Chapter 5 Functional Groups and Nomenclature I Alkanes

- Slides: 28

Chapter 5: Functional Groups and Nomenclature I Alkanes a subgroup of hydrocarbons: contain only C and H only have C-C single bonds Cn. H 2 n+2 DU = 0 no functional group Cycloalkanes have a ring low melting, low boiling, hydrophobic

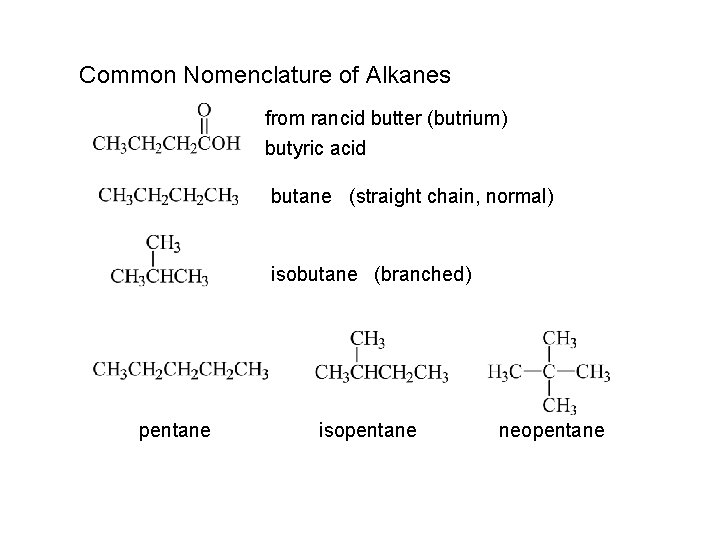
Common Nomenclature of Alkanes from rancid butter (butrium) butyric acid butane (straight chain, normal) isobutane (branched) pentane isopentane neopentane

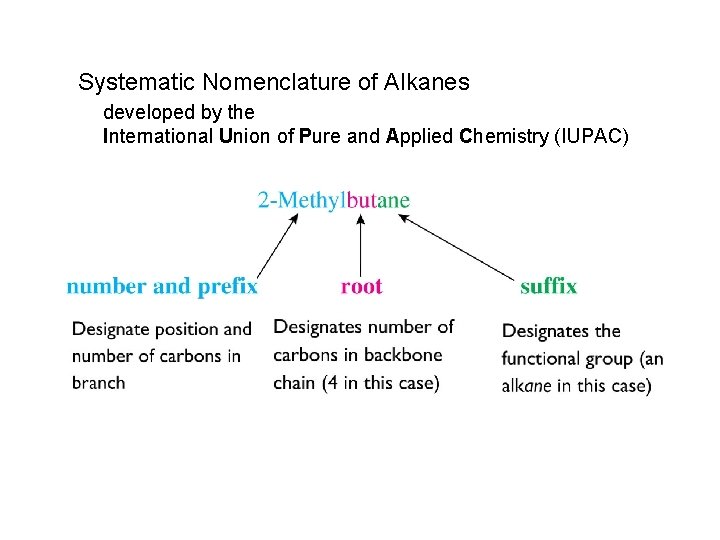
Systematic Nomenclature of Alkanes developed by the International Union of Pure and Applied Chemistry (IUPAC)

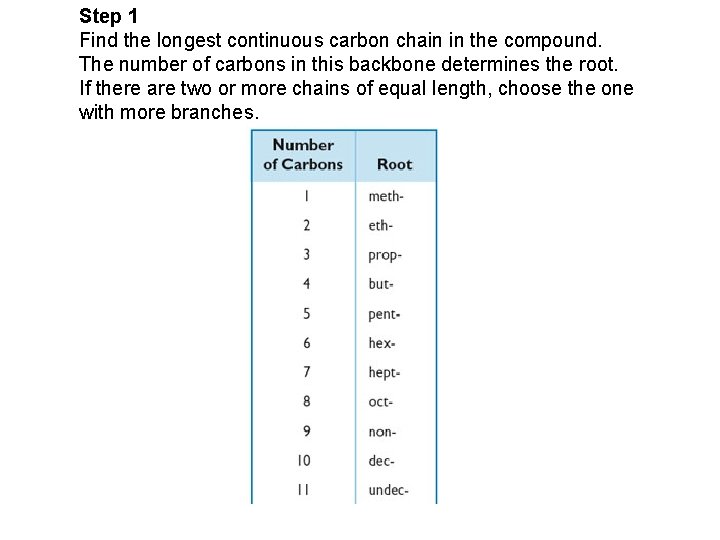
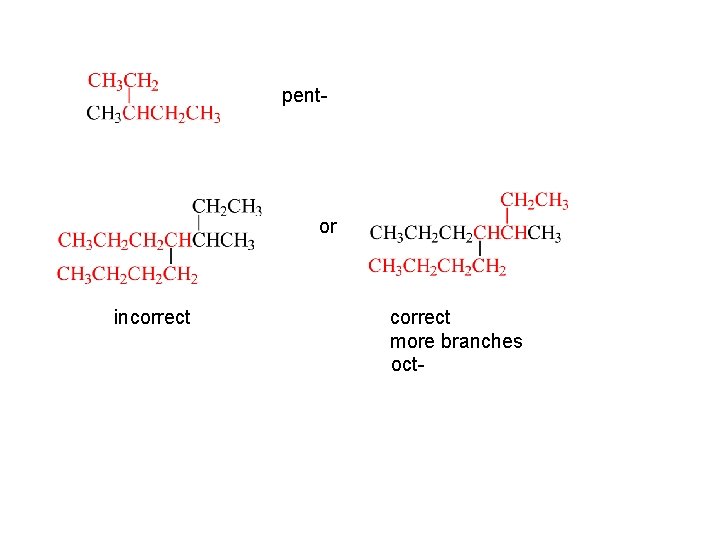
Step 1 Find the longest continuous carbon chain in the compound. The number of carbons in this backbone determines the root. If there are two or more chains of equal length, choose the one with more branches.

