Chemistry 125 Lecture 8 Sept 17 2010 OneDimensional






- Slides: 30

Chemistry 125: Lecture 8 Sept 17, 2010 One-Dimensional Wave Functions The magnitude of the curvature of a wave function relates to the kinetic energy of the system, and the square of the wave function relates to probability density. The requirement that the wavefunction not diverge in areas of negative kinetic energy can constrain total energies to certain values, a property which is explored for the harmonic oscillator, the Morse potential, and the Columbic potential. Consideration of the influence of mass reveals an “isotope effect” on dynamics and on the energy, vibration frequency, and length of bonds. Introducing the double minimum potential leads to the study of bonding. For copyright notice see final page of this file

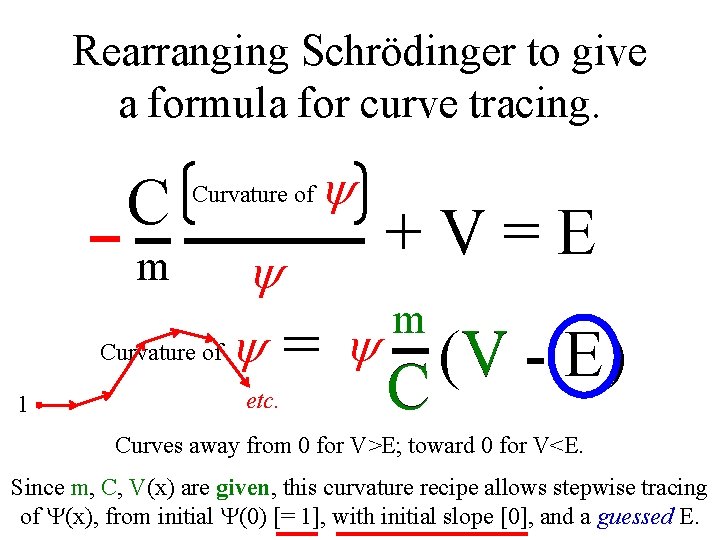
Rearranging Schrödinger to give a formula for curve tracing. C Curvature of y +V=E y m Curvature of y = y V (V m 1 etc. C - E) Curves away from 0 for V>E; toward 0 for V<E. Since m, C, V(x) are given, this curvature recipe allows stepwise tracing of (x), from initial (0) [= 1], with initial slope [0], and a guessed E.

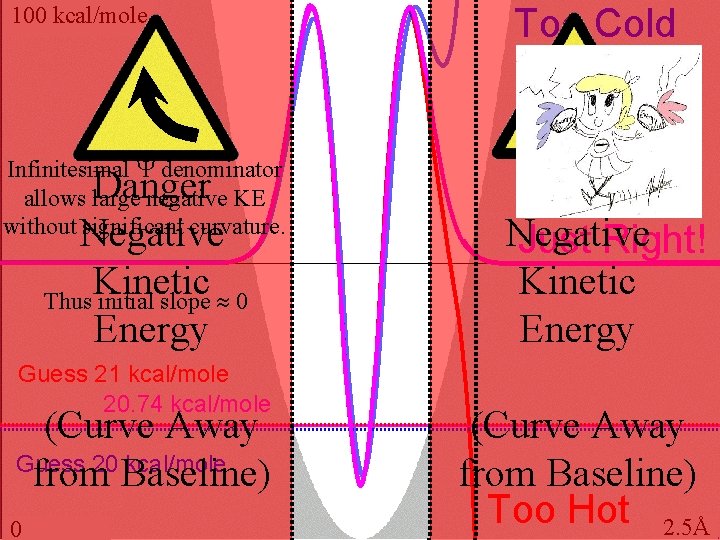
100 kcal/mole Infinitesimal denominator allows large negative KE without significant curvature. Danger Negative Kinetic Thus initial slope 0 Energy Guess 21 kcal/mole 20. 74 kcal/mole (Curve Away Guess 20 kcal/mole from Baseline) 0 Too Cold Danger Negative Just Right! Kinetic Energy (Curve Away from Baseline) Too Hot 2. 5Å

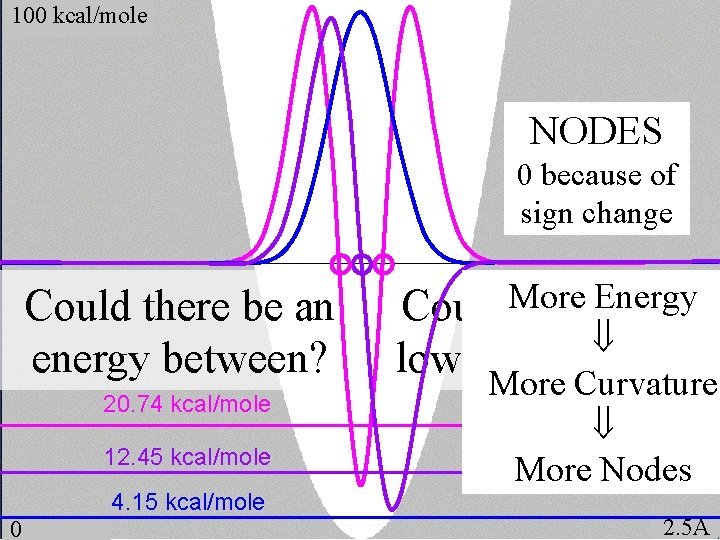
100 kcal/mole NODES 0 because of sign change Could there be an energy between? 20. 74 kcal/mole 12. 45 kcal/mole 4. 15 kcal/mole 0 Could. More there. Energy be a lower-energy ? More Curvature More Nodes 2. 5Å

