Chemistry of Life Chapter 2 Water l Water












- Slides: 25

Chemistry of Life Chapter 2

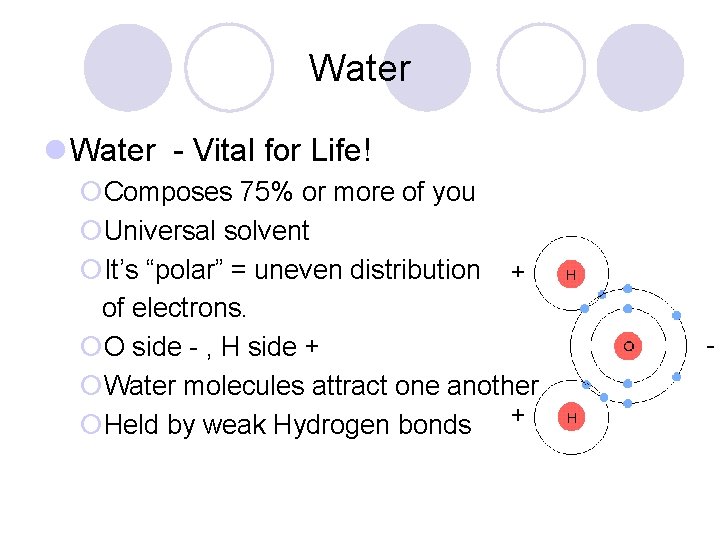
Water l Water - Vital for Life! ¡Composes 75% or more of you ¡Universal solvent ¡It’s “polar” = uneven distribution of electrons. ¡O side - , H side + ¡Water molecules attract one another ¡Held by weak Hydrogen bonds

Sticking Together…Surface Tension Video – Water Strider/Surface Tension Video – Jesus Lizard http: //www. thenakedscientists. com/HTML/ex periments/exp/bending-water-static-attraction/

Bonds ¡Hydrogen Bonds: weak bonds in a charged molecule ¡Cohesion: attraction between molecules of the same substance. ¡Adhesion: attraction between molecules of different substances.

Mixing It Up… l Solutions are composed of a… ¡Solute: substance being dissolved ¡Solvent: substance in which the solute is dissolved ¡Concentration: amount of solute dissolved per volume of solution

Some things don’t mix… l Suspensions - Mixtures of water and nondissolved material. EX: Blood has dissolved compounds, cells, and undissolved particles.

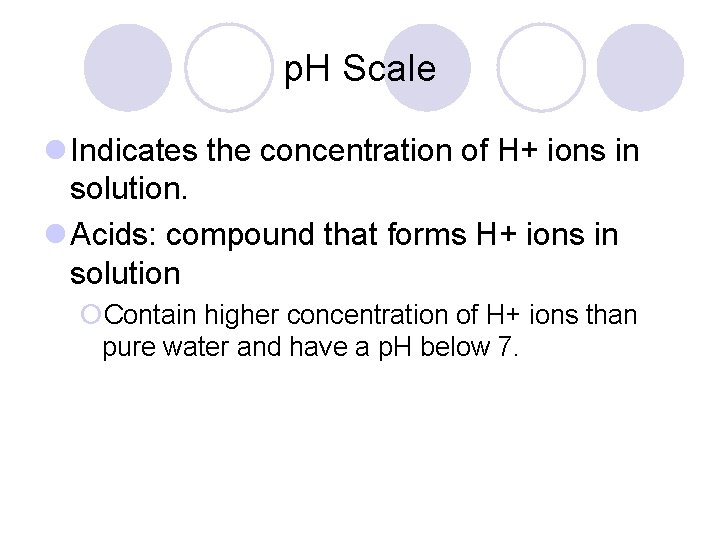
p. H Scale l Indicates the concentration of H+ ions in solution. l Acids: compound that forms H+ ions in solution ¡Contain higher concentration of H+ ions than pure water and have a p. H below 7.

