included with the meeting materials. Adapted from Stahl’s Essential Psychopharmacology, 4 th ed. 2013. 6

5 -HT 2 A Relative Receptor Binding Affinity The ‘-pines’ D 2 clozapine 5 HT 2 A olanzapine quetiapine 5 HT 2 A More Potent than D 2 Less Potent than D 2 Adapted from Stahl’s Essential Psychopharmacology, 4 th ed. 2013. 7

5 -HT 2 A Relative Receptor Binding Affinity The ‘-dones’ and a ‘pip’ D 2 5 HT 2 A risperidone 5 HT 2 A paliperidone 5 HT 2 A lurasidone 5 HT 2 A aripiprazole More Potent than D 2 Less Potent than D 2 Adapted from Stahl’s Essential Psychopharmacology, 4 th ed. 2013. 8

Additional Antipsychotic Binding Properties Hypothetical Mediators of Therapeutic Actions 2 A M 1 T 1 A 5 HT X M 3 H 1 1 5 HT 1 D 2 5 HT 2 C SRI NRI 5 HT 3 D 1 5 H T 7 T 6 H 5 D 4 D 3 D 2 Adapted from Stahl’s Essential Psychopharmacology, 4 th ed. 2013. 9

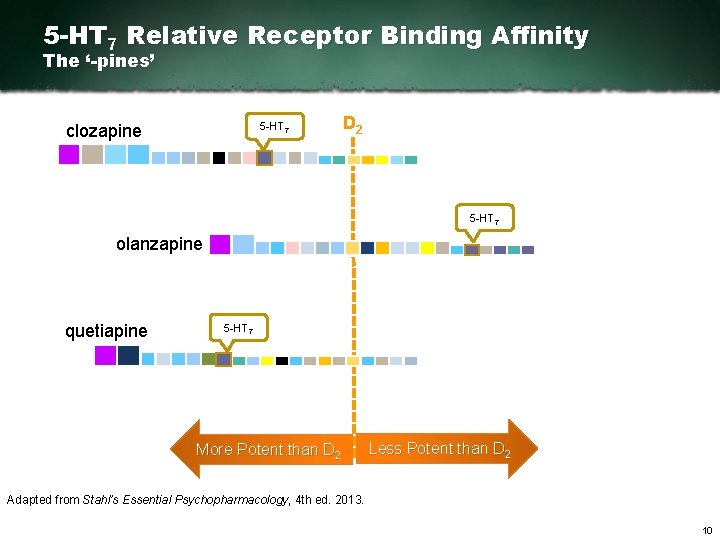
5 -HT 7 Relative Receptor Binding Affinity The ‘-pines’ 5 HT 7 clozapine D 2 5 HT 7 olanzapine quetiapine 5 HT 7 More Potent than D 2 Less Potent than D 2 Adapted from Stahl’s Essential Psychopharmacology, 4 th ed. 2013. 10

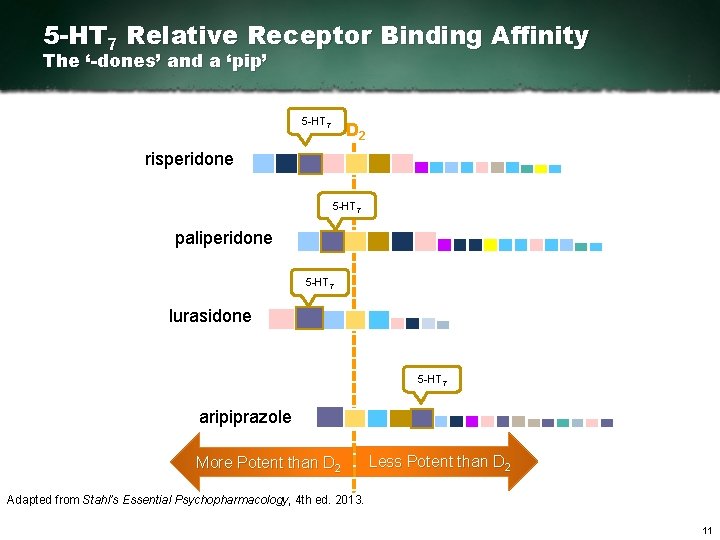
5 -HT 7 Relative Receptor Binding Affinity The ‘-dones’ and a ‘pip’ 5 HT 7 D 2 risperidone 5 HT 7 paliperidone 5 HT 7 lurasidone 5 HT 7 aripiprazole More Potent than D 2 Less Potent than D 2 Adapted from Stahl’s Essential Psychopharmacology, 4 th ed. 2013. 11

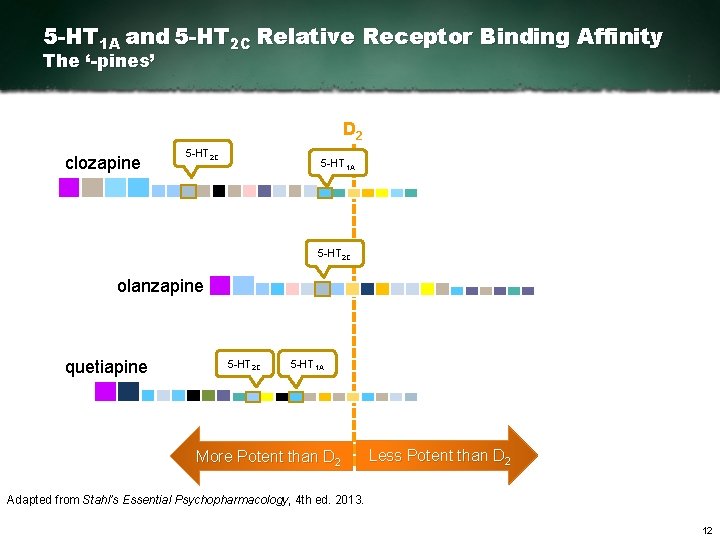
5 -HT 1 A and 5 -HT 2 C Relative Receptor Binding Affinity The ‘-pines’ D 2 clozapine 5 HT 2 C 5 HT 1 A 5 HT 2 C olanzapine quetiapine 5 HT 2 C 5 HT 1 A More Potent than D 2 Less Potent than D 2 Adapted from Stahl’s Essential Psychopharmacology, 4 th ed. 2013. 12

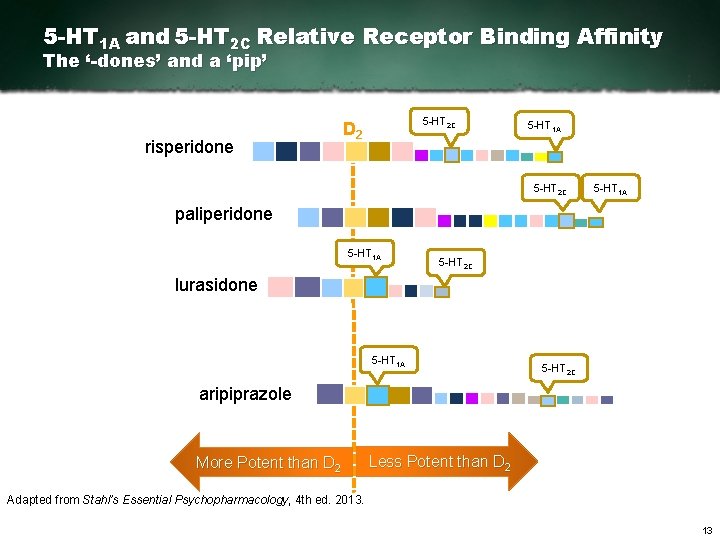
5 -HT 1 A and 5 -HT 2 C Relative Receptor Binding Affinity The ‘-dones’ and a ‘pip’ risperidone 5 HT 2 C D 2 5 HT 1 A 5 HT 2 C 5 HT 1 A paliperidone 5 HT 1 A 5 HT 2 C lurasidone 5 HT 1 A 5 HT 2 C aripiprazole More Potent than D 2 Less Potent than D 2 Adapted from Stahl’s Essential Psychopharmacology, 4 th ed. 2013. 13

Additional Antipsychotic Binding Properties Hypothetical Mediators of Side Effects 5 HT 1 A 5 H T 2 A X M 1 M 3 5 H T 1 D H 1 5 HT 1 2 C 2 5 HT 3 T 6 D 1 5 HT 7 5 H SR I NR I D 4 D 3 D 2 Adapted from Stahl’s Essential Psychopharmacology, 4 th ed. 2013. 14

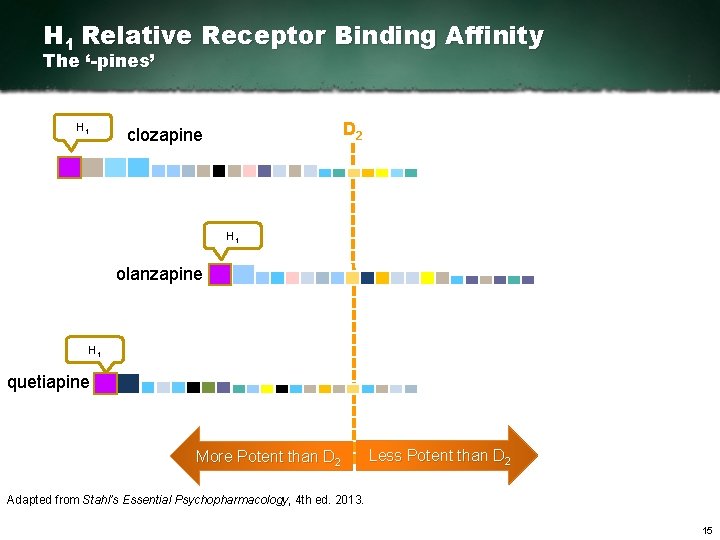
H 1 Relative Receptor Binding Affinity The ‘-pines’ H 1 D 2 clozapine H 1 olanzapine H 1 quetiapine More Potent than D 2 Less Potent than D 2 Adapted from Stahl’s Essential Psychopharmacology, 4 th ed. 2013. 15