Finding Solutions is Much Harder with Many Particles. Is it worth our effort?

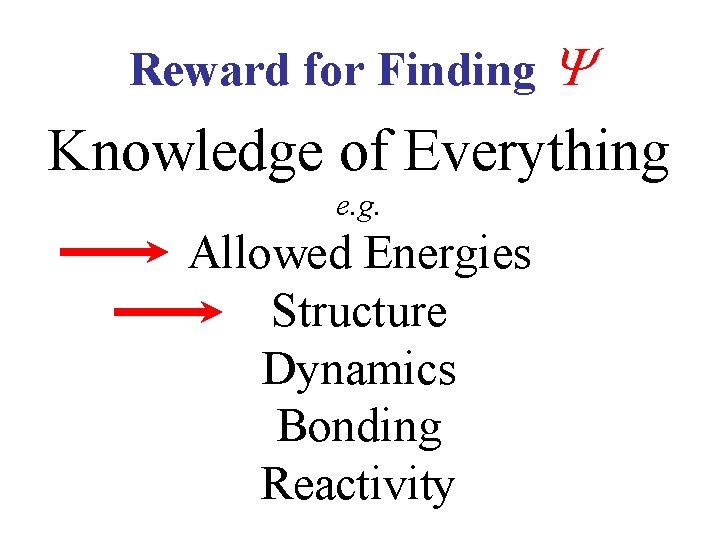
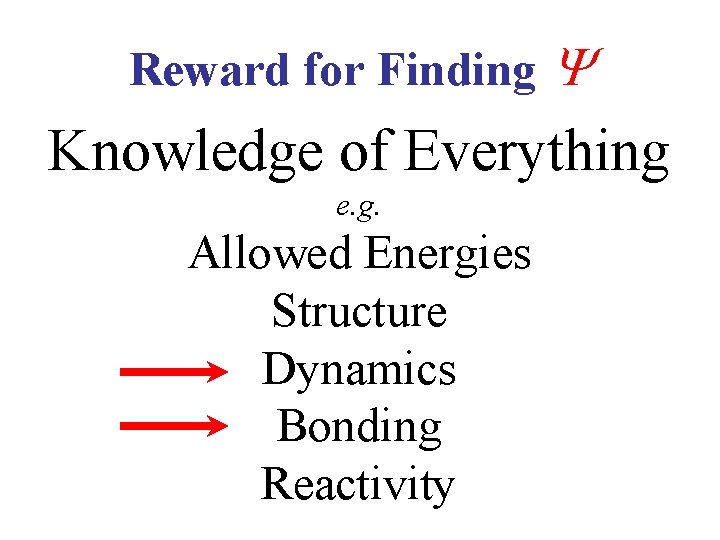
Reward for Finding Knowledge of Everything e. g. Allowed Energies Structure Dynamics Bonding Reactivity

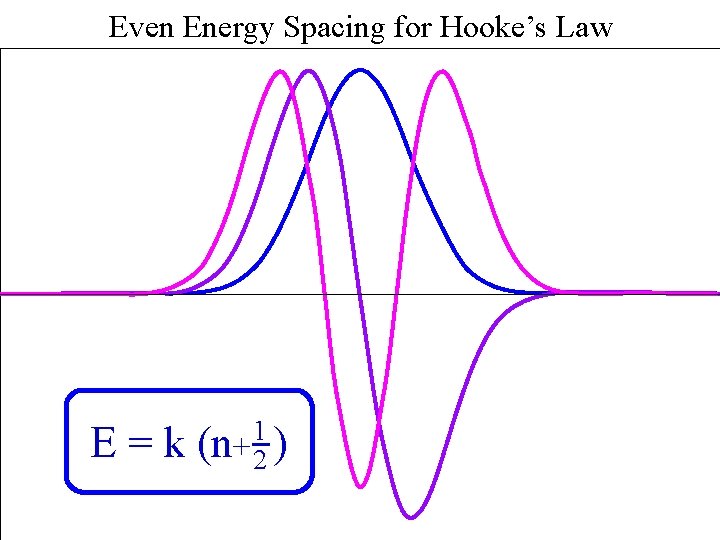
Even Energy Spacing for Hooke’s Law Harmonic Spacing E=k 1 (n+ 2 )