pent- or incorrect more branches oct-

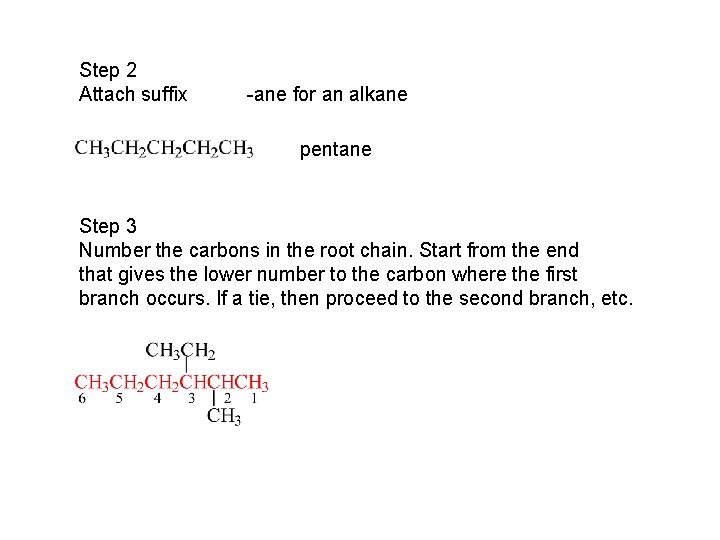
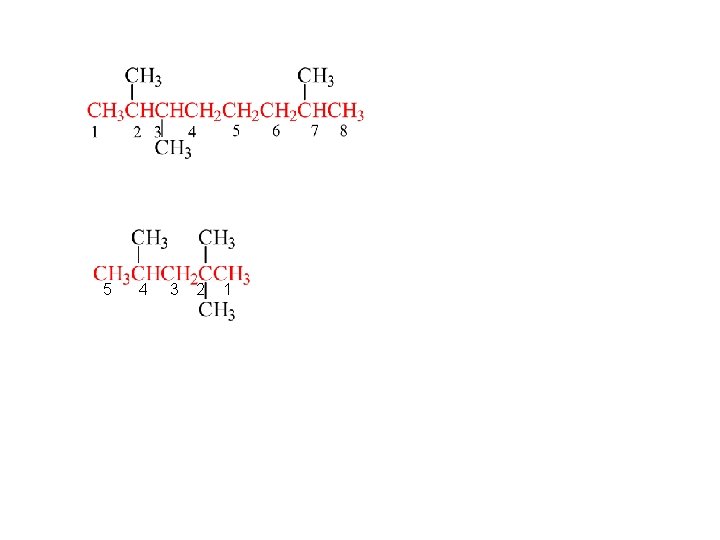
Step 2 Attach suffix -ane for an alkane pentane Step 3 Number the carbons in the root chain. Start from the end that gives the lower number to the carbon where the first branch occurs. If a tie, then proceed to the second branch, etc.