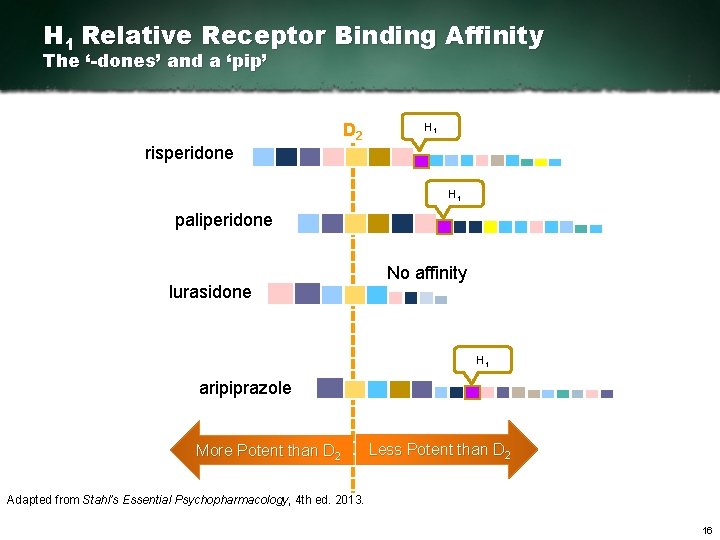
H 1 Relative Receptor Binding Affinity The ‘-dones’ and a ‘pip’ risperidone D 2 H 1 paliperidone lurasidone No affinity H 1 aripiprazole More Potent than D 2 Less Potent than D 2 Adapted from Stahl’s Essential Psychopharmacology, 4 th ed. 2013. 16

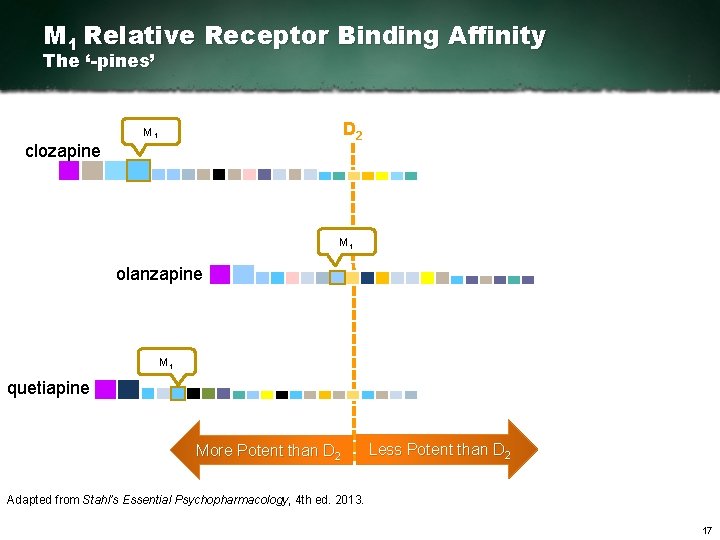
M 1 Relative Receptor Binding Affinity The ‘-pines’ D 2 M 1 clozapine M 1 olanzapine M 1 quetiapine More Potent than D 2 Less Potent than D 2 Adapted from Stahl’s Essential Psychopharmacology, 4 th ed. 2013. 17

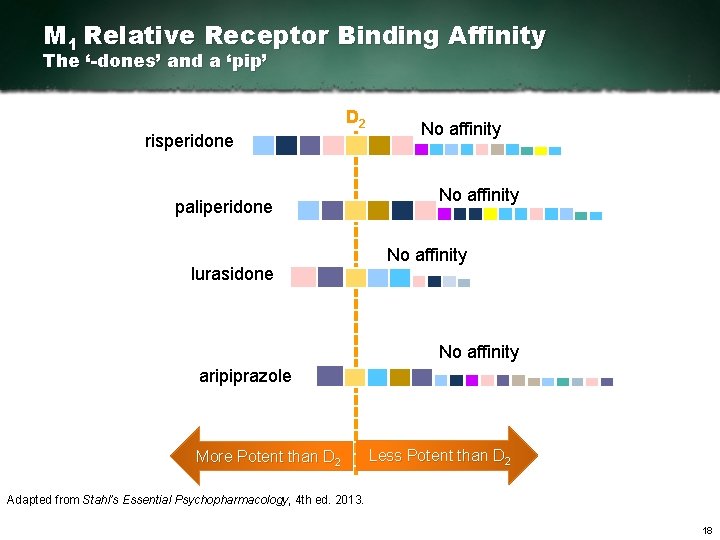
M 1 Relative Receptor Binding Affinity The ‘-dones’ and a ‘pip’ D 2 risperidone paliperidone lurasidone No affinity aripiprazole More Potent than D 2 Less Potent than D 2 Adapted from Stahl’s Essential Psychopharmacology, 4 th ed. 2013. 18

Summary 1: Atypical Antipsychotics as a Class § All atypical antipsychotics have dopamine D 2 and 5 HT 2 A antagonist properties § All atypicals have numerous other binding properties and are linked to various clinical effects*, but no two agents are identical § Differences in binding properties are the most rational explanation for differences in efficacy and tolerability in individual patients* *The correlation between receptor binding affinities and clinical outcomes is uncertain. 19

Outline 1. Atypical Antipsychotics What Makes An Antipsychotic Atypical? – Receptor Pharmacology – Pines, Dones, and More 2. Comparison of Treatment Guidelines How to Understand Treatment Guidelines – Meta-guidelines – Switching 3. Clinical Study Design Considerations – Placebo Response – Fixed and Flexible Dosing – Rating Scales – Noninferiority 20

Perspectives on Treatment Guidelines Recommendations from BAP “The ethos of early intervention services for first-episode psychosis is to reduce duration of untreated psychosis (DUP), and to provide high-quality pharmacotherapeutic, psychological and psychosocial interventions in the critical early phase of the disorder. ” Barnes TRE et al. J Psychopharmacol. 2011; 25(5): 567 620. 21

Perspectives on Treatment Guidelines Recommendations from BAP Rationale for early intervention: § Patients “are accessed at a relatively treatment responsive stage of illness” § Minimise “possible adverse consequences of… untreated psychosis” improving symptomatic and functional outcomes § Potentially arrest progressive structural brain changes associated with psychotic illness Barnes TRE et al. J Psychopharmacol. 2011; 25(5): 567 620. 22

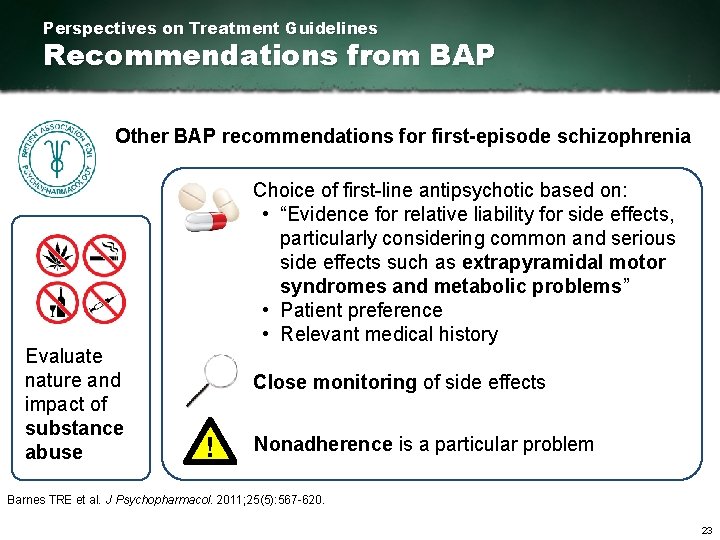
Perspectives on Treatment Guidelines Recommendations from BAP Other BAP recommendations for first episode schizophrenia Evaluate nature and impact of substance abuse Choice of first line antipsychotic based on: • “Evidence for relative liability for side effects, particularly considering common and serious side effects such as extrapyramidal motor syndromes and metabolic problems” • Patient preference • Relevant medical history Close monitoring of side effects ! Nonadherence is a particular problem Barnes TRE et al. J Psychopharmacol. 2011; 25(5): 567 620. 23

The Abandoned Illness A report by the Schizophrenia Commission 14 6 2, 500 experts evidence gathering sessions respondents to survey ‘ Consensus findings on unmet needs included: § Greater partnership and shared decision making with service users § Better prescribing and a right to a second opinion on medication § Extending training for general practitioners in mental illness § A stronger focus on prevention Research has led to an increasing number of effective drugs to choose from. . . We know much more about ‘what works’ than we used to and we have seen inspiring examples of recovery based services in England learnt of better approaches used in other countries. Professor Sir Robin Murray, FRS Chair of the Schizophrenia Commission ’ 24

Royal College of Psychiatrists’ Centre for Quality Improvement National Audit of Schizophrenia The goal was “to collect case record data on 100 service users, living in the community, from each of the Mental Health Trusts/Health Boards in England Wales. ” NAS 1, 2012 – Key findings on areas of significant deficiency • Monitoring and managing of physical health problems in people with schizophrenia • Some aspects of medication prescribing practice • Some aspects of how clinicians communicate with service users and carers NAS 2, 2014 – Key findings on major areas of concern • Poor monitoring of, and intervention for, risk factors for diabetes and cardiovascular disease • Service users whose illness is poorly responsive to standard antipsychotic medications are waiting too long to be switched to newer antipsychotics • Significant gaps in the availability of cognitive behavioural therapy and family interventions • Inadequate provisions of information and support for carers • Inadequacies in information systems Royal College of Psychiatrists. Report of the Second Round of the National Audit of Schizophrenia (NAS 2) 2014. London: Healthcare Quality Improvement Partnership. 2014. 25