“We only wish that we could glean an inkling of what could mean. ”

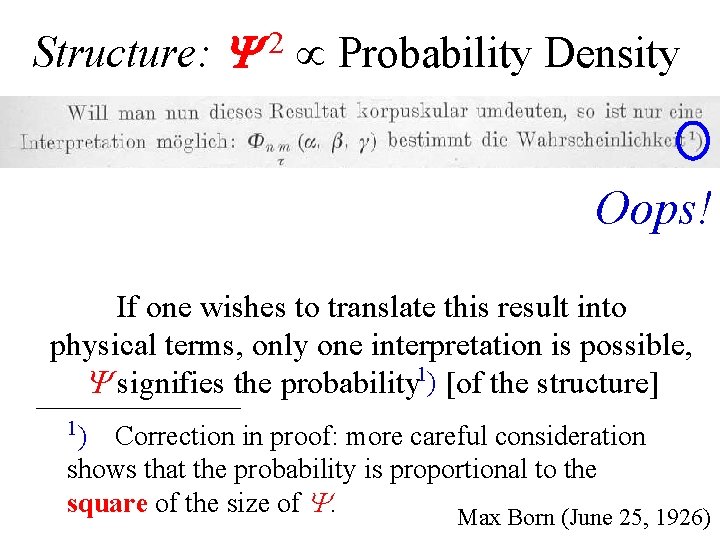
Structure: Y 2 Probability Density Oops! If one wishes to translate this result into physical terms, only one interpretation is possible, signifies the probability 1) [of the structure] 1) Correction in proof: more careful consideration shows that the probability is proportional to the square of the size of . Max Born (June 25, 1926)

Structure: Y 2 Probability Density Albert Einstein to Max Born December 4, 1926 Aber eine innere Stimme sagt mir, dass das doch nicht der wahre Jakob ist. Die Theorie liefert viel, aber dem Geheimnis des Alten bringt sie uns kaum näher. Jedenfalls bin ich überzeugt, dass der nicht würfelt. But an inner voice tells me, that this is not the real thing. The theory yields a great deal, but it brings us no nearer to the secret of the Old One. Anyway I am convinced that He does not play dice.

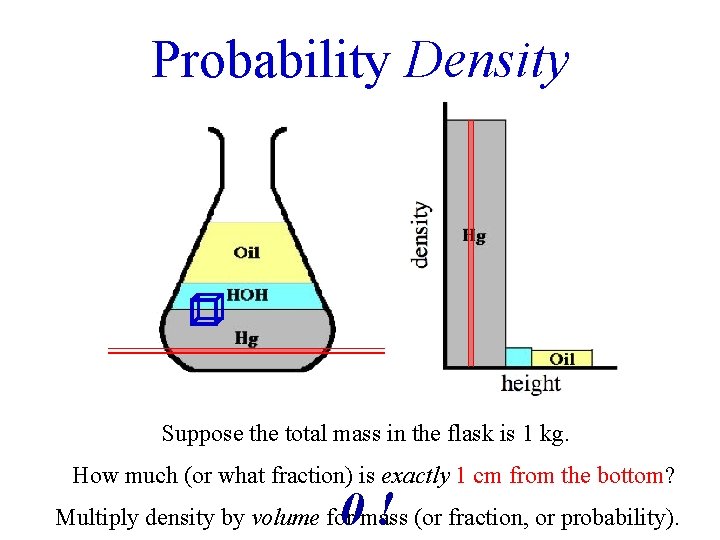
Probability Density Suppose the total mass in the flask is 1 kg. How much (or what fraction) is exactly 1 cm from the bottom? 0! Multiply density by volume for mass (or fraction, or probability).

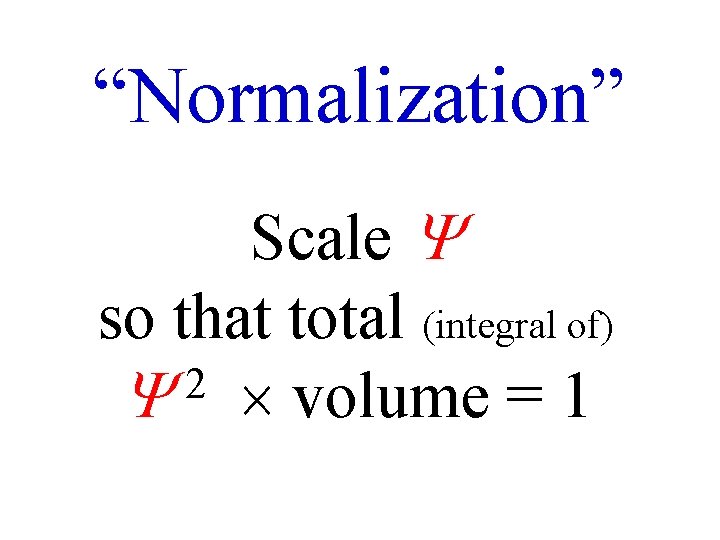
“Normalization” Scale so that total (integral of) 2 volume = 1