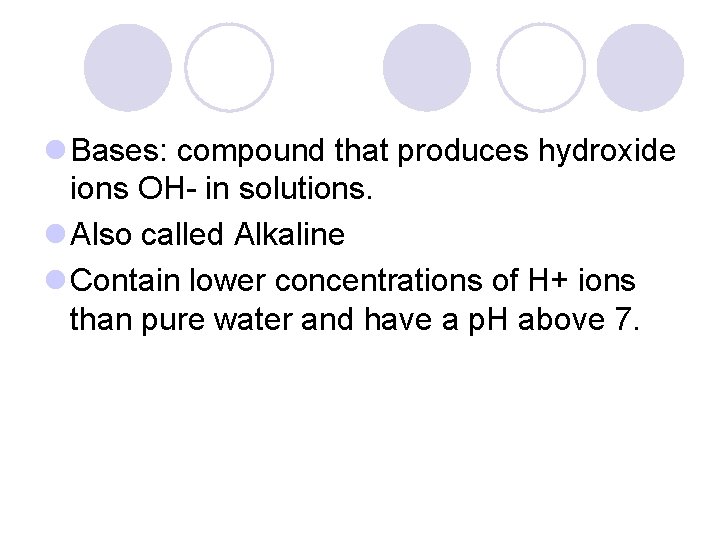
l Bases: compound that produces hydroxide ions OH- in solutions. l Also called Alkaline l Contain lower concentrations of H+ ions than pure water and have a p. H above 7.



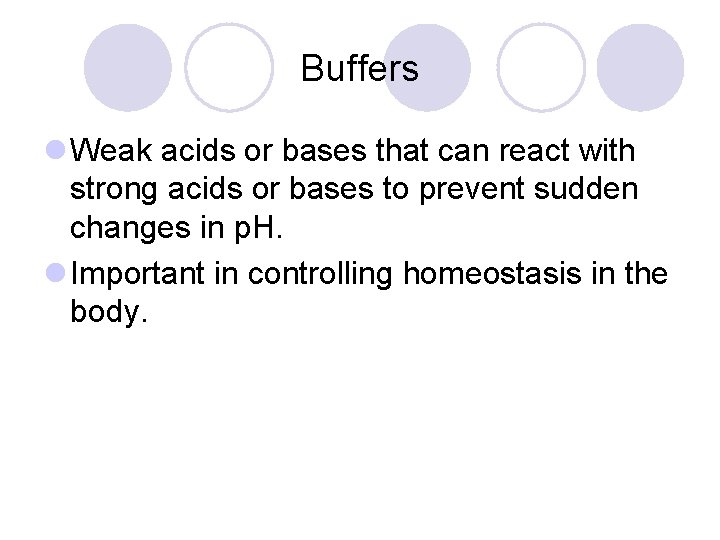
Buffers l Weak acids or bases that can react with strong acids or bases to prevent sudden changes in p. H. l Important in controlling homeostasis in the body.

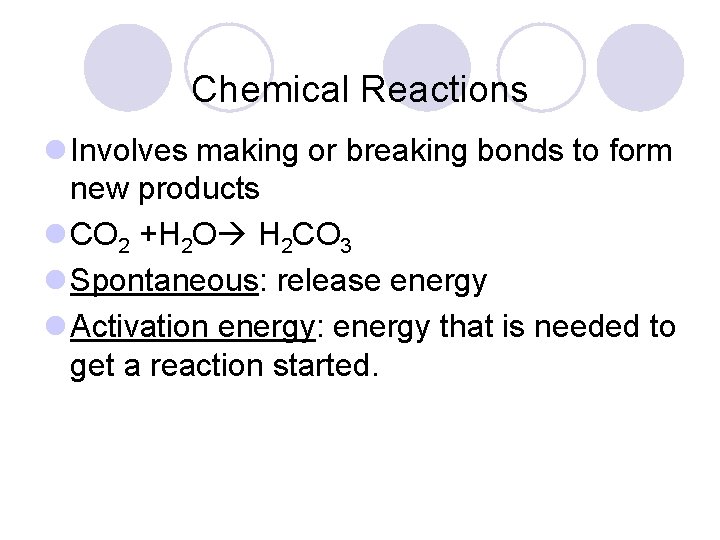
Chemical Reactions l Involves making or breaking bonds to form new products l CO 2 +H 2 O H 2 CO 3 l Spontaneous: release energy l Activation energy: energy that is needed to get a reaction started.

