Alkanes 1 Introduction 2 Nomenclature of Alkanes 3








- Slides: 47

Alkanes 1 Introduction 2 Nomenclature of Alkanes 3 Physical Properties of Alkanes 4 Preparation of Alkanes 5 Reactions of Alkanes Dr Manal F. Abou. Taleb

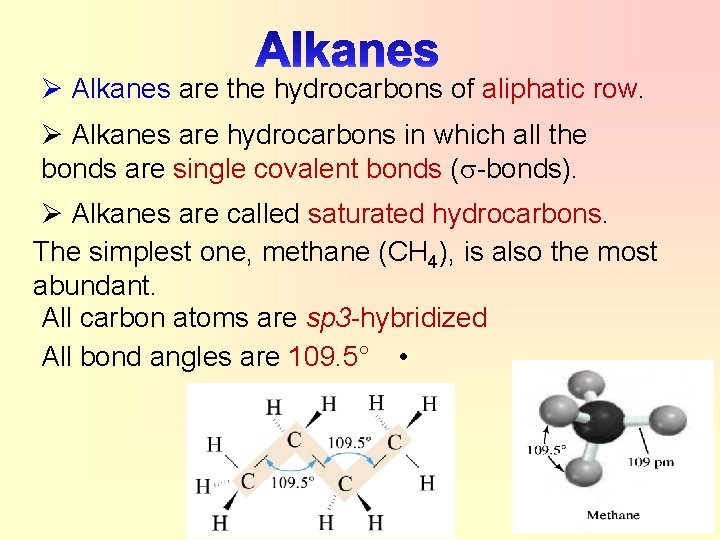
Ø Alkanes are the hydrocarbons of aliphatic row. Ø Alkanes are hydrocarbons in which all the bonds are single covalent bonds ( -bonds). Ø Alkanes are called saturated hydrocarbons. The simplest one, methane (CH 4), is also the most abundant. All carbon atoms are sp 3 -hybridized All bond angles are 109. 5° •

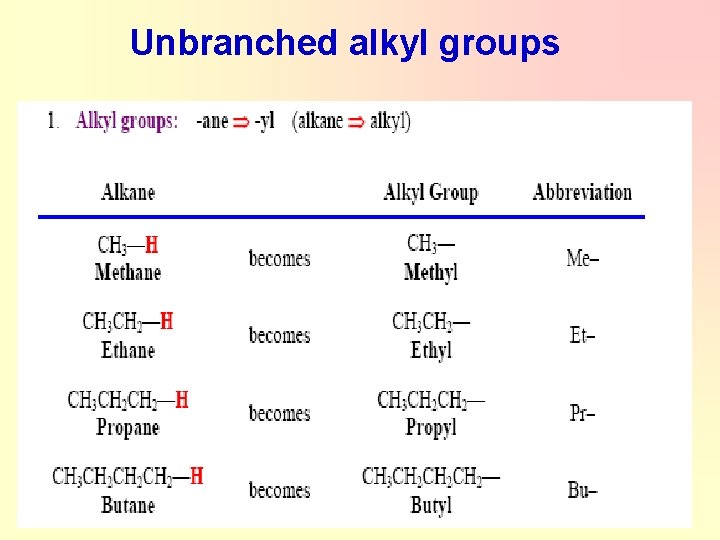
Alkyl groups Ø Alkyl groups are formed by loss of a hydrogen atom from the corresponding alkane. General formula of alkyl group : Cn. H 2 n+1 Ø ( e. g. CH 4 Methane – 1 H = -CH 3 Methyl group ) Ø Alkyl groups are named by dropping the -ane suffix of the alkanes and adding the suffix -yl. Methane becomes a methyl group, ethane an ethyl group, etc.

Unbranched alkyl groups

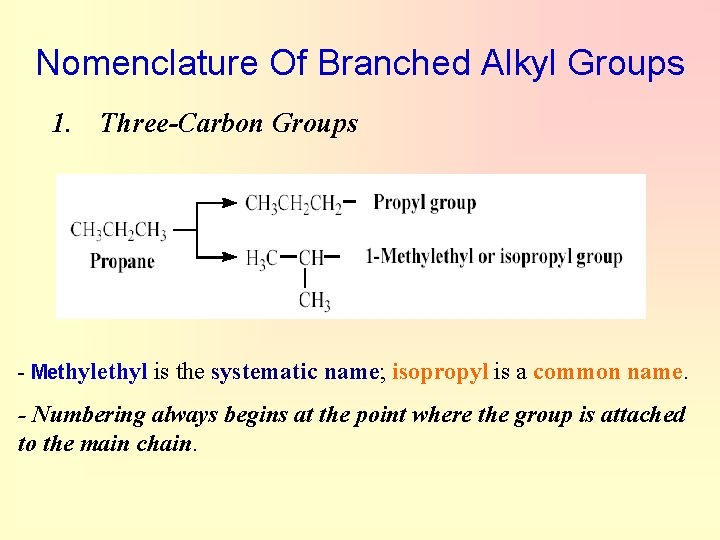
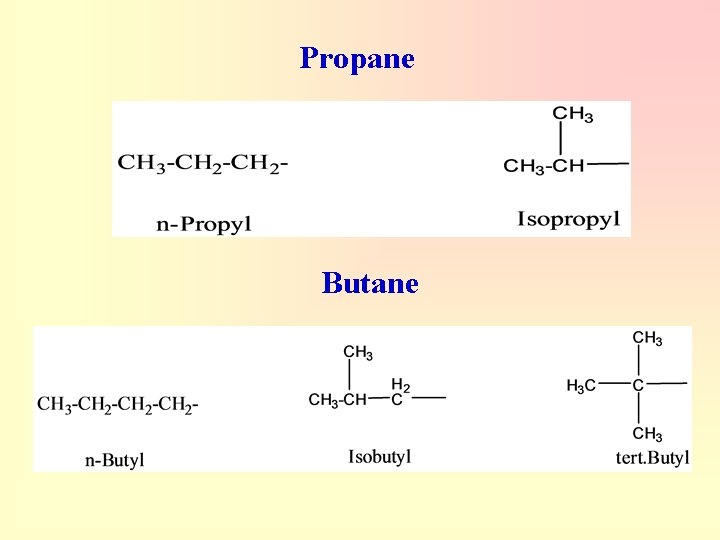
Nomenclature Of Branched Alkyl Groups 1. Three-Carbon Groups - Methyl is the systematic name; isopropyl is a common name. - Numbering always begins at the point where the group is attached to the main chain.