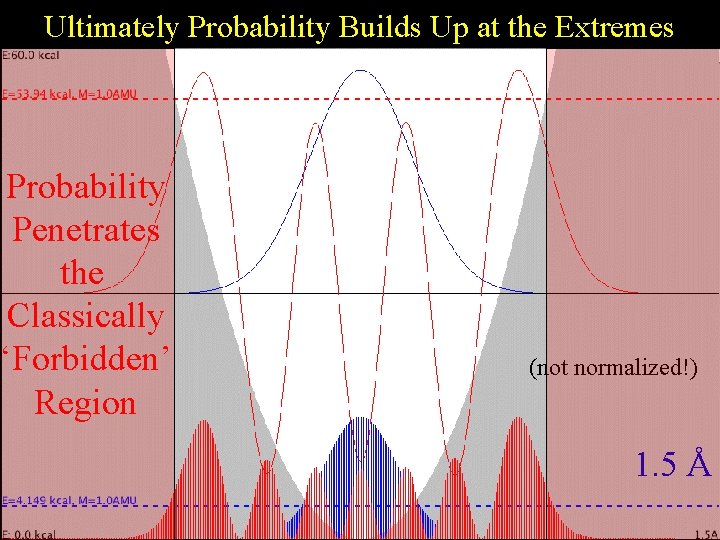
Ultimately Probability Builds Up at the Extremes Harmonic Probability Penetrates the Classically ‘Forbidden’ Region (not normalized!) 1. 5 Å

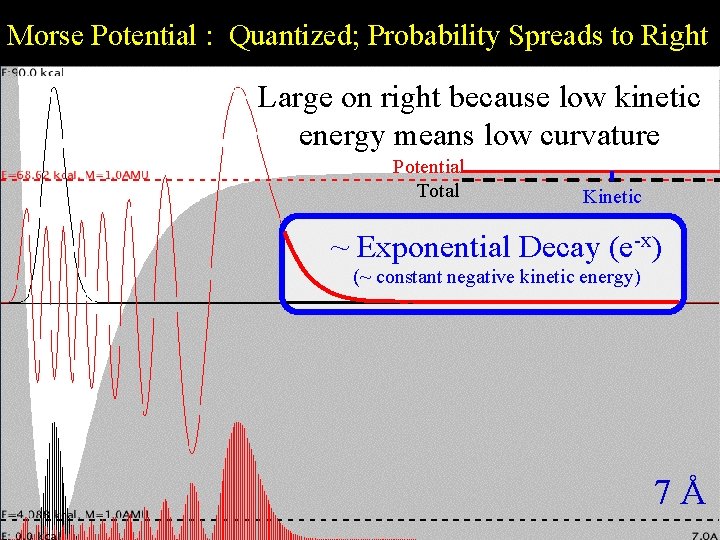
Morse Potential : Quantized; Probability Spreads to Right Large on right because low kinetic Morse Quantization energy means low curvature Potential Total Kinetic ~ Exponential Decay (e-x) (~ constant negative kinetic energy) 7Å

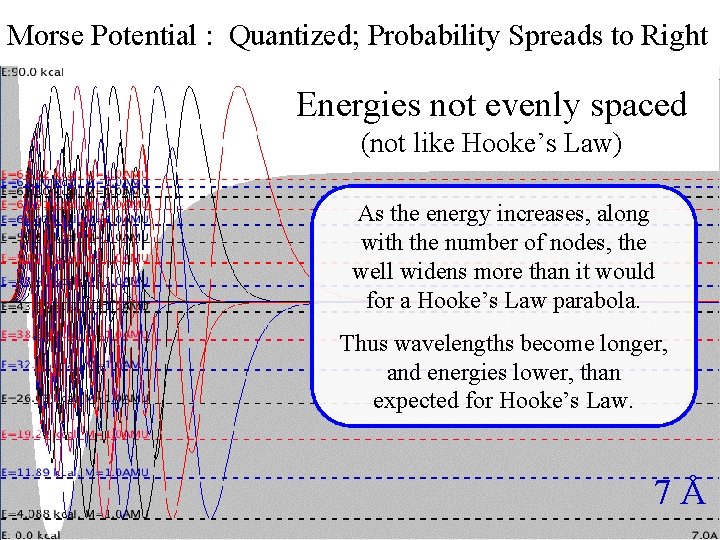
Morse Potential : Quantized; Probability Spreads to Right Morse. Energies Quantization not evenly spaced (not like Hooke’s Law) As the energy increases, along with the number of nodes, the well widens more than it would for a Hooke’s Law parabola. Thus wavelengths become longer, and energies lower, than expected for Hooke’s Law. 7Å