5 4 3 2 1

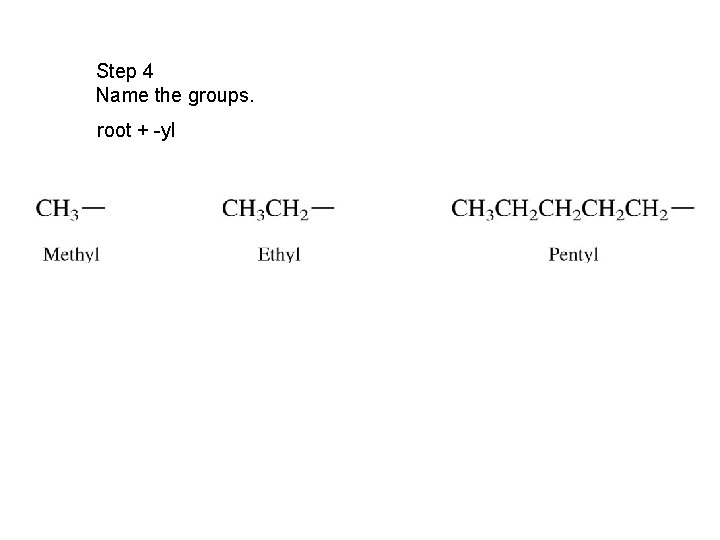
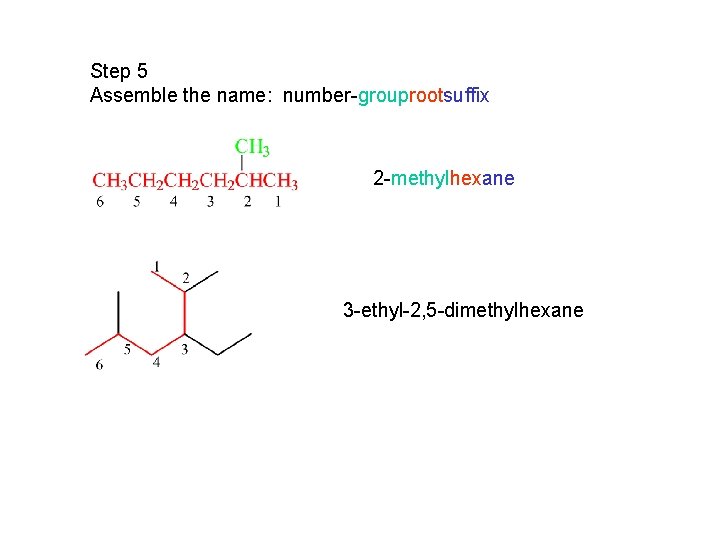
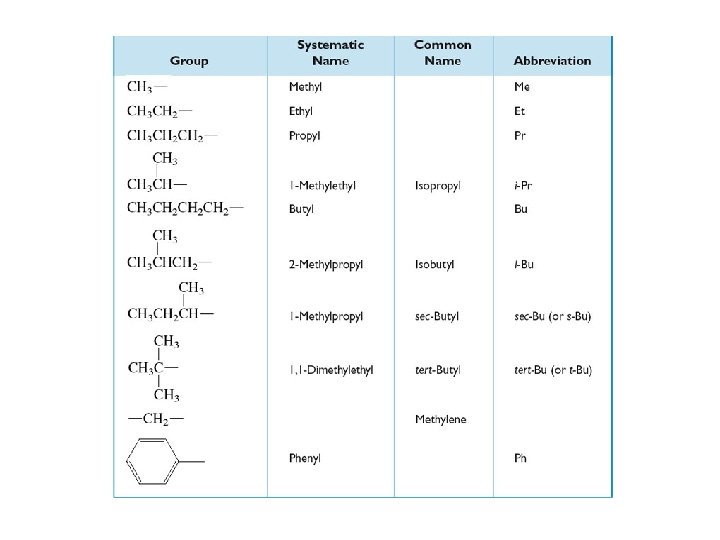
Step 4 Name the groups. root + -yl

Step 5 Assemble the name: number-grouprootsuffix 2 -methylhexane 3 -ethyl-2, 5 -dimethylhexane

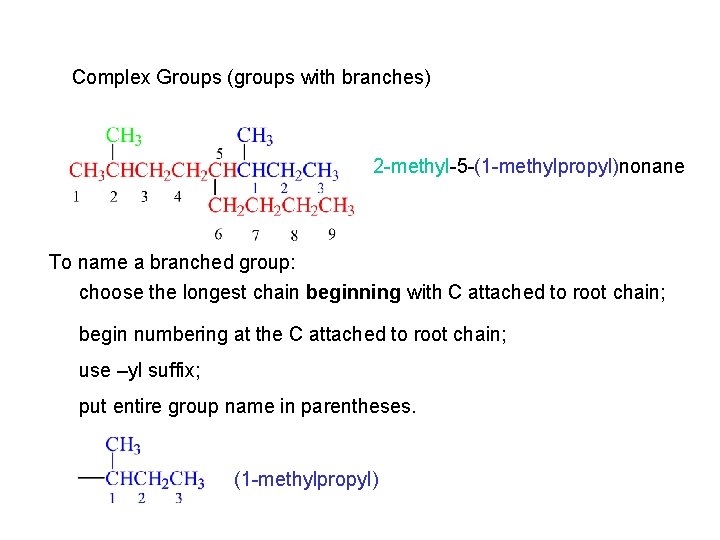
Complex Groups (groups with branches) 2 -methyl-5 -(1 -methylpropyl)nonane To name a branched group: choose the longest chain beginning with C attached to root chain; begin numbering at the C attached to root chain; use –yl suffix; put entire group name in parentheses. (1 -methylpropyl)