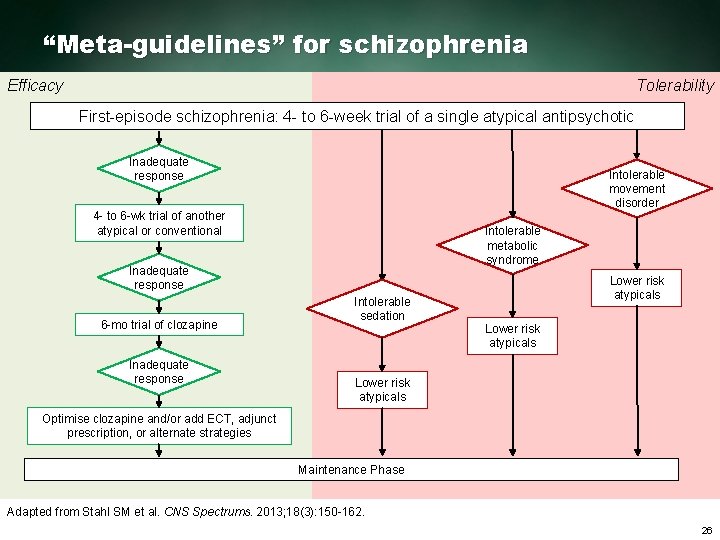
“Meta-guidelines” for schizophrenia Efficacy Tolerability First episode schizophrenia: 4 to 6 week trial of a single atypical antipsychotic Inadequate response Intolerable movement disorder 4 to 6 wk trial of another atypical or conventional Intolerable metabolic syndrome Inadequate response 6 mo trial of clozapine Inadequate response Intolerable sedation Lower risk atypicals Optimise clozapine and/or add ECT, adjunct prescription, or alternate strategies Maintenance Phase Adapted from Stahl SM et al. CNS Spectrums. 2013; 18(3): 150 162. 26

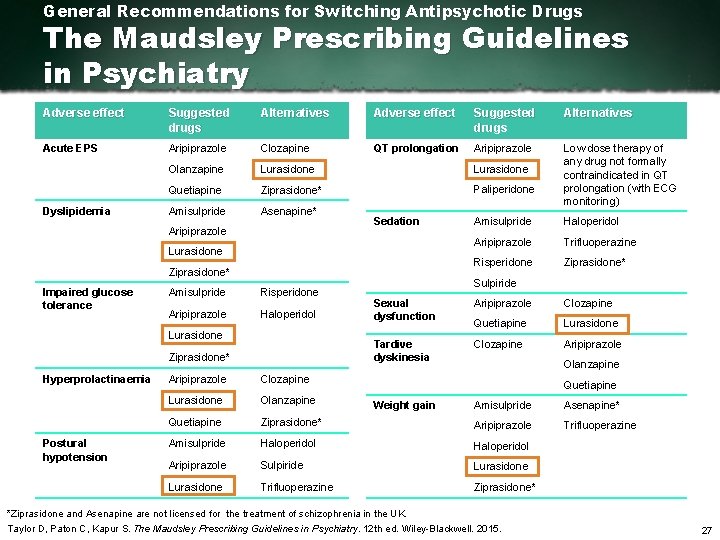
General Recommendations for Switching Antipsychotic Drugs The Maudsley Prescribing Guidelines in Psychiatry Adverse effect Suggested drugs Alternatives Acute EPS Aripiprazole Clozapine QT prolongation Aripiprazole Olanzapine Lurasidone Quetiapine Ziprasidone* Paliperidone Amisulpride Asenapine* Low dose therapy of any drug not formally contraindicated in QT prolongation (with ECG monitoring) Dyslipidemia Aripiprazole Sedation Lurasidone Ziprasidone* Impaired glucose tolerance Amisulpride Risperidone Aripiprazole Haloperidol Lurasidone Ziprasidone* Hyperprolactinaemia Postural hypotension Aripiprazole Clozapine Lurasidone Olanzapine Quetiapine Amisulpride Haloperidol Aripiprazole Trifluoperazine Risperidone Ziprasidone* Sulpiride Sexual dysfunction Aripiprazole Clozapine Quetiapine Lurasidone Tardive dyskinesia Clozapine Aripiprazole Olanzapine Quetiapine Weight gain Amisulpride Asenapine* Ziprasidone* Aripiprazole Trifluoperazine Amisulpride Haloperidol Aripiprazole Sulpiride Lurasidone Trifluoperazine Ziprasidone* *Ziprasidone and Asenapine are not licensed for the treatment of schizophrenia in the UK. Taylor D, Paton C, Kapur S. The Maudsley Prescribing Guidelines in Psychiatry. 12 th ed. Wiley Blackwell. 2015. 27

Antipsychotics in Clinical Practice § Real patients are often more complicated § May have diagnoses that do not neatly meet formal criteria § Generally have much more comorbidity than patients in clinical studies 28

Case Study* Hannah 05346 940 * hypothetical case Single Female 21 nursing student Lives with her parents Rebecca Mother Schizophrenia 21 29

Case Study* Hannah Uncle with mental illness * hypothetical case • First episode • While walking through a park one evening she thought she heard someone following her and threatening to hurt her; she hid in a maintenance building and called the police insisting she was in danger • When the police arrived she was completely alone but was frantic and saying that she could still hear someone threatening her, when the police told her there was nobody there she became very agitated and ultimately the police brought her to the hospital 30

Case Study* Hannah Uncle with bipolar disorder * hypothetical case • First episode • While in the hospital she was clearly terrified of the voices threatening to hurt her so the doctors gave her medication to calm her while they ran tests and brought in a psychiatrist • Interview reveals • She had been hearing voices, whom she thought were her neighbours, in the months prior to the episode in the park • She has been struggling in her nursing classes for the past 2 years 31

Case Study* Hannah * hypothetical case • No evidence of substance use • Nothing abnormal in hospital lab work 32

Case Study* Hannah * hypothetical case • First generation antipsychotic, inpatient • She is showing signs of improvement and she wants to be seen as an outpatient • Was prescribed an atypical antipsychotic, but she complained of side effects • Switched to 37 mg/day lurasidone 33

Case Study* Hannah * hypothetical case • Outpatient for 6 months now • Her symptoms are under control and she wants to go back to school • She is nervous about returning to school since she was struggling academically prior to the episode in the park. • She decides to enroll in a single class so as not to overwhelm herself 34

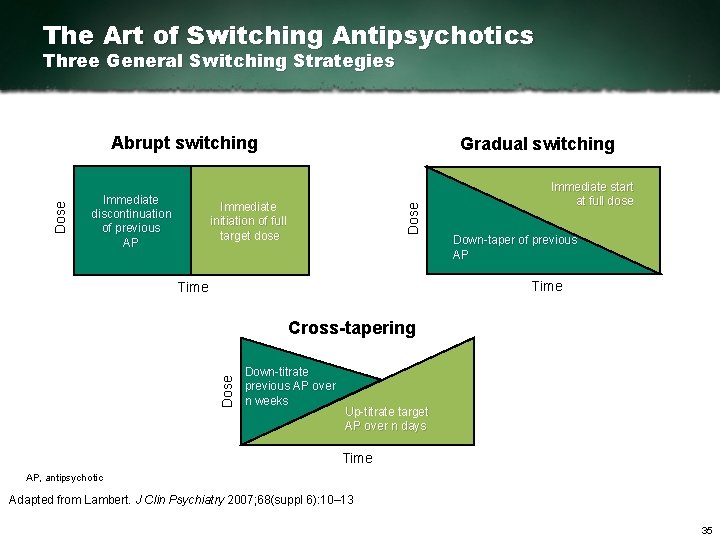
The Art of Switching Antipsychotics Three General Switching Strategies Immediate discontinuation of previous AP Gradual switching Immediate initiation of full target dose Dose Abrupt switching Immediate start at full dose Down taper of previous AP Time Dose Cross tapering Down titrate previous AP over n weeks Up titrate target AP over n days Time AP, antipsychotic Adapted from Lambert. J Clin Psychiatry 2007; 68(suppl 6): 10– 13 35

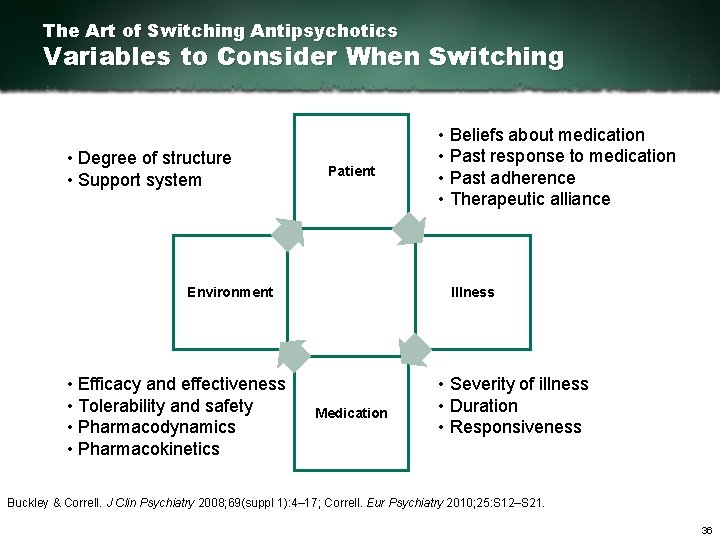
The Art of Switching Antipsychotics Variables to Consider When Switching • Degree of structure • Support system Patient Illness Environment • Efficacy and effectiveness • Tolerability and safety • Pharmacodynamics • Pharmacokinetics • Beliefs about medication • Past response to medication • Past adherence • Therapeutic alliance Medication • Severity of illness • Duration • Responsiveness Buckley & Correll. J Clin Psychiatry 2008; 69(suppl 1): 4– 17; Correll. Eur Psychiatry 2010; 25: S 12–S 21. 36