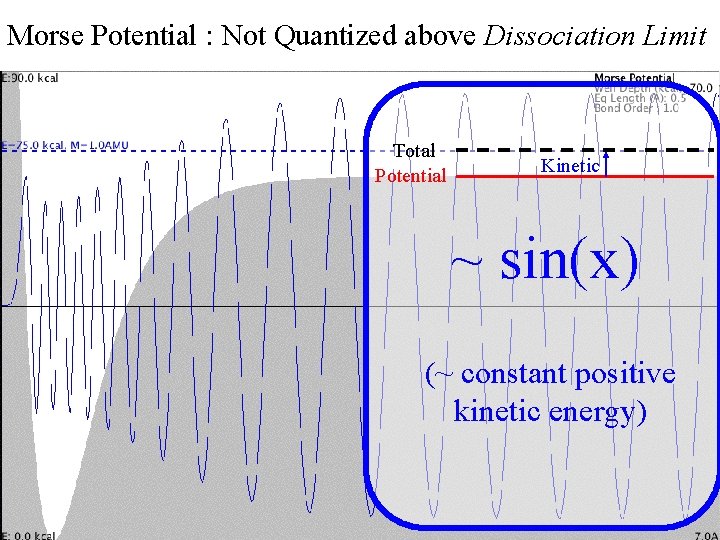
Morse Potential : Not Quantized above Dissociation Limit Morse Continuum Total Potential Kinetic ~ sin(x) (~ constant positive kinetic energy)

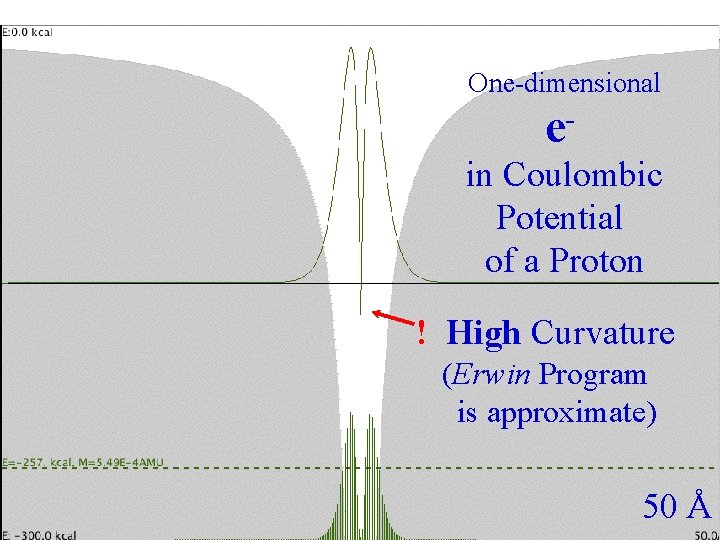
Coulombic One-dimensional Spacing e in Coulombic Potential of a Proton ! High Curvature (Erwin Program is approximate) 50 Å

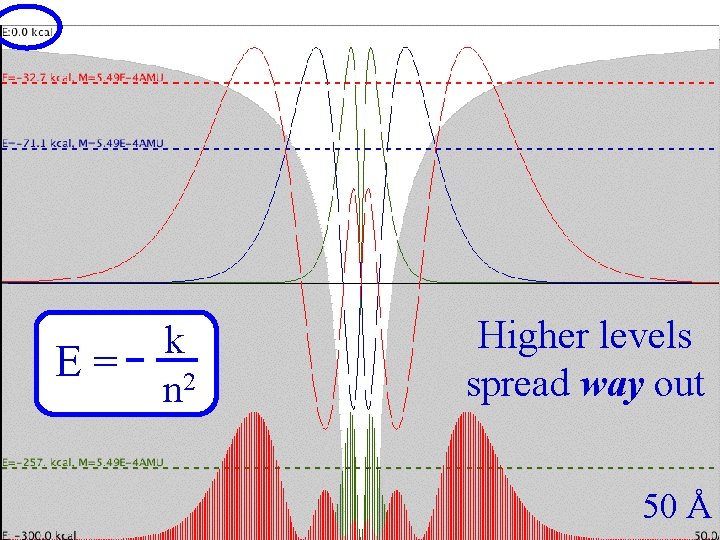
Coulomb Three E= k n 2 Higher levels spread way out 50 Å

Reward for Finding Knowledge of Everything e. g. Allowed Energies Structure Dynamics Bonding Reactivity