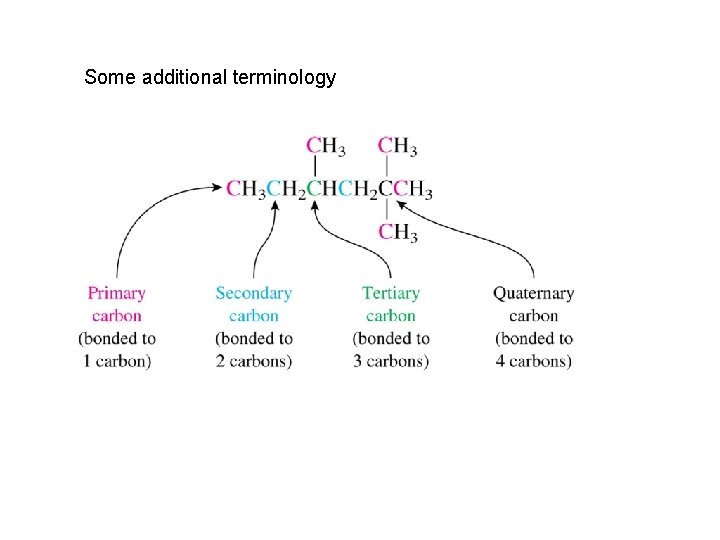
Some additional terminology


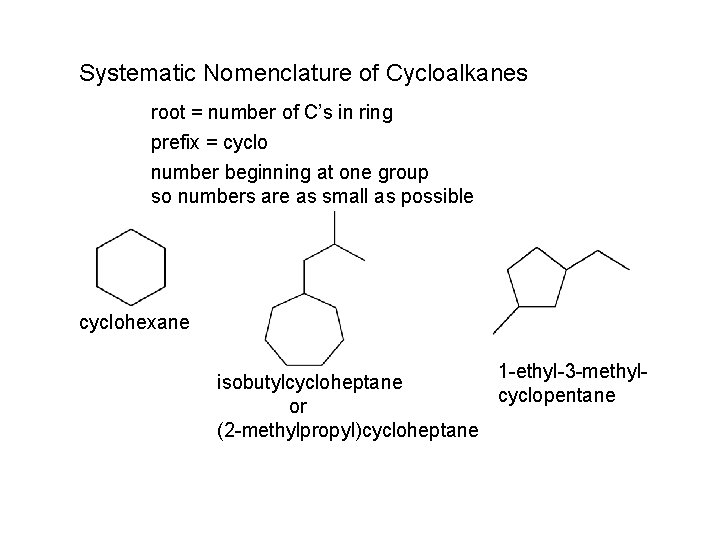
Systematic Nomenclature of Cycloalkanes root = number of C’s in ring prefix = cyclo number beginning at one group so numbers are as small as possible cyclohexane 1 -ethyl-3 -methylisobutylcycloheptane cyclopentane or (2 -methylpropyl)cycloheptane

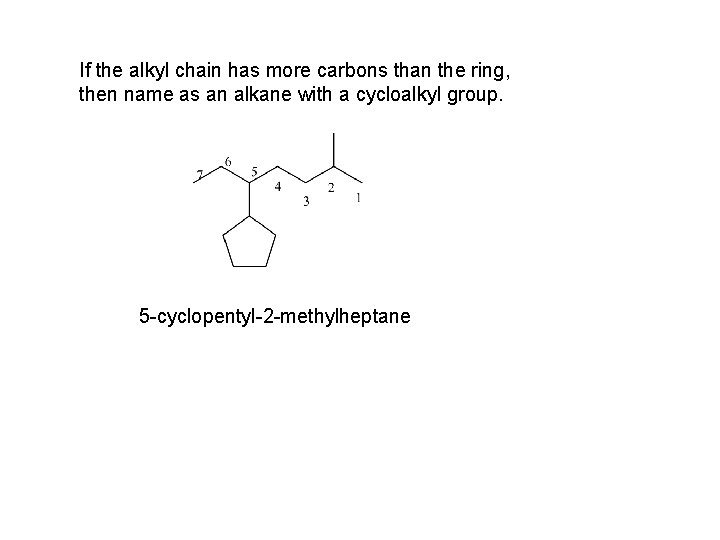
If the alkyl chain has more carbons than the ring, then name as an alkane with a cycloalkyl group. 5 -cyclopentyl-2 -methylheptane