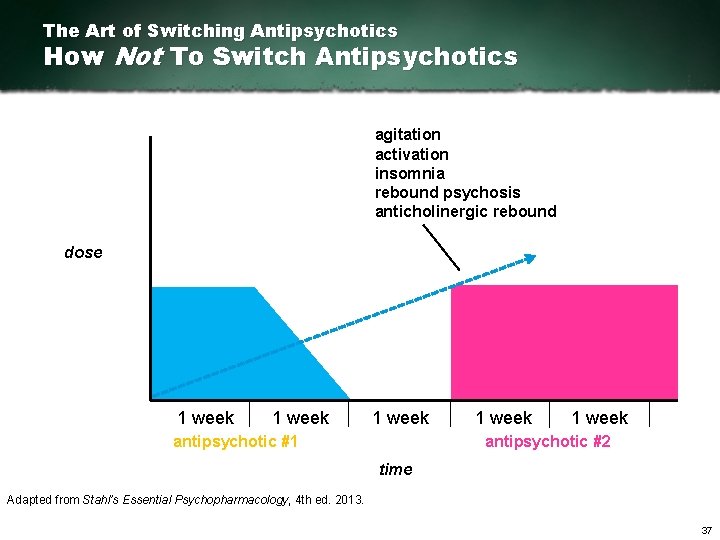
The Art of Switching Antipsychotics How Not To Switch Antipsychotics agitation activation insomnia rebound psychosis anticholinergic rebound dose 1 week antipsychotic #1 1 week antipsychotic #2 time Adapted from Stahl’s Essential Psychopharmacology, 4 th ed. 2013. 37

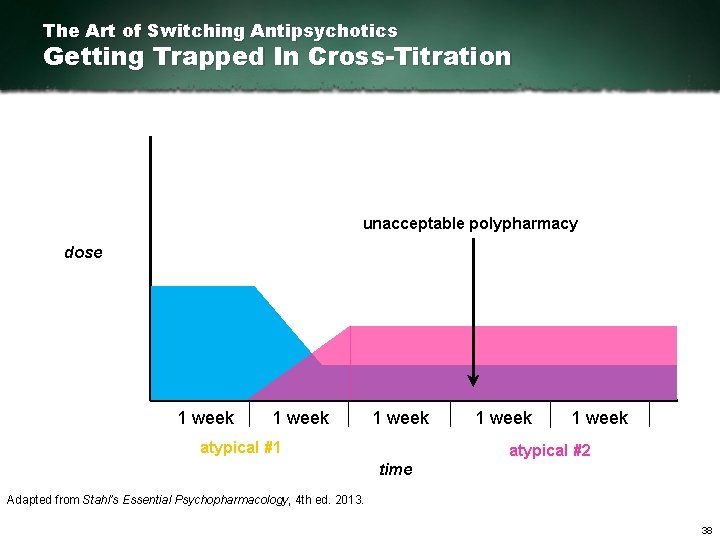
The Art of Switching Antipsychotics Getting Trapped In Cross-Titration unacceptable polypharmacy dose 1 week atypical #1 1 week atypical #2 time Adapted from Stahl’s Essential Psychopharmacology, 4 th ed. 2013. 38

Switching from Oral Antipsychotics to Lurasidone Target dose Dose Target dose aripiprazole paliperidone * lurasidone Dose 1 week Target dose Dose quetiapine olanzapine * lurasidone 1 week Target dose * risperidone lurasidone 1 week Dose * lurasidone clozapine 1 week * Benzodiazepine or anticholinergic medication can be administered during cross titration to help alleviate side effects such as insomnia, agitation, and/or psychosis Adapted from Stahl SM, The Prescriber’s Guide, 5 th ed. 2014. 39

Summary 2 § Guidelines may assist clinicians in choosing the best evidence based treatment strategies; clinical judgment should always be exercised § Real life patients are “messy” – complex diagnoses, comorbidities, etc. – and nonadherence is a particular problem § Switching antipsychotics requires observation and care – otherwise, patients can develop agitation, activation, insomnia, rebound psychosis, and withdrawal effects 40

Outline 1. Atypical Antipsychotics What Makes An Antipsychotic Atypical? – Receptor Pharmacology – Pines, Dones, and More 2. Comparison of Treatment Guidelines How to Understand Treatment Guidelines – Meta-guidelines – Switching 3. Clinical Study Design Considerations – Placebo Response – Fixed and Flexible Dosing – Rating Scales – Noninferiority 41

Challenges in Antipsychotic Clinical Trial Design § High placebo responses and failure of active treatments to demonstrate significant efficacy vs. placebo 1 § High rates of subject discontinuation 2, 3 § Generalisability of results to real world patients 4, 5 § Choosing the right dose in practice § What to expect in terms of efficacy § What to expect in terms of safety and tolerability 1. 2. 3. 4. 5. Kemp AS, et al. Schizophr Bulletin. 2010; 36(3): 504 509. Kemmler G, et al. Arch Gen Psychiatry. 2005; 62(12): 1305 1312. Lieberman JA, et al. N Engl J Med. 2005; 353(12): 1209 1223. Hofer A, et al. J Clin Psychopharmacol. 2000; 20(6): 699 702. Riedel M, et al. Eur Arch Psychiatry Clin Neurosci. 2005; 255(2): 143 148. 42

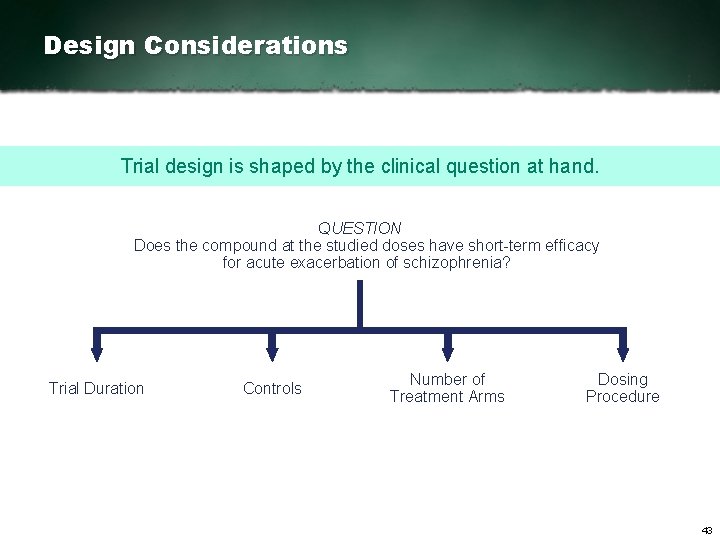
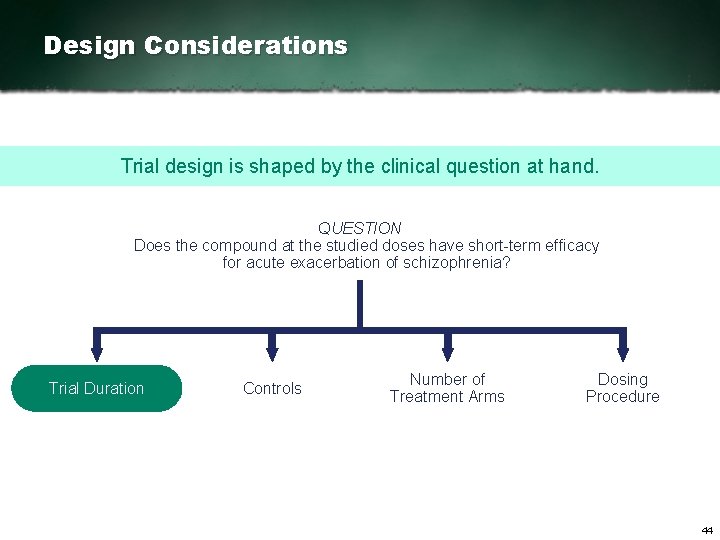
Design Considerations Trial design is shaped by the clinical question at hand. QUESTION Does the compound at the studied doses have short term efficacy for acute exacerbation of schizophrenia? Trial Duration Controls Number of Treatment Arms Dosing Procedure 43

Design Considerations Trial design is shaped by the clinical question at hand. QUESTION Does the compound at the studied doses have short term efficacy for acute exacerbation of schizophrenia? Trial Duration Controls Number of Treatment Arms Dosing Procedure 44

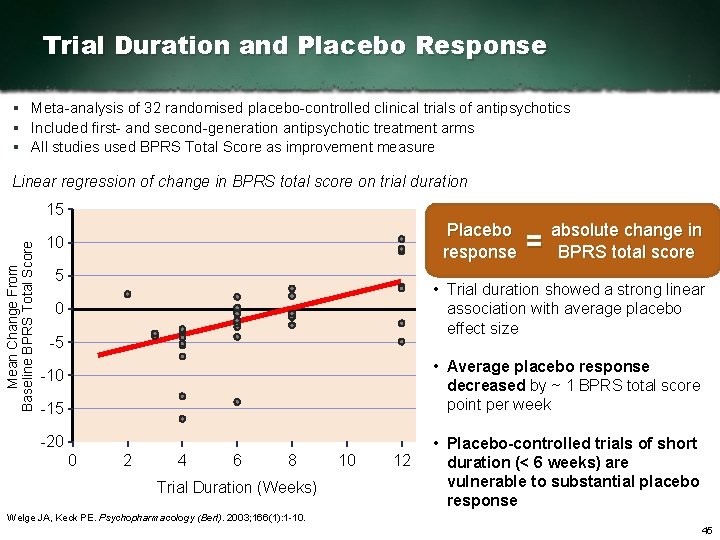
Trial Duration and Placebo Response § Meta analysis of 32 randomised placebo controlled clinical trials of antipsychotics § Included first and second generation antipsychotic treatment arms § All studies used BPRS Total Score as improvement measure Linear regression of change in BPRS total score on trial duration Mean Change From Baseline BPRS Total Score 15 Placebo response 10 5 = absolute change in BPRS total score • Trial duration showed a strong linear association with average placebo effect size 0 5 • Average placebo response decreased by ~ 1 BPRS total score point per week 10 15 20 0 2 4 6 8 Trial Duration (Weeks) 10 12 • Placebo controlled trials of short duration (< 6 weeks) are vulnerable to substantial placebo response Welge JA, Keck PE. Psychopharmacology (Berl). 2003; 166(1): 1 10. 45