Propane Butane

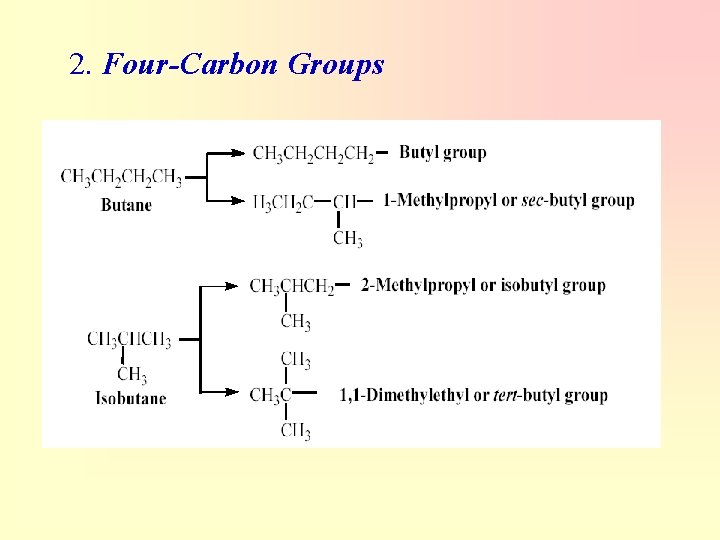
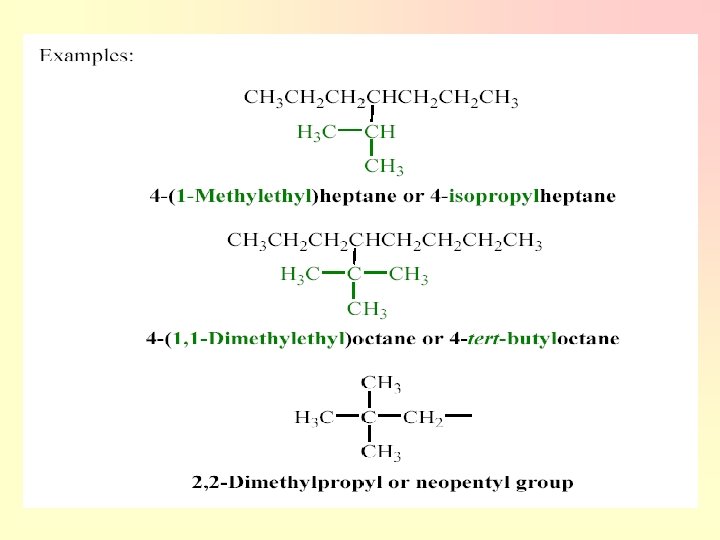
2. Four-Carbon Groups

2. Four-Carbon Groups

IUPAC Nomenclature • IUPAC (International Union of Pure and Applied Chemistry) names: • 1 - The unbranched alkanes (homologous series) • 2 - Branched alkanes • Suffix of Alkanes added ane

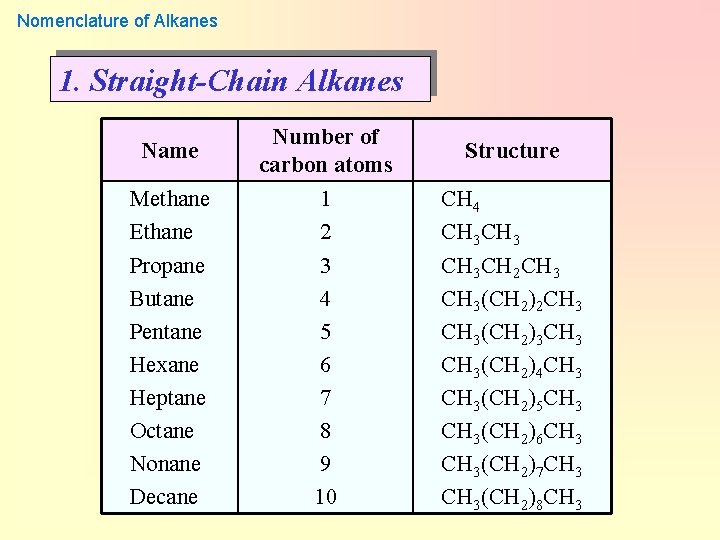
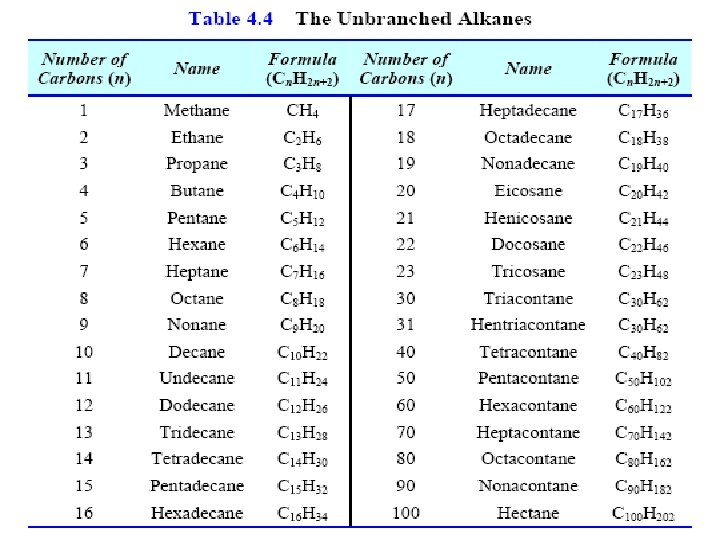
Nomenclature of Alkanes 1. Straight-Chain Alkanes Name Number of carbon atoms Structure Methane Ethane Propane Butane Pentane Hexane Heptane Octane Nonane Decane 1 2 3 4 5 6 7 8 9 10 CH 4 CH 3 CH 2 CH 3(CH 2)3 CH 3(CH 2)4 CH 3(CH 2)5 CH 3(CH 2)6 CH 3(CH 2)7 CH 3(CH 2)8 CH 3