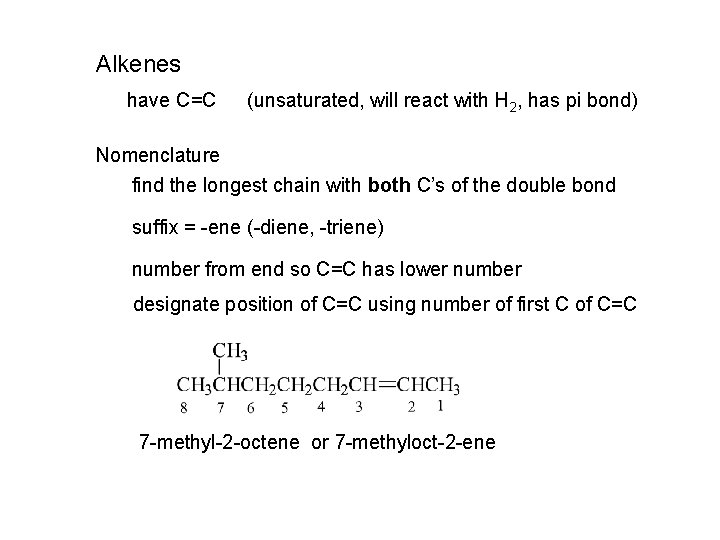
Alkenes have C=C (unsaturated, will react with H 2, has pi bond) Nomenclature find the longest chain with both C’s of the double bond suffix = -ene (-diene, -triene) number from end so C=C has lower number designate position of C=C using number of first C of C=C 7 -methyl-2 -octene or 7 -methyloct-2 -ene

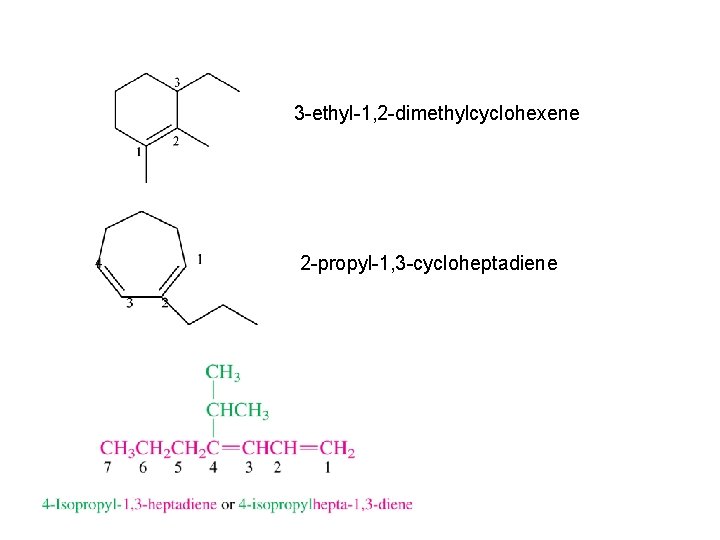
3 -ethyl-1, 2 -dimethylcyclohexene 2 -propyl-1, 3 -cycloheptadiene

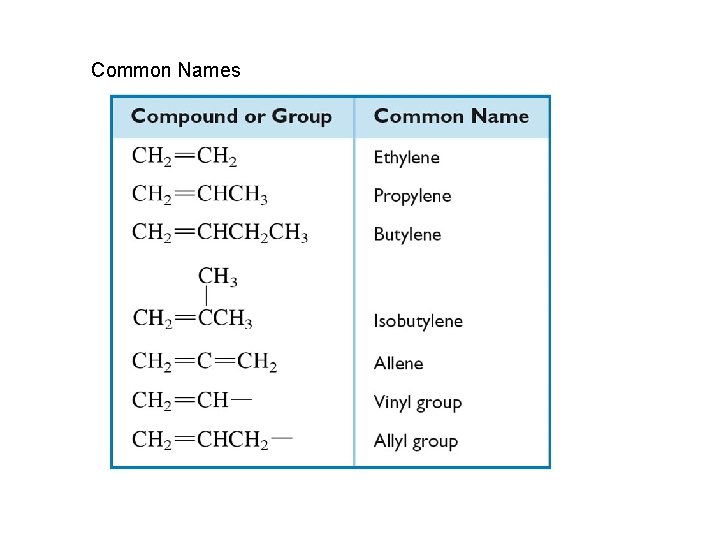
Common Names

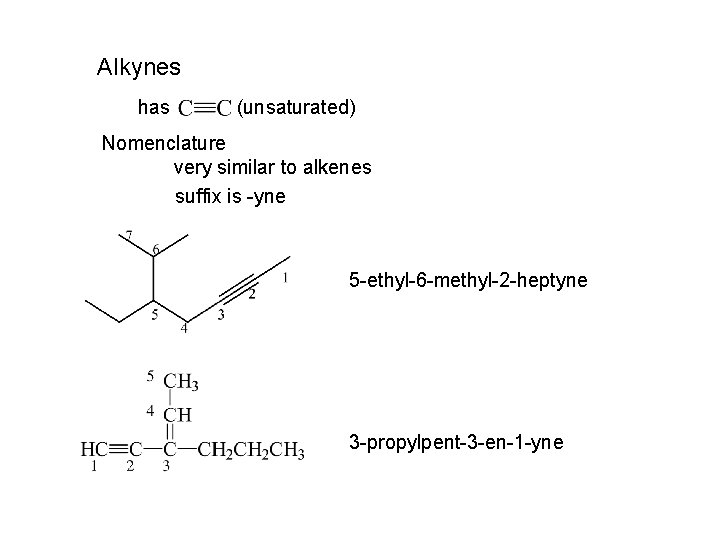
Alkynes has (unsaturated) Nomenclature very similar to alkenes suffix is -yne 5 -ethyl-6 -methyl-2 -heptyne 3 -propylpent-3 -en-1 -yne