Carbon l All organic (living) things contain carbon l Can bond with many elements like hydrogen, oxygen, nitrogen, etc.

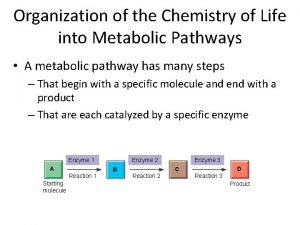
Macromolecules (aka Biomolecules or Organic Compounds) l Large molecules l Made of many, many smaller molecules l Polymerization: process that forms macromolecules l Monomers: smaller units in a polymer

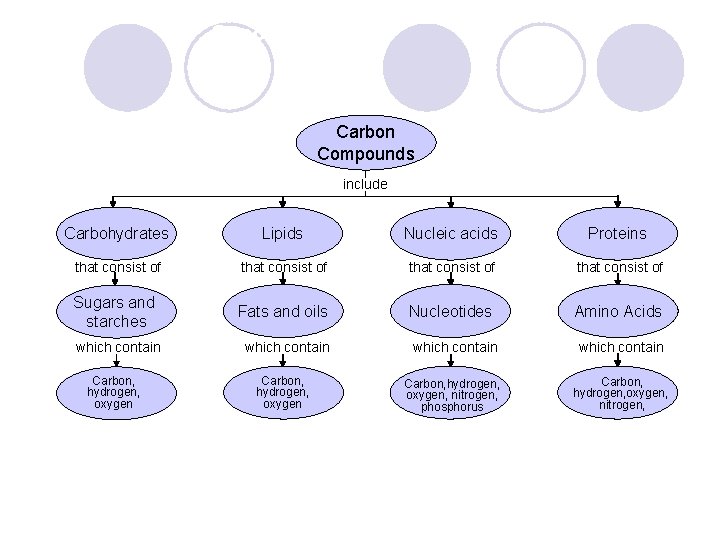
4 Groups of organic compounds found in living things: 1. Carbohydrates 2. Lipids 3. Nucleic acids 4. Proteins

Carbohydrates l Made up of carbon, hydrogen and oxygen atoms l Main source of energy and for structural purposes l Store extra sugar as starches in plants and glycogen in animals.

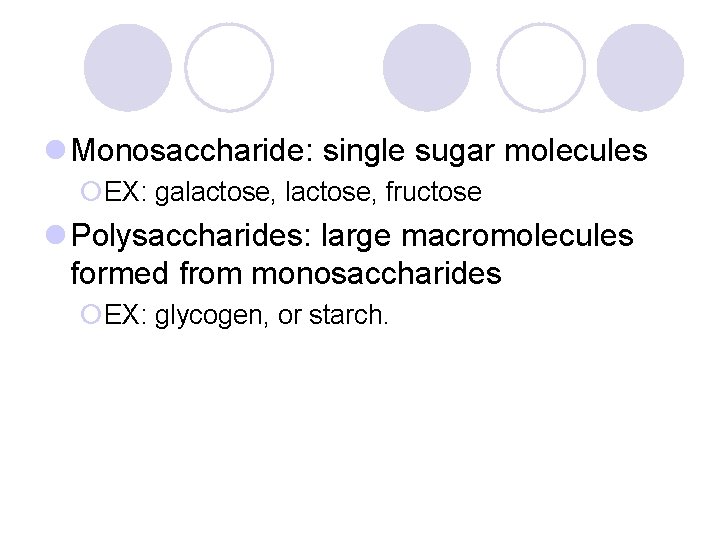
l Monosaccharide: single sugar molecules ¡EX: galactose, fructose l Polysaccharides: large macromolecules formed from monosaccharides ¡EX: glycogen, or starch.