Alkanes are molecules(hydrocarbons) that contain only C-C and C-H bonds. Alkanes are either acyclic or cyclic. Acyclic alkanes (not cyclic) have the formula Cn. H 2 n+2 (where n = an integer)

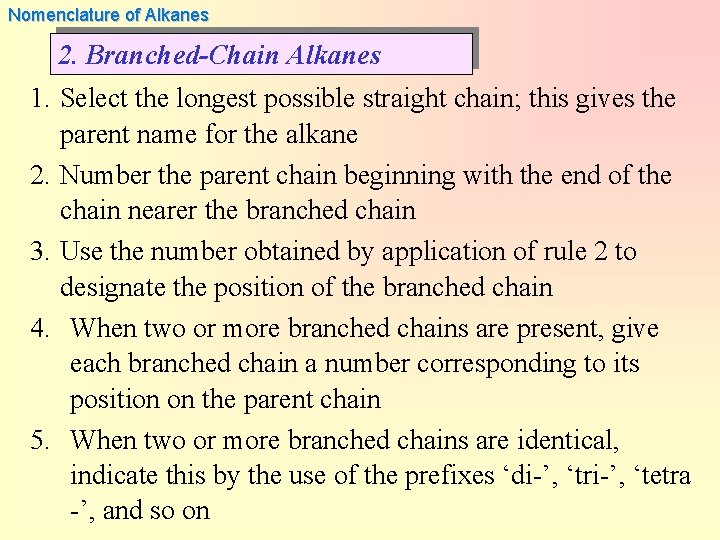
Nomenclature of Alkanes 2. Branched-Chain Alkanes 1. Select the longest possible straight chain; this gives the parent name for the alkane 2. Number the parent chain beginning with the end of the chain nearer the branched chain 3. Use the number obtained by application of rule 2 to designate the position of the branched chain 4. When two or more branched chains are present, give each branched chain a number corresponding to its position on the parent chain 5. When two or more branched chains are identical, indicate this by the use of the prefixes ‘di-’, ‘tri-’, ‘tetra -’, and so on

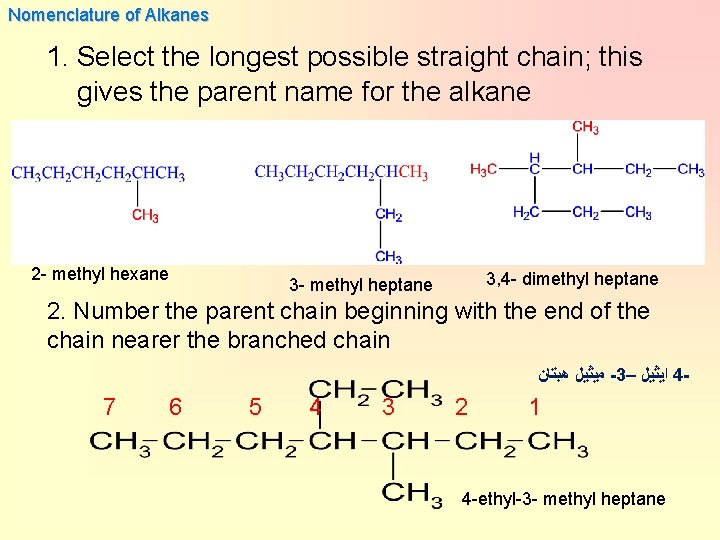
Nomenclature of Alkanes 1. Select the longest possible straight chain; this gives the parent name for the alkane 2 - methyl hexane 3, 4 - dimethyl heptane 3 - methyl heptane 2. Number the parent chain beginning with the end of the chain nearer the branched chain ﻣﻴﺜﻴﻞ ﻫﺒﺘﺎﻥ -3– ﺍﻳﺜﻴﻞ 4 - 7 6 5 4 3 2 1 4 -ethyl-3 - methyl heptane

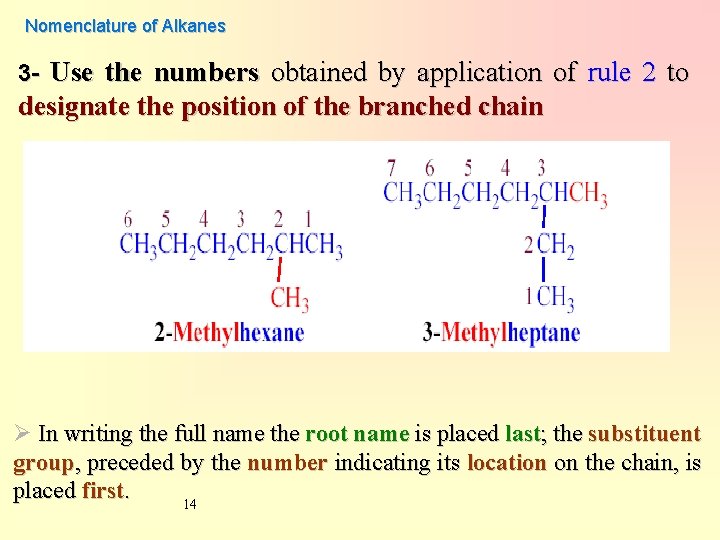
Nomenclature of Alkanes 3 - Use the numbers obtained by application of rule 2 to designate the position of the branched chain Ø In writing the full name the root name is placed last; the substituent group, preceded by the number indicating its location on the chain, is placed first. 14