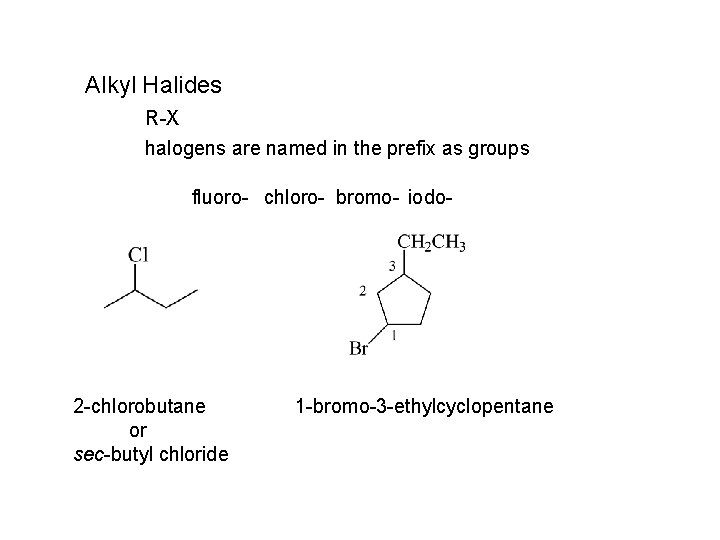
Alkyl Halides R-X halogens are named in the prefix as groups fluoro- chloro- bromo- iodo- 2 -chlorobutane or sec-butyl chloride 1 -bromo-3 -ethylcyclopentane

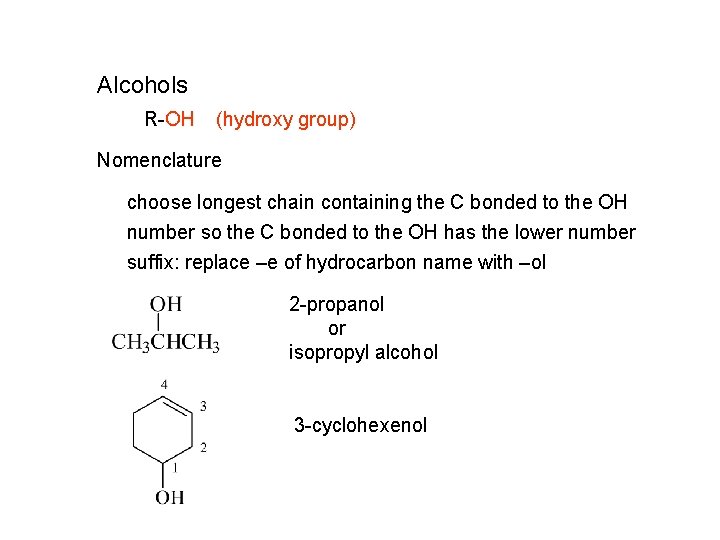
Alcohols R-OH (hydroxy group) Nomenclature choose longest chain containing the C bonded to the OH number so the C bonded to the OH has the lower number suffix: replace –e of hydrocarbon name with –ol 2 -propanol or isopropyl alcohol 3 -cyclohexenol

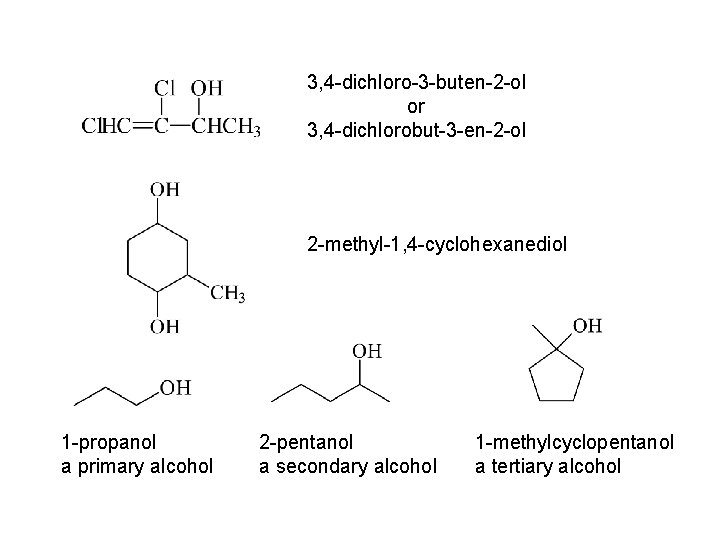
3, 4 -dichloro-3 -buten-2 -ol or 3, 4 -dichlorobut-3 -en-2 -ol 2 -methyl-1, 4 -cyclohexanediol 1 -propanol a primary alcohol 2 -pentanol a secondary alcohol 1 -methylcyclopentanol a tertiary alcohol

physical properties of alcohols -OH group is polar and can form hydrogen bonds CH 3 CH 2 CH 2 CH 3 pentane 72 g/mol mp -130 o. C bp 36 o. C CH 3 CH 2 CH 2 OH 1 -butanol 74 g/mol mp -90 o. C bp 117 o. C Hydrogen bonding has a larger effect on boiling points because the H-bond must be broken for the molecule to get into the gas phase. -OH group is hydrophilic ethanol miscible with water 1 -pentanol 2. 7 g/100 m. L of water 1 -heptanol 0. 1 g/100 m. L of water

