Clinical Practice Guidelines for Gastroesophageal reflux disease Sharon












- Slides: 13

Clinical Practice Guidelines for Gastroesophageal reflux disease Sharon Ronoh Chamberlain College of Nursing NR 511 April , 2018

Introduction Disease and background Publication and applicability in primary care Key action statement and body of evidence Application in clinical rotation (Katz, Gerson, Vela, 2013).

GERD and Background Gastroesophageal reflux(GERD) disease brief statement Incidence and prevalence 10%-20% Pathophysiology Typical clinical presentation (Katz, Gerson, Vela, 2013).

Publication and applicability The author and organization that developed the CPG The year of original guideline Applicability and use of CPG in primary care setting (Katz, Gerson, Vela, 2013).

Key Action statement and body of evidence Establishing the diagnosis of Gastroesophageal Reflux Disease (GERD) a) Presentation with typical symptoms of heartburn and regurgitation (Strong recommendation, moderate level of evidence) b) Rule out cardiac issues (Conditional recommendation, moderate level of evidence. c) Barium sulfate (Strong recommendation, high level of evidence) (Katz, Gerson, Vela, 2013). d) Upper endoscopy (Strong recommendation, moderate level of evidence) (Katz, Gerson, Vela, 2013).

Key Action statement and body of evidence TREATMENT AND MANAGEMENT 1. Lifestyle changes (Conditional recommendation, moderate level of evidence) 2. Pharmacological management 3. Surgical option (Katz, Gerson, Vela, 2013).

Key Action statement and body of evidence TREATMENT AND MANAGEMENT 1. Lifestyle changes Wight loss (Conditional recommendation, moderate level of evidence) b) Diet management: (Conditional recommendation, low level of evidence) c) position and activity : (Conditional recommendation, low level of evidence) (Katz, Gerson, Vela, 2013). a)

Key Action statement and body of evidence TREATMENT AND MANAGEMENT 2. Pharmacological management a) 8 weeks course of proton pump inhibitor (Strong recommendation, high level of evidence), b) First treatment of PPI once a day (Strong recommendation, moderate level of evidence). c) PPI should be administered 30 -60 minutes before meals (Strong recommendation, moderate level of evidence) d) Administer lowest dose possible of PPI (Conditional recommendation, low level of evidence) e) PPI is Safe if used during pregnancy (Conditional recommendation, moderate level of evidence)

Key Action statement and body of evidence TREATMENT AND MANAGEMENT H 2 -receptor antagonist (H 2 RA) (Conditional recommendation, moderate level of evidence). (Katz, Gerson, Vela, 2013). b) H 2 -receptor effective in non-erosive GERD (Conditional recommendation, moderate level of evidence). c) Use of prokinetic and baclofen in addition to PPI(Conditional recommendation, moderate level of evidence d) Refer patient not responding to treatment (Conditional recommendation, low level of evidence(Katz, Gerson, Vela, 2013). a)

Key Action statement and body of evidence TREATMENT AND MANAGEMENT 3. Surgical option a) Treatment option for long term therapy (Strong recommendation, high level of evidence) b) Not a first line treatment in patients not responding to PPI(Strong recommendation, moderate level of evidence) (Katz, Gerson, Vela, 2013).

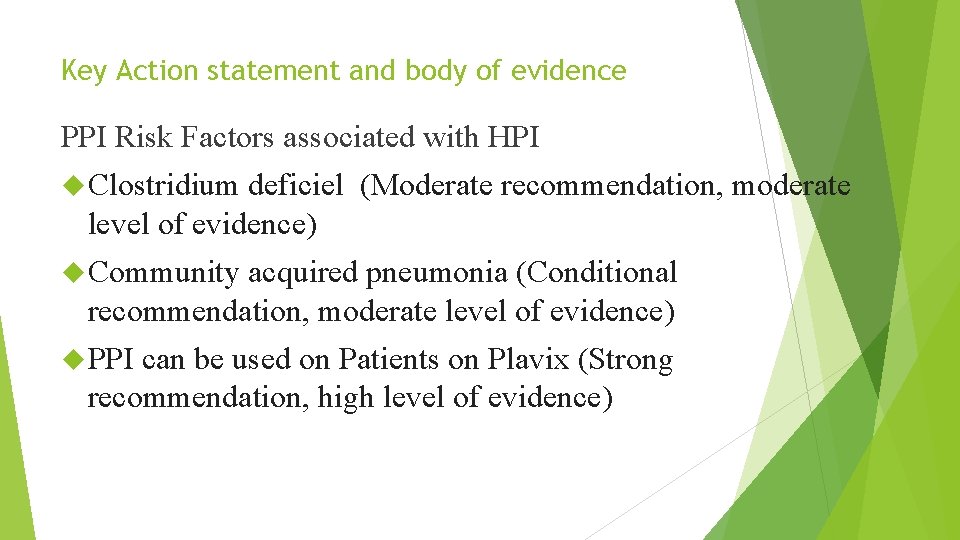
Key Action statement and body of evidence PPI Risk Factors associated with HPI Clostridium deficiel (Moderate recommendation, moderate level of evidence) Community acquired pneumonia (Conditional recommendation, moderate level of evidence) PPI can be used on Patients on Plavix (Strong recommendation, high level of evidence)

Application on clinical rotation Diagnosis : a) heartburn for the last three months b) Heartburn worsens with laying down c) Heart burn relieved with Tums Treatment a) Proton pump inhibitor (Katz, Gerson, Vela, 2013).

Reference Katz, P. O. , Gerson, L. B. , & Vela, M. F. (2013). Guidelines for the diagnosis and management of gastroesophageal reflux disease. The American Journal Of Gastroenterology, 108(3), 308328. doi: 10. 1038/ajg. 2012. 444
 Gerd clinical practice guidelines
Gerd clinical practice guidelines Easl clinical practice guidelines
Easl clinical practice guidelines Site:slidetodoc.com
Site:slidetodoc.com Fetomaternal hemorrhage
Fetomaternal hemorrhage Gastroesophageal sphincter
Gastroesophageal sphincter Exercise 38 anatomy of the digestive system
Exercise 38 anatomy of the digestive system Bedside clinical guidelines partnership
Bedside clinical guidelines partnership Clinical guidelines
Clinical guidelines Pyramid of evidence
Pyramid of evidence Bharathi viswanathan
Bharathi viswanathan Calculate minimum reflux ratio
Calculate minimum reflux ratio Minimum reflux ratio
Minimum reflux ratio Mylantah
Mylantah Condenser energy balance
Condenser energy balance

