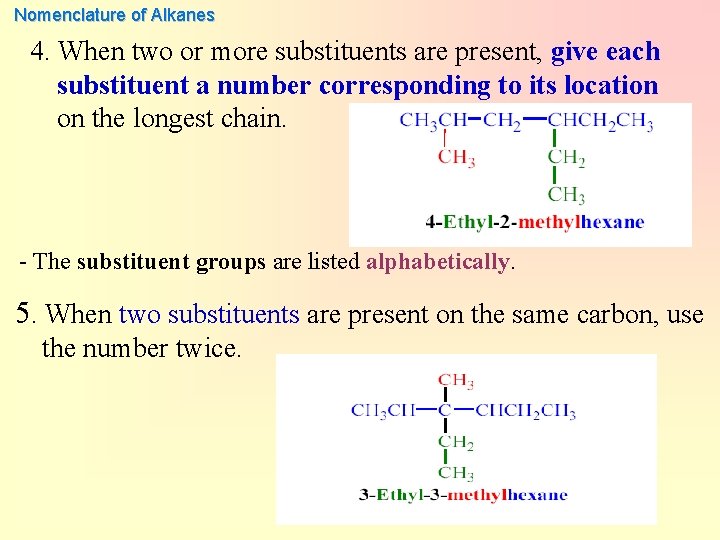
Nomenclature of Alkanes 4. When two or more substituents are present, give each substituent a number corresponding to its location on the longest chain. - The substituent groups are listed alphabetically. 5. When two substituents are present on the same carbon, use the number twice.

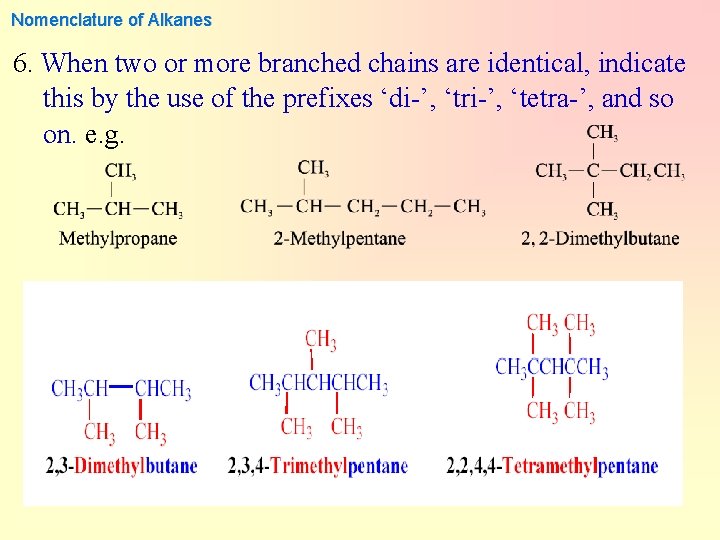
Nomenclature of Alkanes 6. When two or more branched chains are identical, indicate this by the use of the prefixes ‘di-’, ‘tri-’, ‘tetra-’, and so on. e. g.

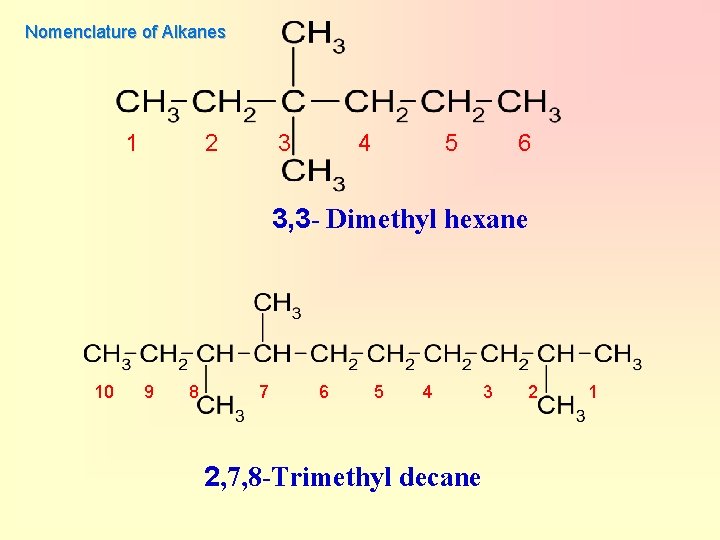
Nomenclature of Alkanes 1 2 3 4 5 6 3, 3 - Dimethyl hexane 10 9 8 7 6 5 4 2, 7, 8 -Trimethyl decane 3 2 1

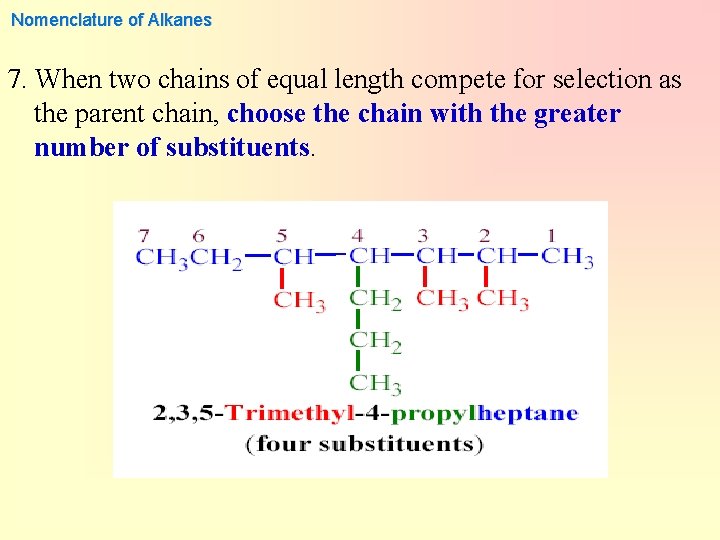
Nomenclature of Alkanes 7. When two chains of equal length compete for selection as the parent chain, choose the chain with the greater number of substituents.