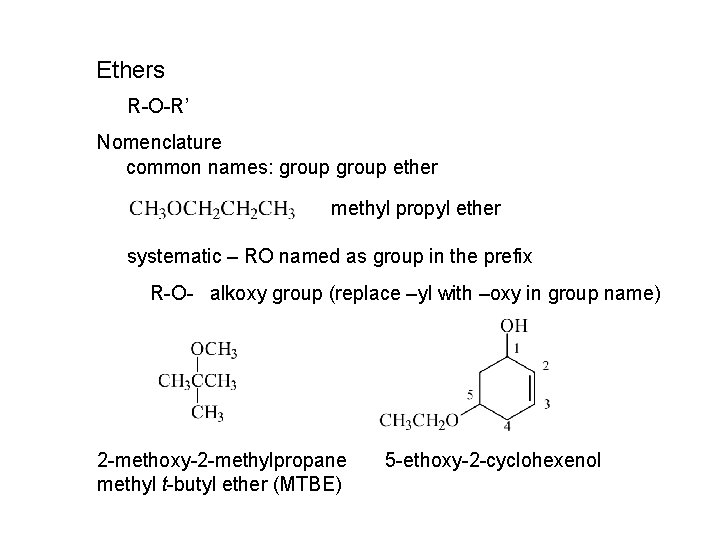
Ethers R-O-R’ Nomenclature common names: group ether methyl propyl ether systematic – RO named as group in the prefix R-O- alkoxy group (replace –yl with –oxy in group name) 2 -methoxy-2 -methylpropane methyl t-butyl ether (MTBE) 5 -ethoxy-2 -cyclohexenol

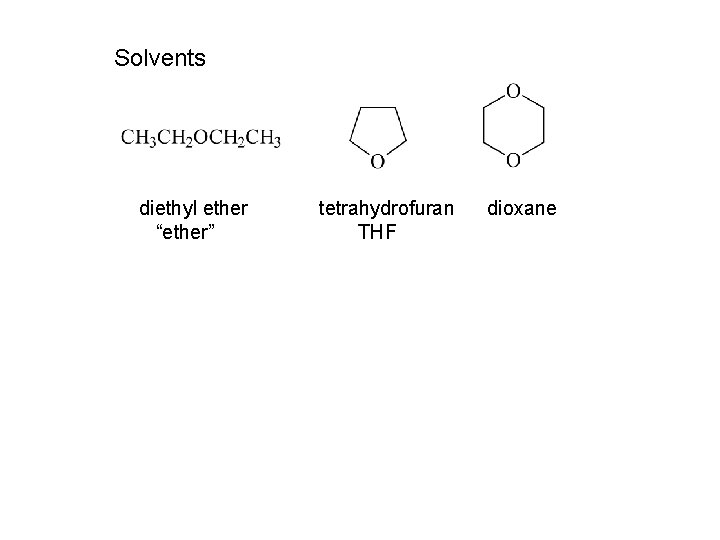
Solvents diethyl ether “ether” tetrahydrofuran THF dioxane

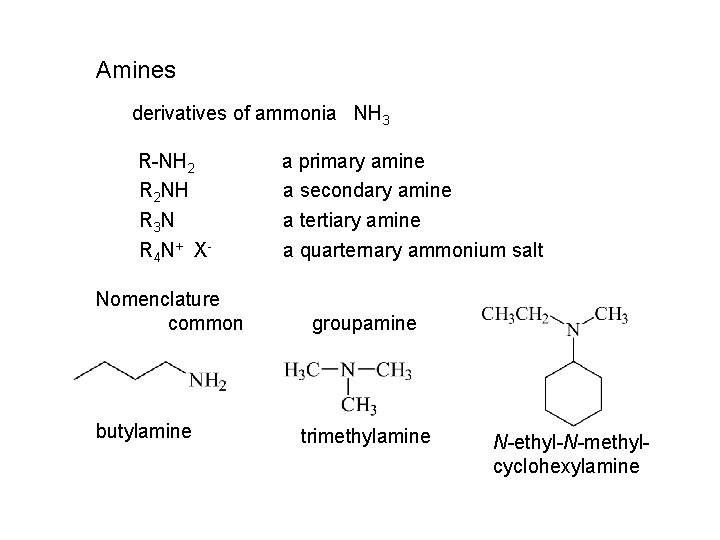
Amines derivatives of ammonia NH 3 R-NH 2 R 2 NH R 3 N R 4 N+ X Nomenclature common butylamine a primary amine a secondary amine a tertiary amine a quarternary ammonium salt groupamine trimethylamine N-ethyl-N-methylcyclohexylamine