Design Considerations Trial design is shaped by the clinical question at hand. QUESTION Does the compound at the studied doses have short term efficacy for acute exacerbation of schizophrenia? Trial Duration Controls Number of Treatment Arms Dosing Procedure 46

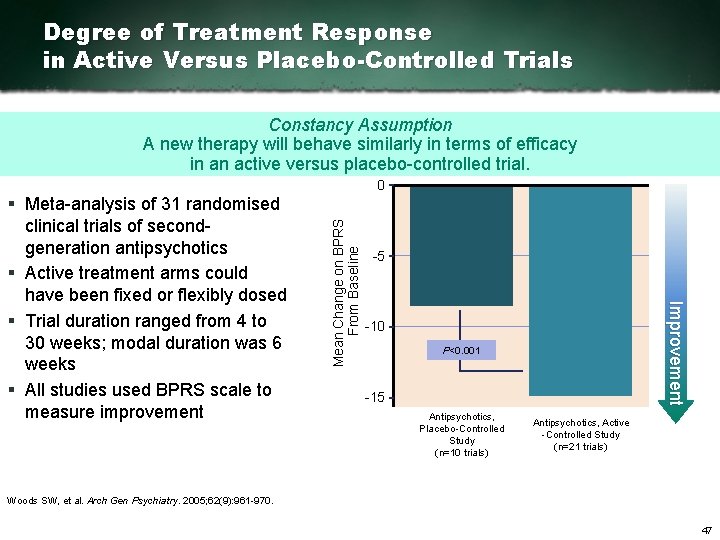
Degree of Treatment Response in Active Versus Placebo-Controlled Trials Constancy Assumption A new therapy will behave similarly in terms of efficacy in an active versus placebo controlled trial. 5 Improvement § Meta analysis of 31 randomised clinical trials of second generation antipsychotics § Active treatment arms could have been fixed or flexibly dosed § Trial duration ranged from 4 to 30 weeks; modal duration was 6 weeks § All studies used BPRS scale to measure improvement Mean Change on BPRS From Baseline 0 10 P<0. 001 15 Antipsychotics, Placebo Controlled Study (n=10 trials) Antipsychotics, Active Controlled Study (n=21 trials) Woods SW, et al. Arch Gen Psychiatry. 2005; 62(9): 961 970. 47

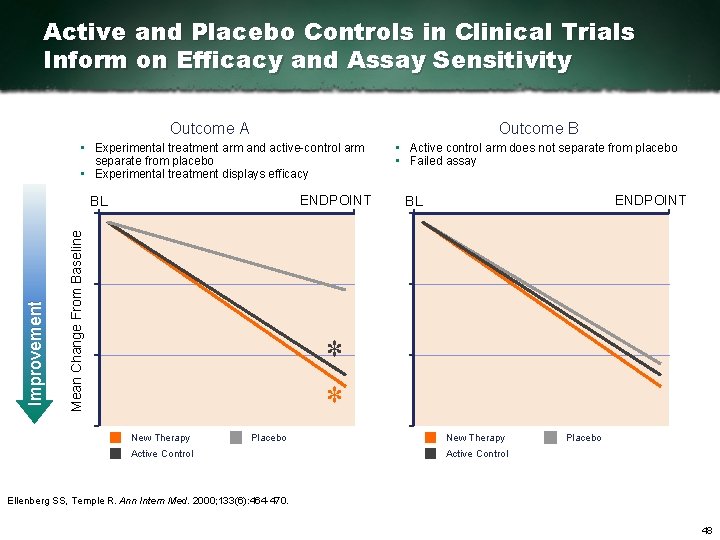
Active and Placebo Controls in Clinical Trials Inform on Efficacy and Assay Sensitivity Outcome B Outcome A • Experimental treatment arm and active control arm separate from placebo • Experimental treatment displays efficacy ENDPOINT Mean Change From Baseline Improvement BL • Active control arm does not separate from placebo • Failed assay ENDPOINT BL * * New Therapy Placebo Active Control Ellenberg SS, Temple R. Ann Intern Med. 2000; 133(6): 464 470. 48

Design Considerations Trial design is shaped by the clinical question at hand. QUESTION Does the compound at the studied doses have short term efficacy for acute exacerbation of schizophrenia? Trial Duration Controls Number of Treatment Arms Dosing Procedure 49

Percentage of Subjects Randomised to Placebo and Placebo Response § Meta analysis of 23 randomised placebo controlled clinical trials of antipsychotics § All studies used PANSS scale to measure changes § Trial duration ranged from 2 to 6 weeks Mean Change From PANSS Baseline Improvement Mean placebo change 0 0. 6 5 7. 6 10 • As the percentage of patients randomised to placebo increased, mean placebo improvement decreased 7. 0 9. 4 • Reasons for this effect are not clear from the study 12. 9 15 18. 2% • Percentage randomised to placebo influenced trial outcomes, specifically mean placebo change 20% 22. 2% 25% 33. 3% % Subjects Randomised to Placebo Significant association (P<0. 05) by mixed effects model meta regression analysis Mallinckrodt CH, et al. Psychopharmacol Bull. 2010; 43(1): 53 72. 50

Design Considerations Trial design is shaped by the clinical question at hand. QUESTION Does the compound at the studied doses have short term efficacy for acute exacerbation of schizophrenia? Trial Duration Controls Number of Treatment Arms Dosing Procedure 51

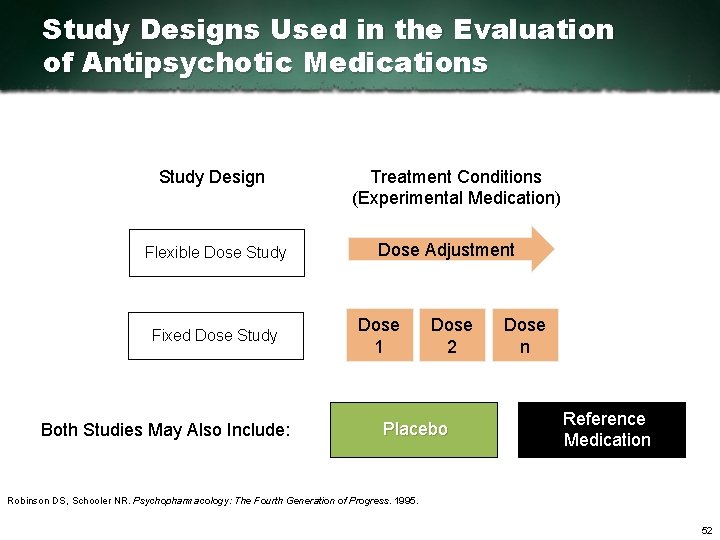
Study Designs Used in the Evaluation of Antipsychotic Medications Study Design Flexible Dose Study Fixed Dose Study Both Studies May Also Include: Treatment Conditions (Experimental Medication) Dose Adjustment Dose 1 Dose 2 Placebo Dose n Reference Medication Robinson DS, Schooler NR. Psychopharmacology: The Fourth Generation of Progress. 1995. 52

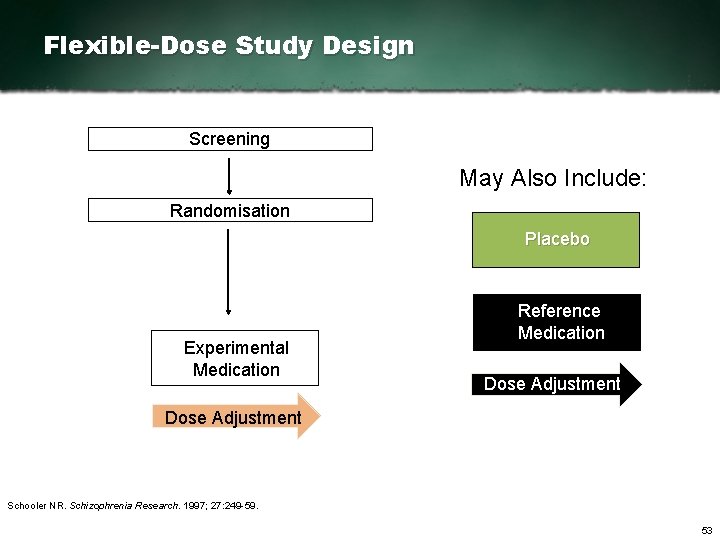
Flexible-Dose Study Design Screening May Also Include: Randomisation Placebo Experimental Medication Reference Medication Dose Adjustment Schooler NR. Schizophrenia Research. 1997; 27: 249 59. 53

Advantages and Limitations of Flexible-Dose Studies Advantages § Adjusting the dose to achieve the desired clinical effect, and decreasing the dose if side effects occur, more closely resembles clinical practice Limitations § Dose adjustment prevents determination of an optimal dose within the range studied § The speed of dosage adjustment may affect the ability to correlate symptom improvement with a specific dosage Schooler NR. Schizophrenia Research. 1997; 27: 249 59. 54