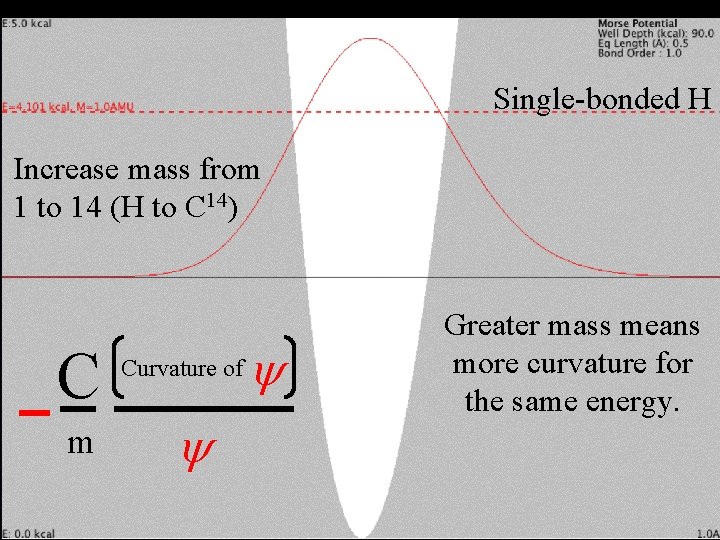
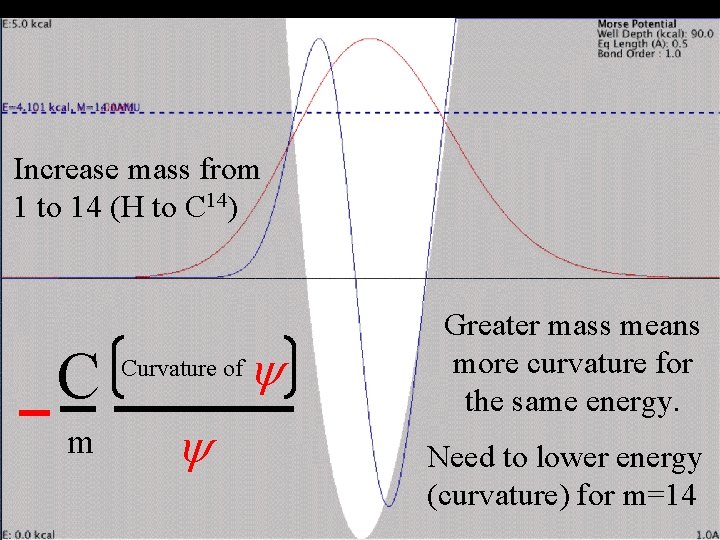
Change mass Single-bonded H Increase mass from 1 to 14 (H to C 14) C m Curvature of y y Greater mass means more curvature for the same energy.

Change mass Single-bonded H Increase mass from 1 to 14 (H to C 14) C m Curvature of y y Greater mass means more curvature for the same energy. Need to lower energy (curvature) for m=14

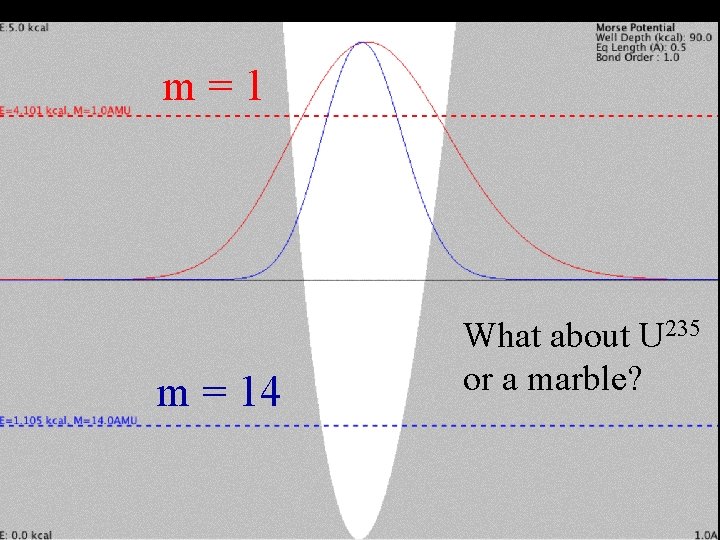
m = 1 Mass Effect m = 14 What about U 235 or a marble?