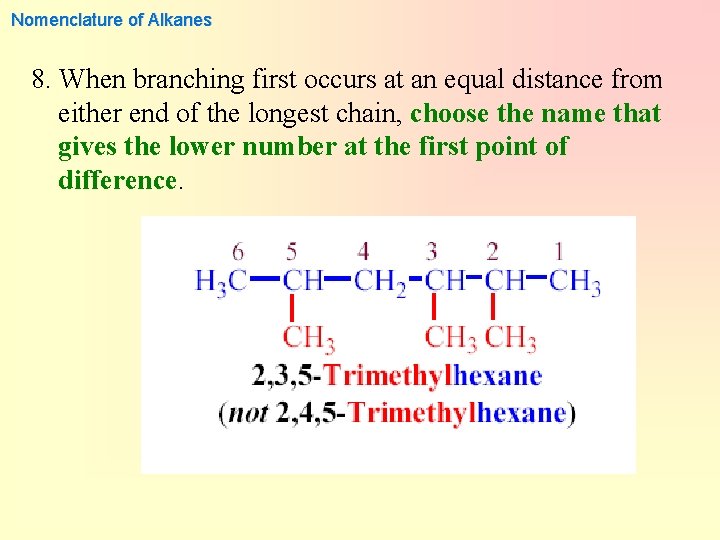
Nomenclature of Alkanes 8. When branching first occurs at an equal distance from either end of the longest chain, choose the name that gives the lower number at the first point of difference.

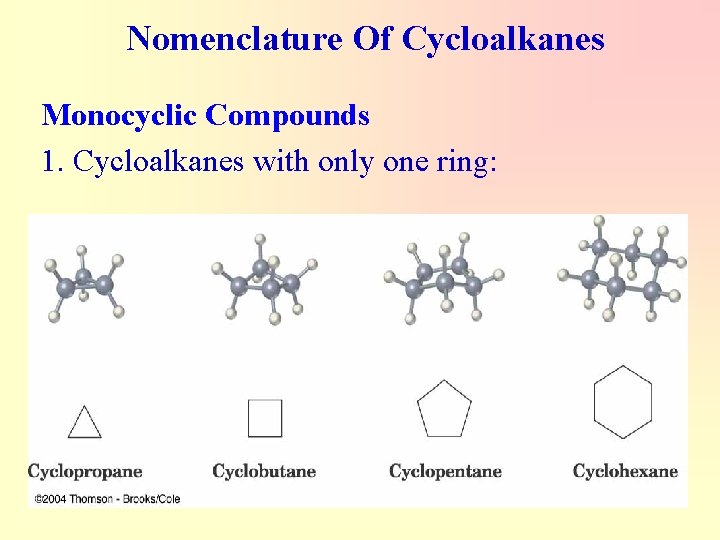
Nomenclature Of Cycloalkanes Monocyclic Compounds 1. Cycloalkanes with only one ring:

Substituted cycloalkanes: 1) Number the ring beginning with the substituent first in the alphabet, and number in the direction that gives the next substituent the lower number possible. 2) When three or more substituents are present, begin at the substituent that leads to the lowest set of locants.


Physical Properties Of Alkanes and Cycloalkanes • 1. A series of compounds, where each member differs from the next member by a constant unit, is called a homologous series. Members of a homologous series are called homologs. • 2. At room temperature ( 25 °C) and 1 atm pressure, the C 1 -C 4 unbranched alkanes are gases; the C 5 -C 17 unbranched alkanes are liquids; the unbranched alkanes with 18 or more carbon atoms are solids.

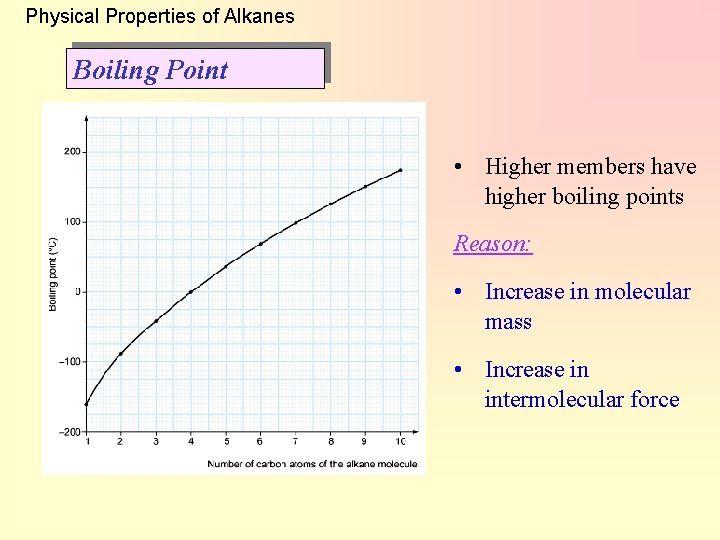
Physical Properties of Alkanes Boiling Point • Higher members have higher boiling points Reason: • Increase in molecular mass • Increase in intermolecular force

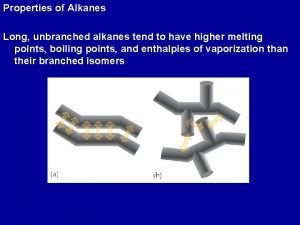
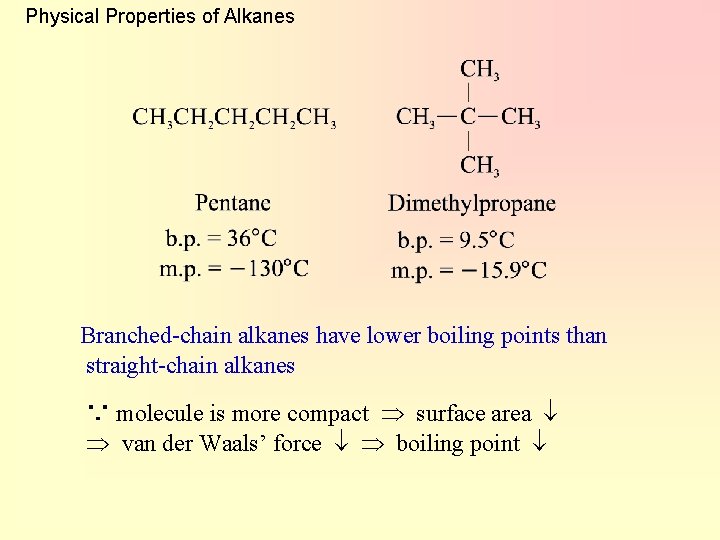
Physical Properties of Alkanes Branched-chain alkanes have lower boiling points than straight-chain alkanes ∵ molecule is more compact surface area van der Waals’ force boiling point