Fixed-Dose Study Design: Multiple Doses of Experimental Medication Screening May Also Include: Randomisation Placebo Dose 1 Dose 2 Dose n Reference Medication Experimental Medication Schooler NR. Schizophrenia Research. 1997; 27: 249 59. 55

Advantages of Fixed-Dose Study Design: Multiple Doses of Experimental Medication § It is not necessary to determine equivalence between the experimental and reference medications prior to initiating the trial § Dose and time are independent of one another for the study medication, making it possible to assess the time to response § Using multiple fixed doses allows investigators to determine an optimal dose within the range of doses studied § The relationship between plasma concentrations and both clinical response and side effects can be determined Schooler NR. Schizophrenia Research. 1997; 27: 249 59. 56

Limitations of Fixed-Dose Study Design: Multiple Doses of Experimental Medication § The conclusions drawn are limited to the doses of the study medication investigated § Patients may be maintained on a suboptimal dose or a dose with intolerable side effects § Study design does not resemble clinical practice 1. Schooler NR. Schizophrenia Research. 1997; 27: 249 59. 2. Lipkovitch I, et al. BMC Psychiatry. 2008; 8(3). 57

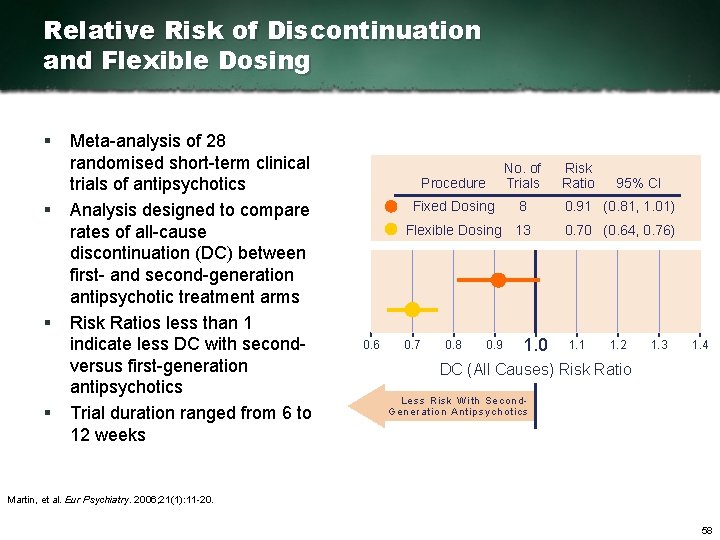
Relative Risk of Discontinuation and Flexible Dosing § § Meta analysis of 28 randomised short term clinical trials of antipsychotics Analysis designed to compare rates of all cause discontinuation (DC) between first and second generation antipsychotic treatment arms Risk Ratios less than 1 indicate less DC with second versus first generation antipsychotics Trial duration ranged from 6 to 12 weeks 0. 6 Procedure No. of Trials Fixed Dosing 8 0. 91 (0. 81, 1. 01) Flexible Dosing 13 0. 70 (0. 64, 0. 76) 0. 7 0. 8 0. 9 1. 0 Risk Ratio 1. 1 95% CI 1. 2 1. 3 1. 4 DC (All Causes) Risk Ratio Less Risk With Se con d Ge ne ration Ant ipsychotics Martin, et al. Eur Psychiatry. 2006; 21(1): 11 20. 58

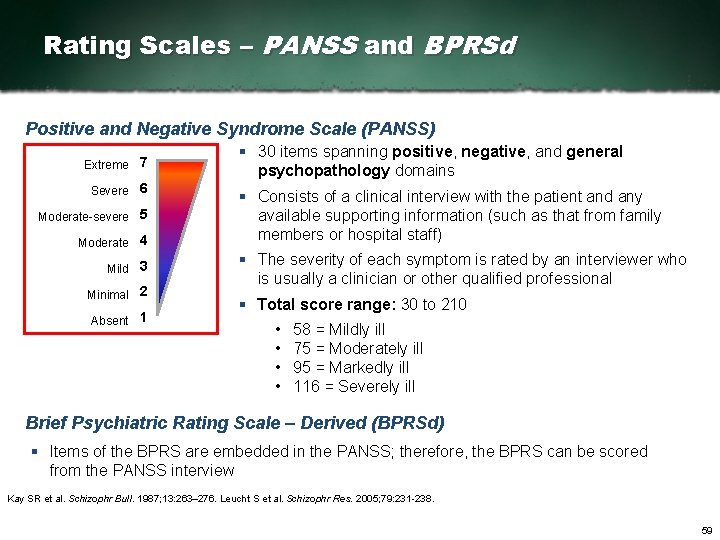
Rating Scales – PANSS and BPRSd Positive and Negative Syndrome Scale (PANSS) Extreme 7 Severe 6 Moderate severe 5 Moderate 4 Mild 3 Minimal 2 Absent 1 § 30 items spanning positive, negative, and general psychopathology domains § Consists of a clinical interview with the patient and any available supporting information (such as that from family members or hospital staff) § The severity of each symptom is rated by an interviewer who is usually a clinician or other qualified professional § Total score range: 30 to 210 • • 58 = Mildly ill 75 = Moderately ill 95 = Markedly ill 116 = Severely ill Brief Psychiatric Rating Scale – Derived (BPRSd) § Items of the BPRS are embedded in the PANSS; therefore, the BPRS can be scored from the PANSS interview Kay SR et al. Schizophr Bull. 1987; 13: 263– 276. Leucht S et al. Schizophr Res. 2005; 79: 231 238. 59

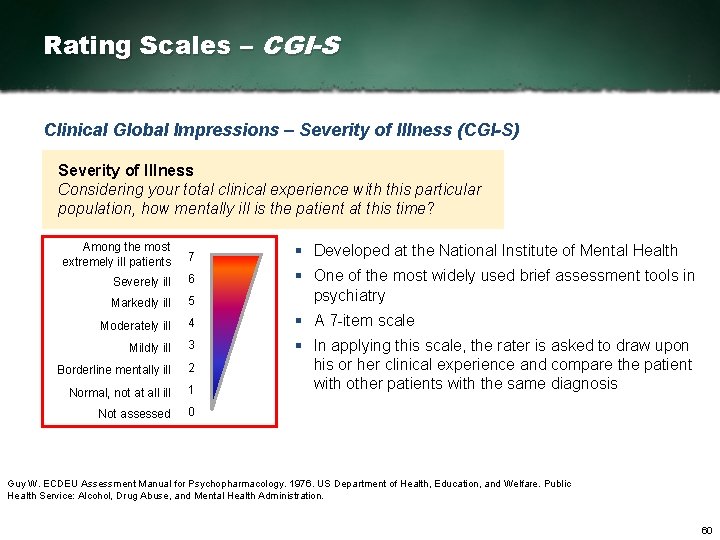
Rating Scales – CGI-S Clinical Global Impressions – Severity of Illness (CGI-S) Severity of Illness Considering your total clinical experience with this particular population, how mentally ill is the patient at this time? Among the most extremely ill patients 7 Severely ill 6 Markedly ill 5 § One of the most widely used brief assessment tools in psychiatry Moderately ill 4 § A 7 item scale Mildly ill 3 Borderline mentally ill 2 Normal, not at all ill 1 § In applying this scale, the rater is asked to draw upon his or her clinical experience and compare the patient with other patients with the same diagnosis Not assessed 0 § Developed at the National Institute of Mental Health Guy W. ECDEU Assessment Manual for Psychopharmacology. 1976. US Department of Health, Education, and Welfare. Public Health Service: Alcohol, Drug Abuse, and Mental Health Administration. 60

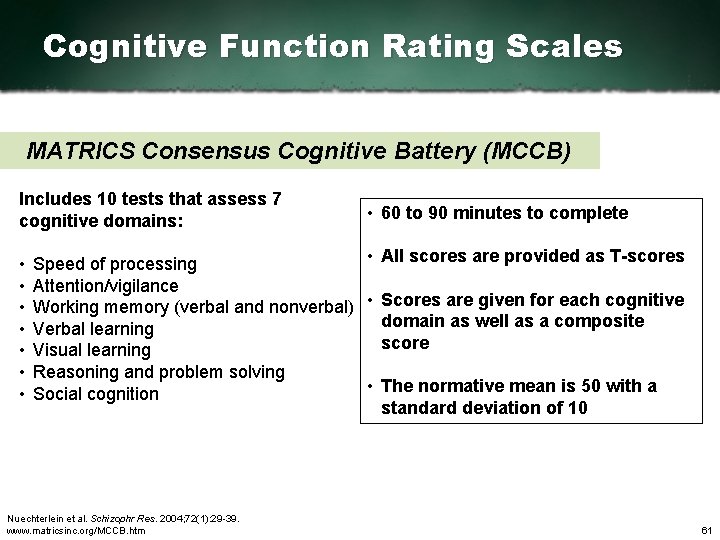
Cognitive Function Rating Scales MATRICS Consensus Cognitive Battery (MCCB) Includes 10 tests that assess 7 cognitive domains: • • 60 to 90 minutes to complete • All scores are provided as T scores Speed of processing Attention/vigilance Working memory (verbal and nonverbal) • Scores are given for each cognitive domain as well as a composite Verbal learning score Visual learning Reasoning and problem solving • The normative mean is 50 with a Social cognition standard deviation of 10 Nuechterlein et al. Schizophr Res. 2004; 72(1): 29 39. www. matricsinc. org/MCCB. htm 61