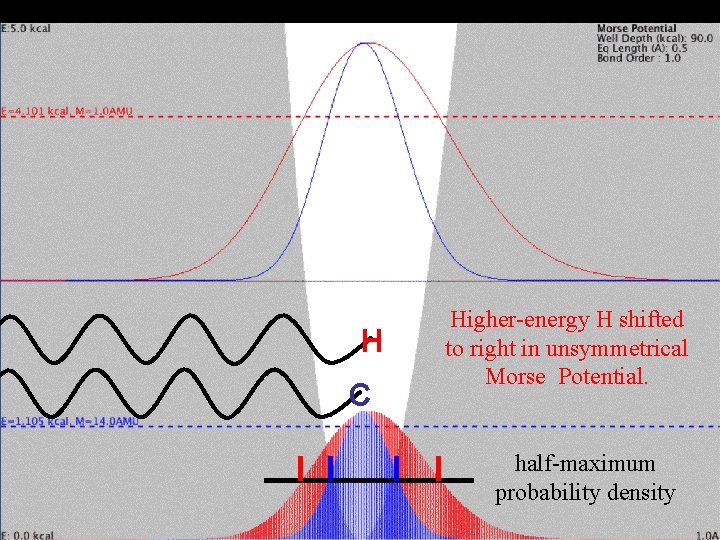
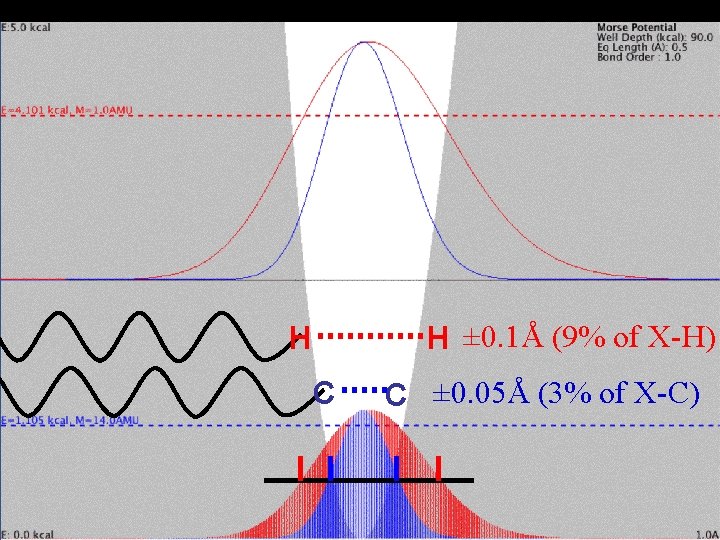
Mass Effect and Vibration H C Higher-energy H shifted to right in unsymmetrical Morse Potential. half-maximum probability density

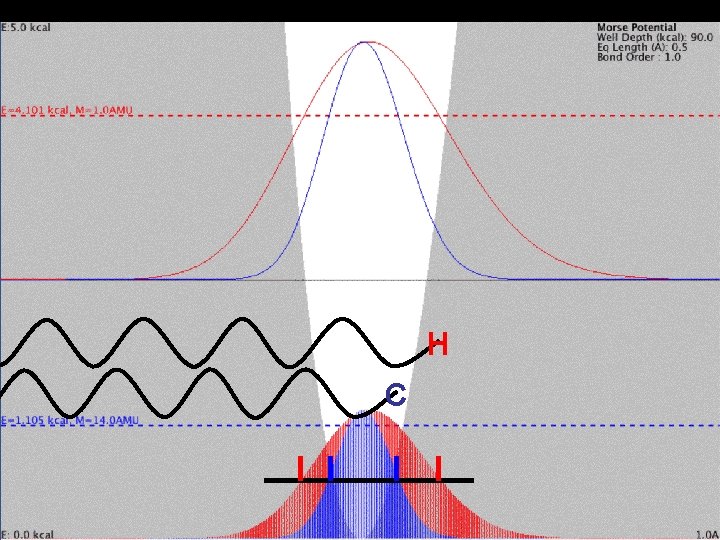
Mass Effect and Vibration H C

Mass Effect and Vibration H ± 0. 1Å (9% of X-H) H C C ± 0. 05Å (3% of X-C)

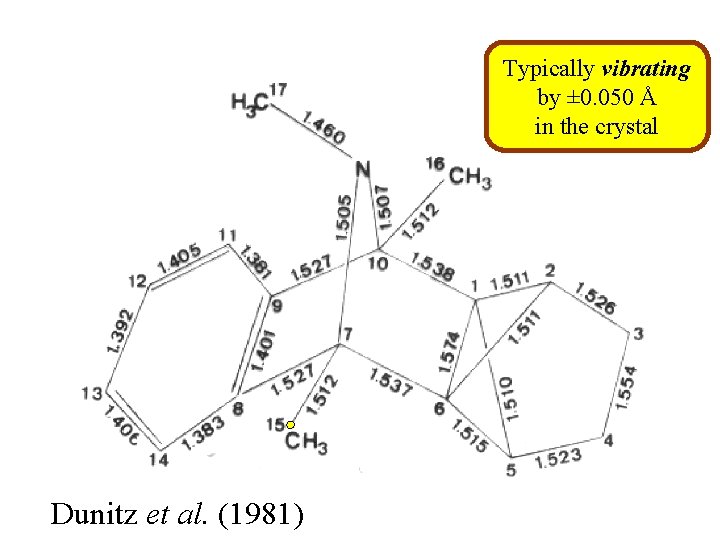
Typically vibrating by ± 0. 050 Å in the crystal Dunitz et al. (1981)

Reward for Finding Knowledge of Everything e. g. Allowed Energies Structure Dynamics Bonding Reactivity