Lipids l Fats, Oils, Waxes l Made from carbon and hydrogen l Important in cell membranes and waterproof coverings l Made of a Glycerol molecule and fatty acids

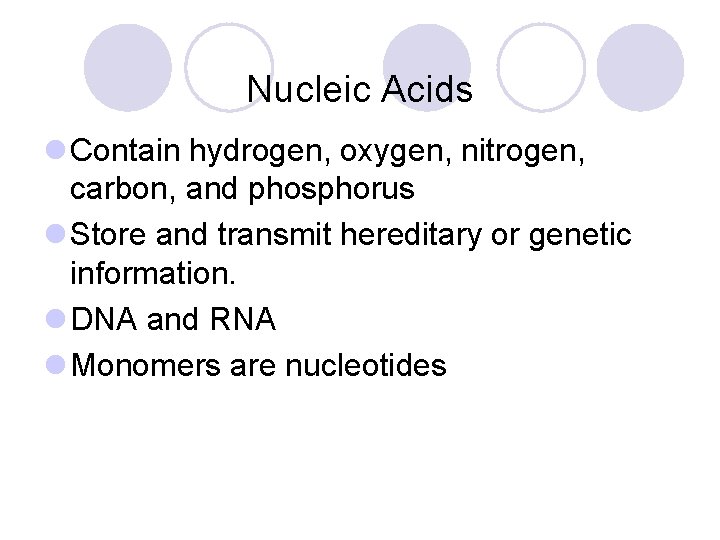
Nucleic Acids l Contain hydrogen, oxygen, nitrogen, carbon, and phosphorus l Store and transmit hereditary or genetic information. l DNA and RNA l Monomers are nucleotides

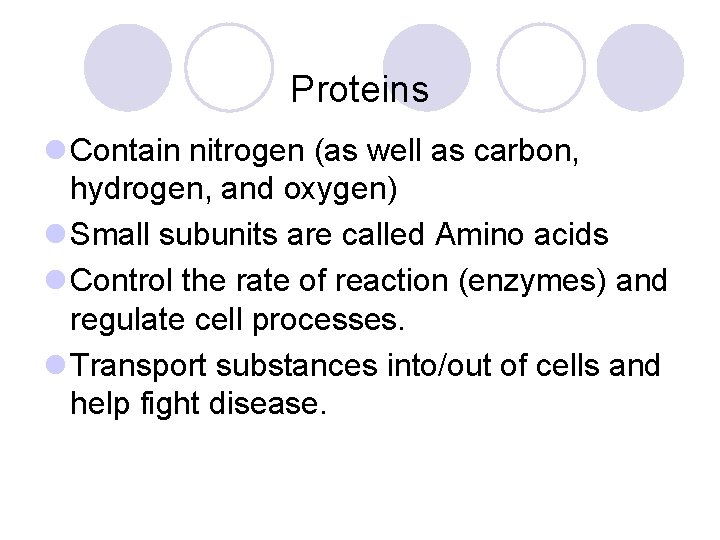
Proteins l Contain nitrogen (as well as carbon, hydrogen, and oxygen) l Small subunits are called Amino acids l Control the rate of reaction (enzymes) and regulate cell processes. l Transport substances into/out of cells and help fight disease.