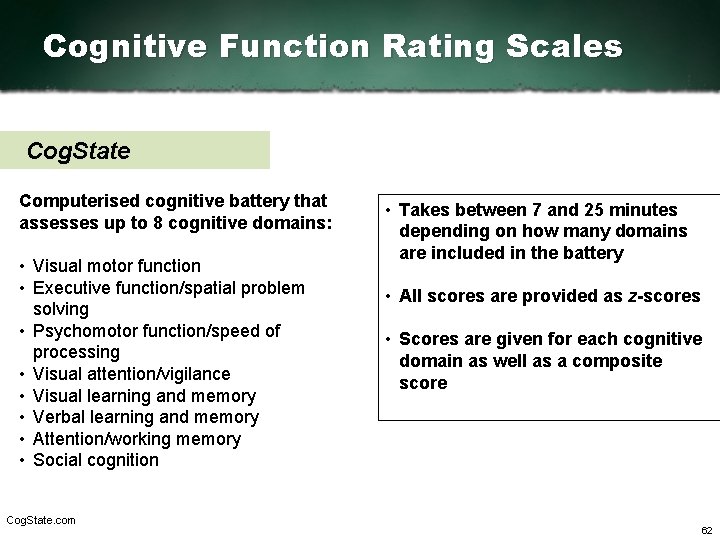
Cognitive Function Rating Scales Cog. State Computerised cognitive battery that assesses up to 8 cognitive domains: • Visual motor function • Executive function/spatial problem solving • Psychomotor function/speed of processing • Visual attention/vigilance • Visual learning and memory • Verbal learning and memory • Attention/working memory • Social cognition Cog. State. com • Takes between 7 and 25 minutes depending on how many domains are included in the battery • All scores are provided as z scores • Scores are given for each cognitive domain as well as a composite score 62

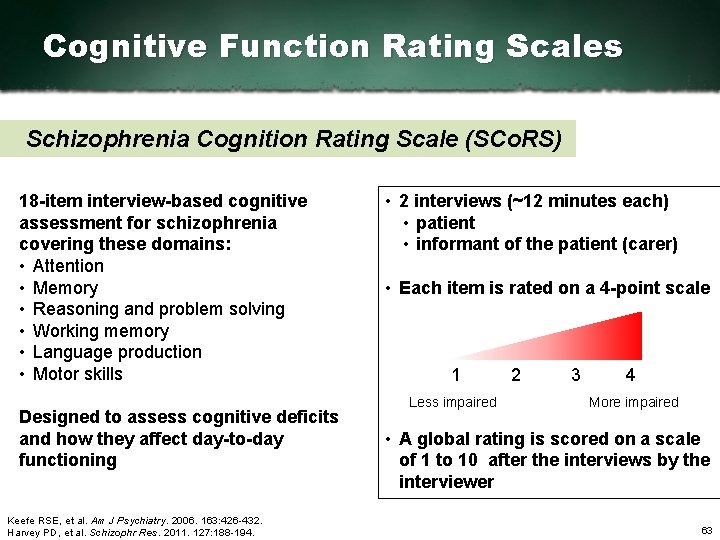
Cognitive Function Rating Scales Schizophrenia Cognition Rating Scale (SCo. RS) 18 item interview based cognitive assessment for schizophrenia covering these domains: • Attention • Memory • Reasoning and problem solving • Working memory • Language production • Motor skills Designed to assess cognitive deficits and how they affect day to day functioning Keefe RSE, et al. Am J Psychiatry. 2006. 163: 426 432. Harvey PD, et al. Schizophr Res. 2011. 127: 188 194. • 2 interviews (~12 minutes each) • patient • informant of the patient (carer) • Each item is rated on a 4 point scale 1 Less impaired 2 3 4 More impaired • A global rating is scored on a scale of 1 to 10 after the interviews by the interviewer 63

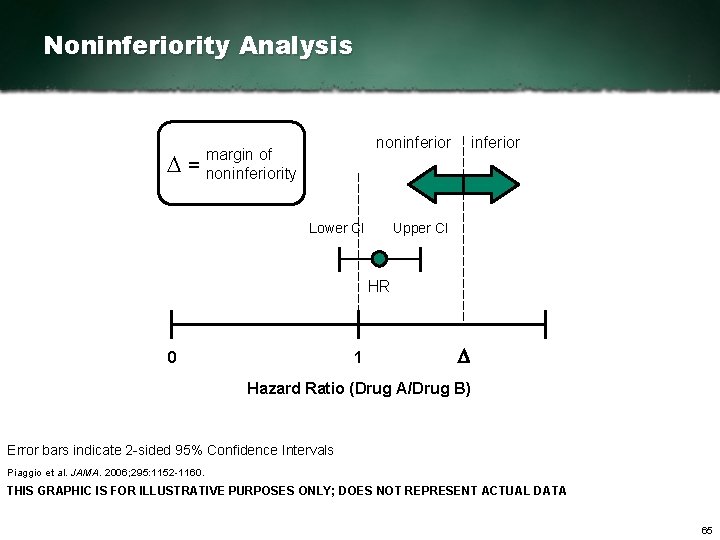
Noninferiority Study A noninferiority study is designed to show that an experimental treatment is not significantly worse than a reference (comparator) treatment. A noninferiority study is not designed to demonstrate superiority. Piaggio et al. JAMA. 2006; 295: 1152 1160. 64

Noninferiority Analysis D= noninferior margin of noninferiority Lower CI inferior Upper CI HR 0 1 D Hazard Ratio (Drug A/Drug B) Error bars indicate 2 sided 95% Confidence Intervals Piaggio et al. JAMA. 2006; 295: 1152 1160. THIS GRAPHIC IS FOR ILLUSTRATIVE PURPOSES ONLY; DOES NOT REPRESENT ACTUAL DATA 65

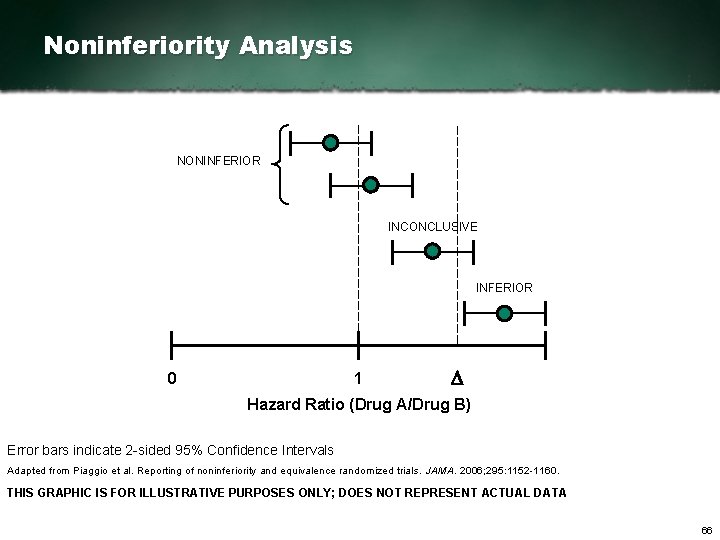
Noninferiority Analysis NONINFERIOR INCONCLUSIVE INFERIOR 0 1 D Hazard Ratio (Drug A/Drug B) Error bars indicate 2 sided 95% Confidence Intervals Adapted from Piaggio et al. Reporting of noninferiority and equivalence randomized trials. JAMA. 2006; 295: 1152 1160. THIS GRAPHIC IS FOR ILLUSTRATIVE PURPOSES ONLY; DOES NOT REPRESENT ACTUAL DATA 66

Summary 3 § Active treatment control arms added to a placebo controlled study can assess for assay sensitivity § Fixed dose trial designs allow for assessment of optimal dosing and time to treatment effect, but do not reflect clinical practice and are associated with higher discontinuation rates § Flexible dose trial designs more accurately reflect clinical practice, but titration prevents the determination of optimal dosing § Trial duration and total number of treatment arms have been associated with magnitude of placebo response in antipsychotic trials 67