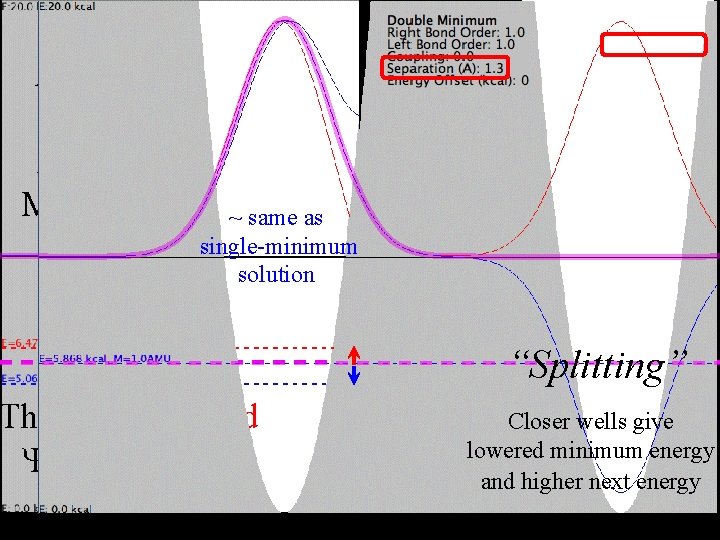
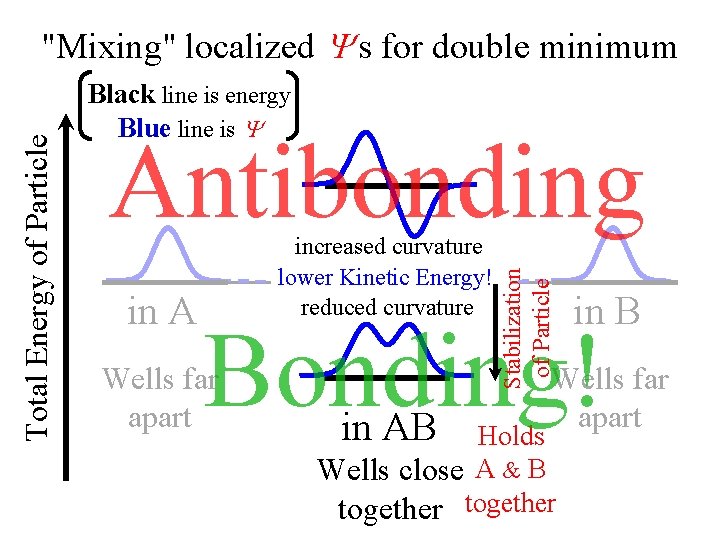
For Hooke's Law Actually Singlevs. Double Minimum the Blue Energy this is a is too Low Doubleand Minimum. ~ same as single-minimum the Red Energy solution is too High. The Blue and Red s are correct! Single-Mimimum “Splitting” The Correct Lowest What if wells give Closer Energy must lie thelowered wells were minimum energy between values. further apart? these and higher next energy

Black line is energy Blue line is Antibonding in A increased curvature lower Kinetic Energy! reduced curvature Stabilization of Particle Total Energy of Particle "Mixing" localized s for double minimum in B Bonding! Wells far apart in AB Wells far apart Holds Wells close A & B together

End of Lecture 8 Sept 17, 2010 Copyright © J. M. Mc. Bride 2009. Some rights reserved. Except for cited third-party materials, and those used by visiting speakers, all content is licensed under a Creative Commons License (Attribution-Non. Commercial-Share. Alike 3. 0). Use of this content constitutes your acceptance of the noted license and the terms and conditions of use. Materials from Wikimedia Commons are denoted by the symbol . Third party materials may be subject to additional intellectual property notices, information, or restrictions. The following attribution may be used when reusing material that is not identified as third-party content: J. M. Mc. Bride, Chem 125. License: Creative Commons BY-NC-SA 3. 0
 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad Damon poole
Damon poole Candide ou l optimisme
Candide ou l optimisme Sept comme setteur questionnaire
Sept comme setteur questionnaire Sept commandements
Sept commandements Sept prefix words
Sept prefix words Cities on hills
Cities on hills Cnn 10 2018
Cnn 10 2018 Zero un deux trois quatre
Zero un deux trois quatre Sept
Sept Poésie le blaireau sans gêne
Poésie le blaireau sans gêne Sept heure moins le quart
Sept heure moins le quart La guerre de sept ans
La guerre de sept ans I sept
I sept Ecrivez les sept jours de la semaine
Ecrivez les sept jours de la semaine Danswer
Danswer Advanced inorganic chemistry lecture notes
Advanced inorganic chemistry lecture notes An introduction to atmospheric physics
An introduction to atmospheric physics Inorganic vs organic chemistry
Inorganic vs organic chemistry Functional groups ib chemistry
Functional groups ib chemistry Ao 125
Ao 125 12-5 areas and volumes of similar solids
12-5 areas and volumes of similar solids Energi listrik menjadi energi cahaya
Energi listrik menjadi energi cahaya Ts designation
Ts designation Adenexal
Adenexal The body of a 1275 kg car is supported
The body of a 1275 kg car is supported 125 years ago today
125 years ago today Timedisc
Timedisc Square roots notes
Square roots notes Numeri divisibili per 62
Numeri divisibili per 62 Gov bom radar brisbane
Gov bom radar brisbane