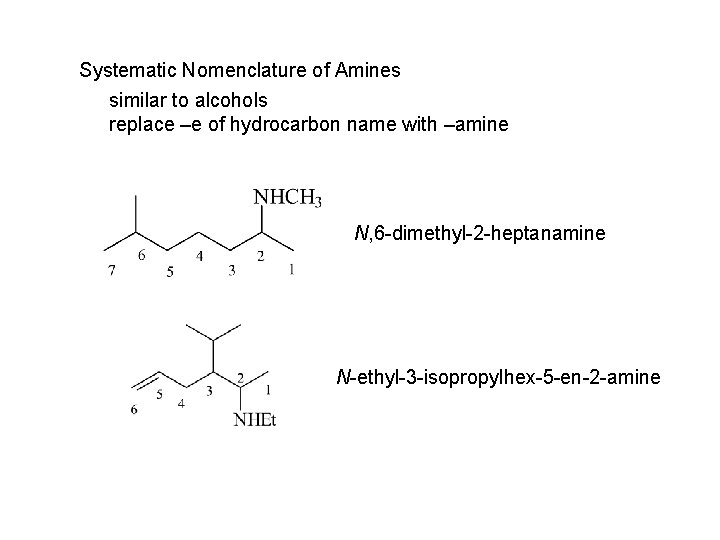
Systematic Nomenclature of Amines similar to alcohols replace –e of hydrocarbon name with –amine N, 6 -dimethyl-2 -heptanamine N-ethyl-3 -isopropylhex-5 -en-2 -amine

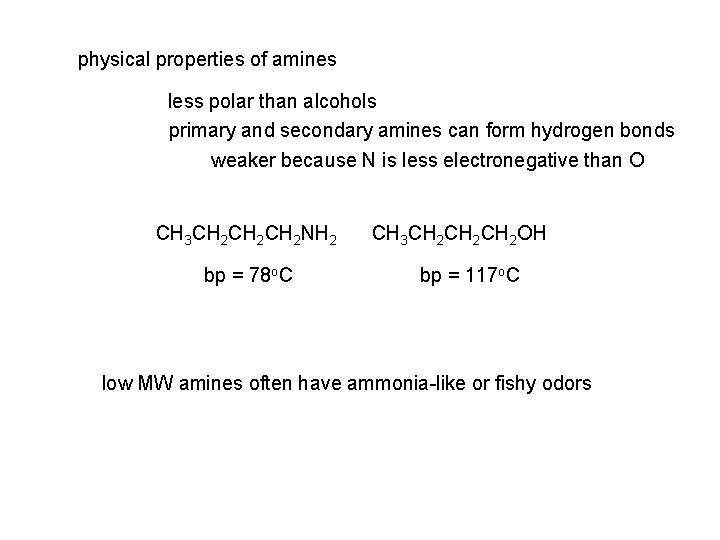
physical properties of amines less polar than alcohols primary and secondary amines can form hydrogen bonds weaker because N is less electronegative than O CH 3 CH 2 CH 2 NH 2 bp = 78 o. C CH 3 CH 2 CH 2 OH bp = 117 o. C low MW amines often have ammonia-like or fishy odors

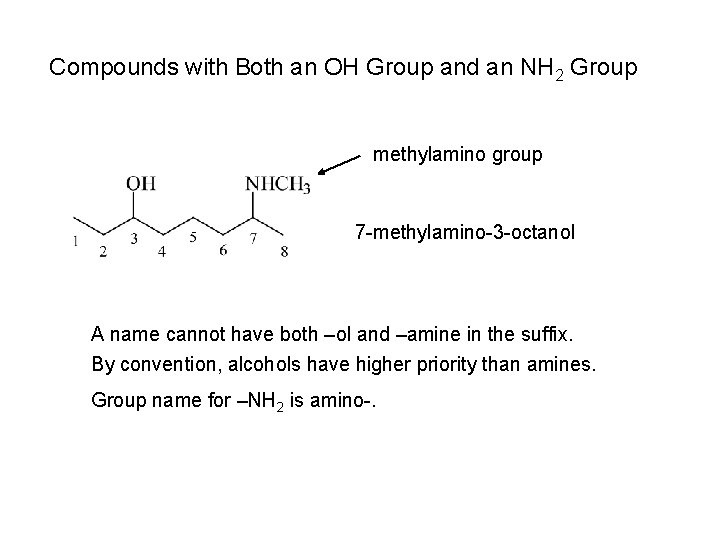
Compounds with Both an OH Group and an NH 2 Group methylamino group 7 -methylamino-3 -octanol A name cannot have both –ol and –amine in the suffix. By convention, alcohols have higher priority than amines. Group name for –NH 2 is amino-.