Latuda® (lurasidone) Prescribing Information s. P yl ema ps te o m i cu mmma al ri yg no af n Pt r os dy un cd t r o r es f e or f t on e t uh re o fl ue l pl t S C m h ae r. a c. C t oe nr is si t di e c sr d t ienf uo ar et i op nr e is f c rs i i bg i nn sg , o p f at ri dc iu vl e e a ar d. v e. Mr a y ( Si s. P c. Co) n b a r d l yy si kn i nr e ls ai ta i oa np pt o s e e e r eu cn a du et ir ol ny si n agn dp ac or kn ti rn as i on d n ii cs amt i os ny sm. p. P tr oe m s n. t Ra it si ok n os f: r ex aa c ct e i or nb sa, t p s e e x t ur ad p s d y mt pa tb ol em t ss. , C s L a a y f r i al m i cd oa al t e c oa nu tt ai o i nn i nagn dl u rc al is ni di co an le m h yo dn ri ot oc rh il no gr i di e re eq cu oi m a m t i ge , n t 3 s 7. w 2 i m t hg aa n h d i s 7 t 4 o. r 5 y m og f l su er i az su i rd eo s n eo. r v am l ee nn dt e td o i 1 n 8 p. 6 c. I n o nd di ic t ai ot n p a o t ei sn t ii na dl l i yc ar te ed du c f eo r s et ih ze u rt er e t ah t r me es h d f, i os n w: h. L i ac th u d n ot l o cs ac r hd ii oz vo aps hc ur le a nr i da i s io nr d ea r ds u, l ot r st h o( s≥ t 1 a 8 t i c y he yap ro st e) n. s. Di oo n s, ad i ga be e t ea s n odr r. A i ds m k i f na ic st ot r sa t f i oor n d. Fi oa rb eo t reasl aa nd dm i wn ei si gt rh at t g i n A. d. Mu al yt s e: l Re ve ac t oe m pmr oe l na dc te i dn i oa n: ls et av re tl i sn. g A dl ol s rei : s k 3 7 f m a cg t o n r sc e f od r a i vl ey n w o iu t sh t ah r mo emabl o. e Nmob oi nl ii st ima l ( Vd T o s. E e) st i ht ro au t li d d f f be ec ft io v r ee d ao ns ed r da un rg ie n: g 3 7 t r t eo a 1 t 4 m 8 emn g t o an nc de o n b i es ri ed qe un i tr ie fd i. e E p t ao ku el nd. b Ce a u d ra ei lv ye. n t. Da ot si ve e i m n ce ra es au sr ee s s h b ta i so en d i n o n p ap th i ye sn i t cs i awni t jh u da g feammei nl yt h r ys e or vf e Q r oi cl ao ln gr ea st pi oo n s, e h. y Mp ao xk i aml ua m e md i oas ea: n 1 d 4 8 c m o ng c po emr i td aa ny t. a ni sd t o b d T c l pi n m h eh e Qn T t ri ne ta et ri vn ag l. w i. Ct l ho sh ei lgyh es ru pdeor sv ei s e. E le dd ei cr al yt i (o≥n 6 5 k n yo ewanr st )o : p. Cr ao ul ot in og n t w h s kn pa an t di e a n td so lf eo sr c r ei sn kt s o (f < s 1 u 8 i c yi ed e C i hg i hl dr ri e a. r s. A) v: o i. Nd o tg r ra ep ce of rmu mi t e nj ud i ec de , . P e sr es s ar e f eg tny a na cn yd ae nf fd i cl aa cc yt a nt oi ot n e: s. Dt oa bn l oi ts hu es de. d u. Dr oi ns ge pa r de j gu nsat nmc ey n ut sn l a p g h hs e pp oa tt ei cn t ai an ld r ri es nk a tl o i m t hpea if ro me et un st , . r eo qt eu ni rt ei ad l i bn e n m eo f di et r ca l t ee a ra l ny d o su et w v ee ri e B f e Ce d if nog r s hf ou ur l td h bee r c od nes ti da ei r l e sd. o. Cn loy n i ft rt ha e i pn o dt ei n ct i aa lt ib eo n ne fs i t: s re e ea s t. S P o t r ee ra st e m ne sn ti t j i uv si t t i yf i et so t th he e p o c it ei on nt ss. : H fy p a tc et ni tv i ea l s r ui sb ks tt ao n t ch ee co hr i l ad n. y. I n et ex rc ai p C a n i ti sa n at d a v id sme i dn iws ht er an t ic oo nm bo i f n isnt gr o wn i gt h C a. Y l. Pc 3 o Ah 4 o l i o e o un tc i oo m n rh i Cb N i t So ras c tai nv d m a tr is o. n. Ws , a ra nn id n m e d a i cni d n e P s r ken co aw un t it oo nc sa : u C s el i n. Q i Tc ap l r oi lmo pn rg oa vt ei omne; n Pt i nedd ui c e g s mgapy a t na dk e. B Ca R f. Pe wi n h s ia dt oi en n e t, d ia by i st o t r os smoa my e i nwcer ee ka ss ; e ce l xo ps oe sl uy r em ot on i lt uo rr a p ld uu rr ai ns gi d toh ni es pi se r ai on d. i n Uh si e b i w t oi tr h o cf a P S t. Ps C w fi ot hr u t igopn ai n d e l. Bd Ce R r l. Py , p sa et ei e n d e m t ae i nl ts i. a Dw oh so e h aa dv je u rs i t smk e fnat c ti os r sr e f co or ms mt re onk de e. d N io nt sc to umd bi ei nd a i t ni o enl d we ir tl hy C p a t i e. Y n t s Pw i t h 3 d e m. A e n t i 4 a. D i s c oi n t i n u e hi f p ai t i e nb t d ei v e l to p s o s i g nr s o rs

Latuda® (lurasidone) Prescribing Information and inducers, see SPC for details. Monitoring recommended when lurasidone and CYP 3 A 4 substrates known to have a narrow therapeutic index are coadministered. Undesirable effects: In clinical trials, the following adverse drug reactions were reported: very common (≥ 10%): akathisia, somnolence; common (≥ 1% to <10%): weight increased, insomnia, agitation, anxiety, restlessness, parkinsonism, dizziness, dystonia, dyskinesia, nausea, vomiting, dyspepsia, salivary hypersecretion, dry mouth, upper abdominal pain, stomach discomfort, musculoskeletal stiffness, blood creatinine phosphokinase increase, serum creatinine increase, fatigue; uncommon (≥ 0. 1% to <1%): decreased appetite, blood glucose increased, catatonia, tardive dyskinesia, tachycardia, hypertension, hypotension, alanine aminotransferase increase, blood prolactin increased; rare (≥ 0. 01% to <0. 1%): eosinophilia, rhabdomyolysis, neuroleptic malignant syndrome (NMS). This is not a complete list of adverse reactions. Prescribers should consult the SPC in relation to all adverse reactions. Special precautions for storage: Store in the original package in order to protect from light. Special precautions for disposal and other handling: Any unused medicinal product or waste material should be disposed of in accordance with local requirements. Legal classification: Prescription Only Medicine (POM). Package Quantities and Basic NHS Costs: Latuda 18. 5 mg, 37 mg and 74 mg £ 90. 72 per pack of 28 tablets. Marketing Authorisation Holder: Sunovion Pharmaceuticals Europe Ltd, Southside, 97 – 105 Victoria Street, London, SW 1 E 6 QT. Latuda is a registered trade mark. Marketing Authorisation Number(s): EU/1/14/913/001 021. Date of Preparation: February 2016 (MI LAT 000781). This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions Adverse reactions should be reported. Reporting forms and information can be found at www. mhra. gov. uk/yellowcard Adverse reactions should also be reported to Sunovion Pharmaceuticals Europe Ltd. on 020 7821 2899 Date of preparation: September 2014. UK/LUR/14/0188 a
 Lithium mechanism of action
Lithium mechanism of action Mesolimbic
Mesolimbic Second generation vs first generation antipsychotics
Second generation vs first generation antipsychotics Trazodone and qtc prolongation
Trazodone and qtc prolongation Antipsychotics brain damage
Antipsychotics brain damage Atypical pneumonia
Atypical pneumonia Ecg in hypokalemia
Ecg in hypokalemia Odg guidelines by state
Odg guidelines by state Sbp paracentesis
Sbp paracentesis Management of allergic rhinitis
Management of allergic rhinitis Management of allergic rhinitis
Management of allergic rhinitis Pressure management
Pressure management Rifaximin in liver failure
Rifaximin in liver failure Atypical bacteria
Atypical bacteria Melanoma texture
Melanoma texture Typical vs atypical chest pain
Typical vs atypical chest pain Etiologic agent
Etiologic agent Atypical bacteria
Atypical bacteria Atypical provider examples
Atypical provider examples Atypical psychology definition
Atypical psychology definition Atypical film
Atypical film Atypical worker meaning
Atypical worker meaning Louis angle
Louis angle Types of lung abscess
Types of lung abscess Classification of pneumonia
Classification of pneumonia Atypical odontalgia
Atypical odontalgia Atypical behavior
Atypical behavior Febrile convulsion
Febrile convulsion An atypical accumulation of fluid in the interstitial space
An atypical accumulation of fluid in the interstitial space An atypical accumulation of fluid in the interstitial space
An atypical accumulation of fluid in the interstitial space Atypical cartilaginous tumor
Atypical cartilaginous tumor Bedside clinical guidelines partnership
Bedside clinical guidelines partnership Gerd clinical practice guidelines
Gerd clinical practice guidelines Clinical guidelines
Clinical guidelines Clinical guidelines
Clinical guidelines Easl clinical practice guidelines
Easl clinical practice guidelines Social action theories
Social action theories Professional nursing practice concepts and perspectives
Professional nursing practice concepts and perspectives Historical and contemporary perspectives ppt
Historical and contemporary perspectives ppt What are the 4 principles of child development
What are the 4 principles of child development Tok essay 2022
Tok essay 2022 What is perspective of anthropology
What is perspective of anthropology Sociological perspectives in health and social care unit 10
Sociological perspectives in health and social care unit 10 Perspective and methodology of economics
Perspective and methodology of economics The social and ethical perspectives of entrepreneurship
The social and ethical perspectives of entrepreneurship Data integration problems approaches and perspectives
Data integration problems approaches and perspectives Personological and life story perspectives
Personological and life story perspectives New evidence and perspectives on mergers
New evidence and perspectives on mergers Family matters definition
Family matters definition Language paper 2 writers' viewpoints and perspectives
Language paper 2 writers' viewpoints and perspectives Political astuteness in nursing
Political astuteness in nursing Paper 2 writers’ viewpoints and perspectives
Paper 2 writers’ viewpoints and perspectives Perspectives on appeasement interactive notebook
Perspectives on appeasement interactive notebook Strategy as a perspective
Strategy as a perspective Sociological.imagination
Sociological.imagination Three major sociological perspectives
Three major sociological perspectives Theoretical perspectives on the family
Theoretical perspectives on the family Four theoretical perspectives
Four theoretical perspectives Six personal perspectives
Six personal perspectives 7 contemporary psychological perspectives
7 contemporary psychological perspectives Hand mnemonic for the 7 perspectives of psychology
Hand mnemonic for the 7 perspectives of psychology Poems with two perspectives
Poems with two perspectives Four perspectives of curriculum
Four perspectives of curriculum Three major theoretical perspectives in sociology
Three major theoretical perspectives in sociology 7 perspectives in psychology
7 perspectives in psychology Perspectives in health information management
Perspectives in health information management How to answer global perspective questions
How to answer global perspective questions What causes pica
What causes pica Psychology perspectives
Psychology perspectives 5 psychological perspectives
5 psychological perspectives