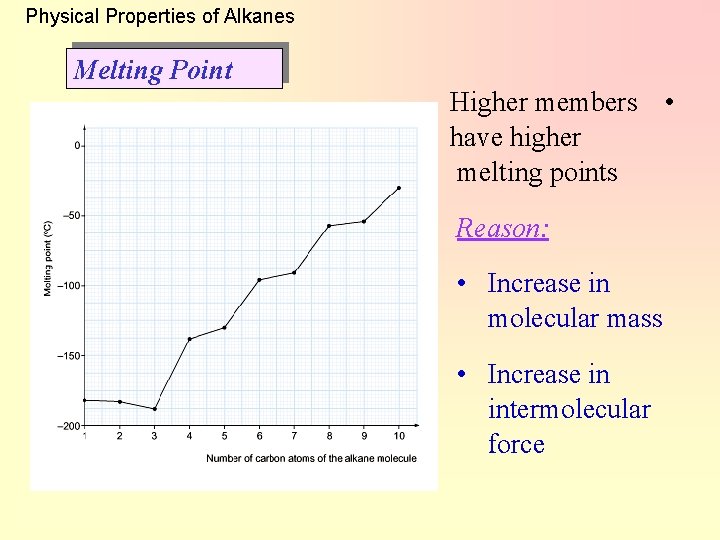
Physical Properties of Alkanes Melting Point Higher members • have higher melting points Reason: • Increase in molecular mass • Increase in intermolecular force

Physical Properties of Alkanes Solubility Alkanes: • non-polar compounds • insoluble in water and highly polar solvents • soluble in non-polar solvents like benzene, 1, 1, 1 -trichloroethane Density All alkanes and cycloalkanes have densities less than 1 g cm– 3 at 20°C. Petroleum floats on water surface Alkanes are less dense than water and swim on top of water

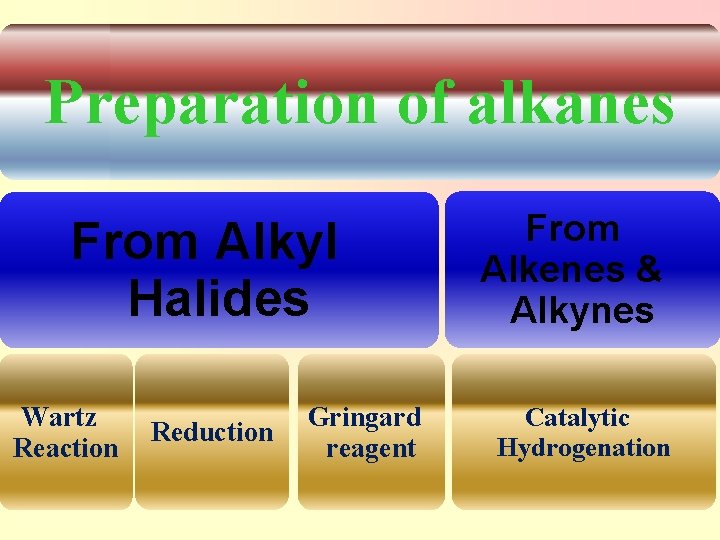
Preparation of alkanes From Alkyl Halides Wartz Reaction Reduction Gringard reagent From Alkenes & Alkynes Catalytic Hydrogenation

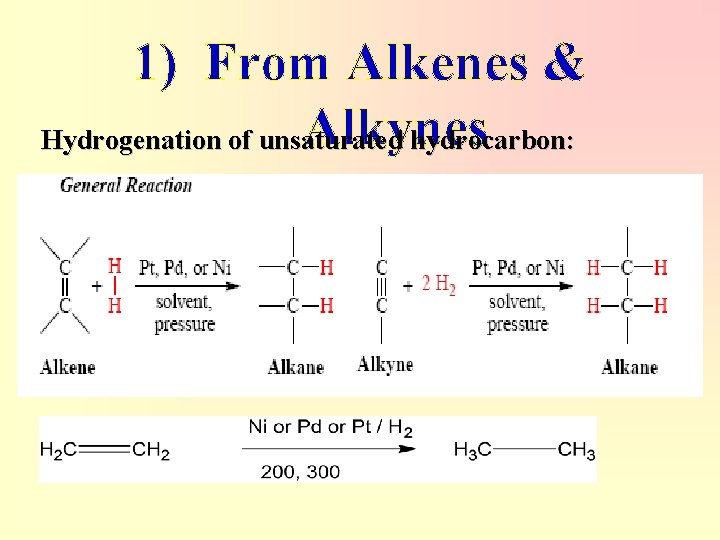
1) From Alkenes & Alkynes Hydrogenation of unsaturated hydrocarbon:


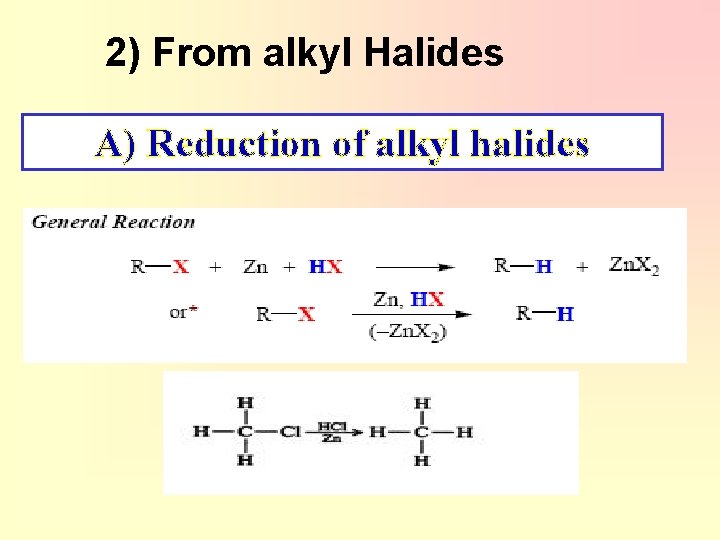
2) From alkyl Halides A) Reduction of alkyl halides