Enzymes l Enzymes are classified as Proteins! l Speed up chemical reactions l They lower activation energy l They work on a specific substance l An enzyme’s name usually comes from the reaction it catalyzes https: //www. youtube. com/watch? v=my. ORDWVz. Nhc http: //highered. mheducation. com/sites/0072495855/student_view 0/chapter 2/animation__how_enzymes_work. html

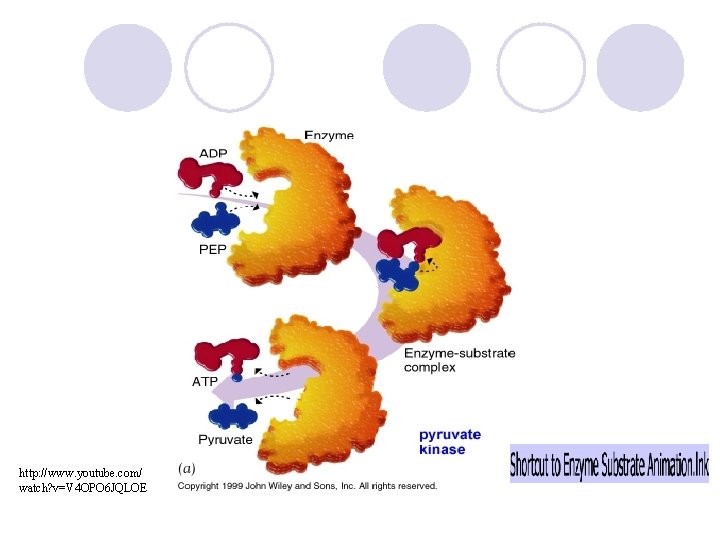
Enzyme Action l Enzyme-Substrate Complex: where reactants are brought together to react. l Substrates: the reactants of enzymecatalyzed reactions. l Active site: where substrate binds

How it works l Substrate fits into the active site on the enzyme forming the enzyme-substrate complex l They stay together till the reaction is over l Then, the enzyme is free to start the process again (its re-usable)

http: //www. youtube. com/ watch? v=V 4 OPO 6 JQLOE

Concept Map Carbon Compounds include Carbohydrates Lipids Nucleic acids Proteins that consist of Sugars and starches Fats and oils Nucleotides Amino Acids which contain Carbon, hydrogen, oxygen, nitrogen, phosphorus Carbon, hydrogen, oxygen, nitrogen,
 Water and water and water water
Water and water and water water Chapter 2 the chemistry of life section review 2-2
Chapter 2 the chemistry of life section review 2-2 Ib organic chemistry functional groups
Ib organic chemistry functional groups Organic vs inorganic chemistry
Organic vs inorganic chemistry Chapter 15 ocean water and ocean life
Chapter 15 ocean water and ocean life Properties of water summary
Properties of water summary Water covalent bond
Water covalent bond Class 8 english chapter 7 water water everywhere
Class 8 english chapter 7 water water everywhere You light up my life chemistry lab answer key
You light up my life chemistry lab answer key Chemical kinetics half life
Chemical kinetics half life Chemistry
Chemistry Concept 2 chemistry of life
Concept 2 chemistry of life Concept 2 chemistry of life
Concept 2 chemistry of life Chemistry of life summary
Chemistry of life summary How is thermal energy generated
How is thermal energy generated Chemistry for life
Chemistry for life Ocean water chemistry
Ocean water chemistry Chemistry in biology section 3 water and solutions
Chemistry in biology section 3 water and solutions Chapter 9 surface water chapter assessment answer key
Chapter 9 surface water chapter assessment answer key Approach chemistry chalk chapter
Approach chemistry chalk chapter Chemistry chapter 9 stoichiometry
Chemistry chapter 9 stoichiometry Organic chemistry (3rd) edition chapter 1 problem 16s
Organic chemistry (3rd) edition chapter 1 problem 16s Introduction to organic chemistry
Introduction to organic chemistry Modern chemistry chapter 9 stoichiometry
Modern chemistry chapter 9 stoichiometry Chapter 7 review modern chemistry answers
Chapter 7 review modern chemistry answers Modern chemistry chapter 15
Modern chemistry chapter 15