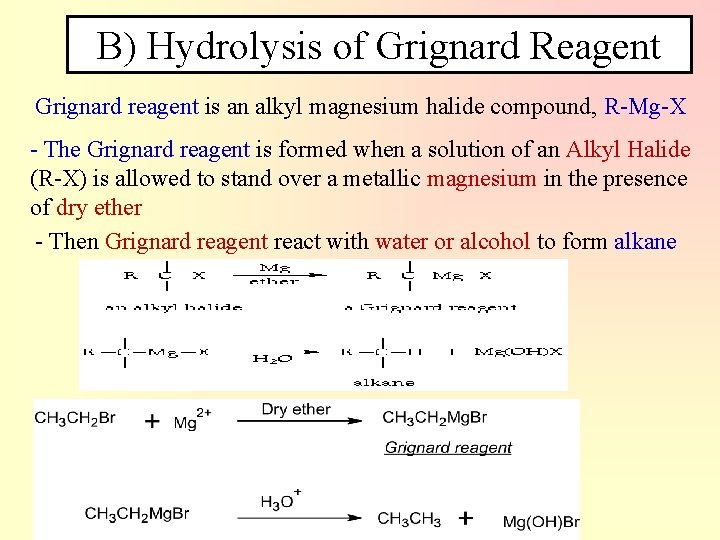
B) Hydrolysis of Grignard Reagent Grignard reagent is an alkyl magnesium halide compound, R-Mg-X - The Grignard reagent is formed when a solution of an Alkyl Halide (R-X) is allowed to stand over a metallic magnesium in the presence of dry ether - Then Grignard reagent react with water or alcohol to form alkane

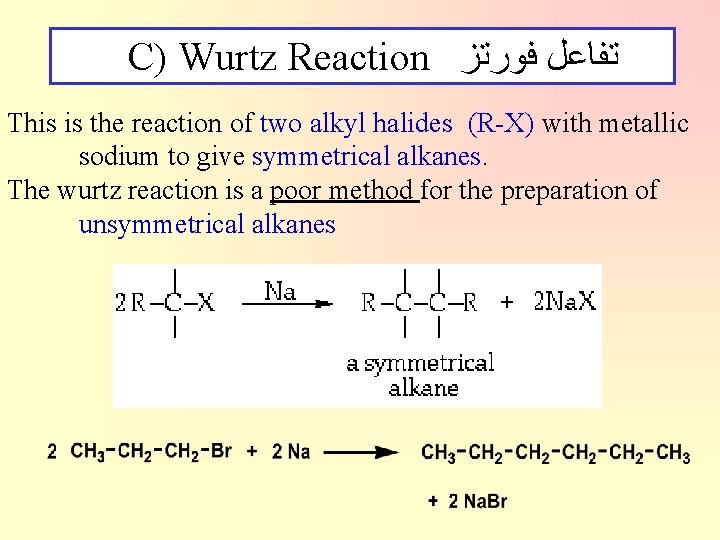
C) Wurtz Reaction ﺗﻔﺎﻋﻞ ﻓﻮﺭﺗﺰ This is the reaction of two alkyl halides (R-X) with metallic sodium to give symmetrical alkanes. The wurtz reaction is a poor method for the preparation of unsymmetrical alkanes

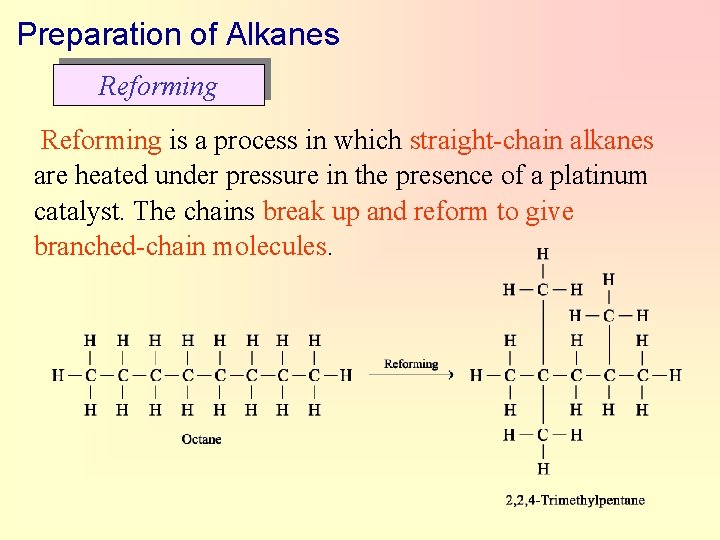
Preparation of Alkanes Reforming is a process in which straight-chain alkanes are heated under pressure in the presence of a platinum catalyst. The chains break up and reform to give branched-chain molecules.

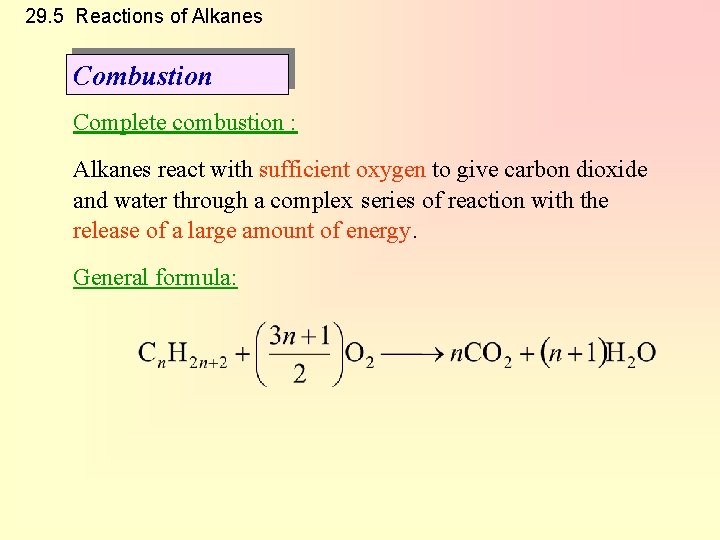
29. 5 Reactions of Alkanes Combustion Complete combustion : Alkanes react with sufficient oxygen to give carbon dioxide and water through a complex series of reaction with the release of a large amount of energy. General formula:

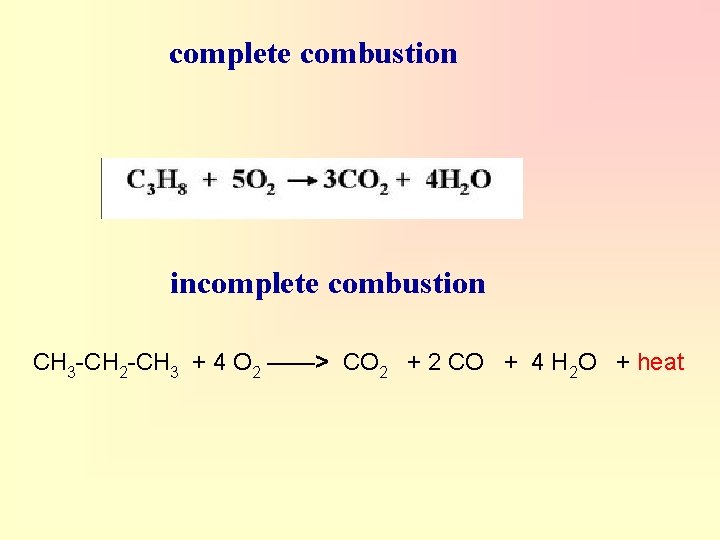
complete combustion incomplete combustion CH 3 -CH 2 -CH 3 + 4 O 2 ——> CO 2 + 2 CO + 4 H 2 O + heat

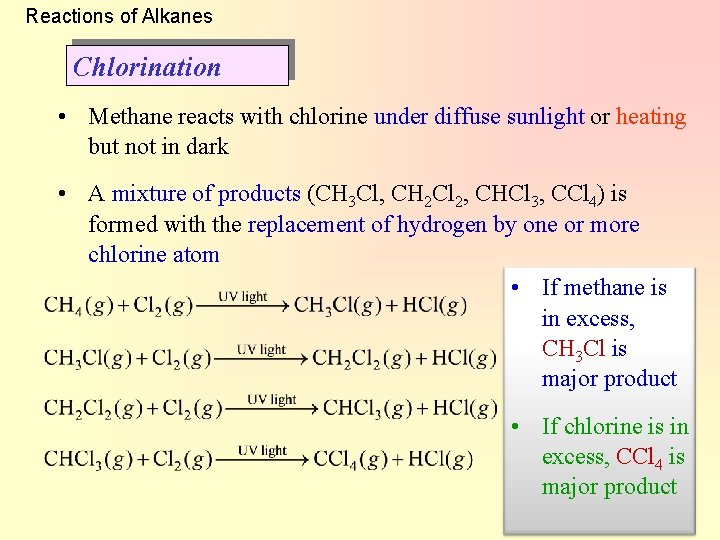
Reactions of Alkanes Chlorination • Methane reacts with chlorine under diffuse sunlight or heating but not in dark • A mixture of products (CH 3 Cl, CH 2 Cl 2, CHCl 3, CCl 4) is formed with the replacement of hydrogen by one or more chlorine atom • If methane is in excess, CH 3 Cl is major product • If chlorine is in excess, CCl 4 is major product

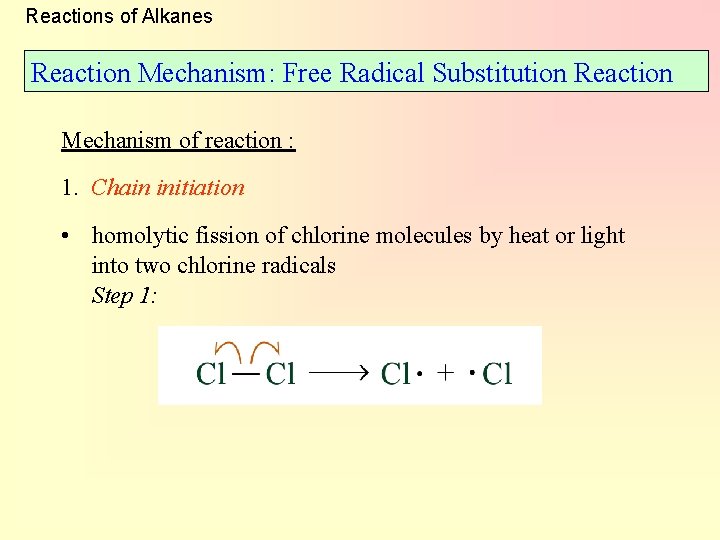
Reactions of Alkanes Reaction Mechanism: Free Radical Substitution Reaction Mechanism of reaction : 1. Chain initiation • homolytic fission of chlorine molecules by heat or light into two chlorine radicals Step 1:

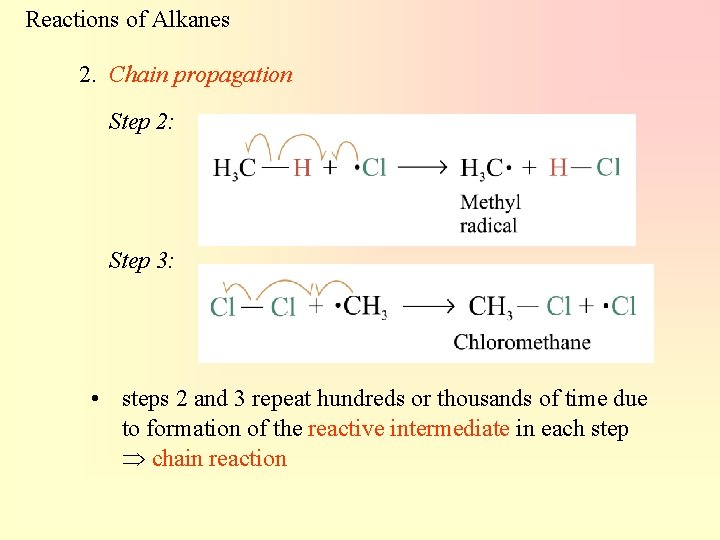
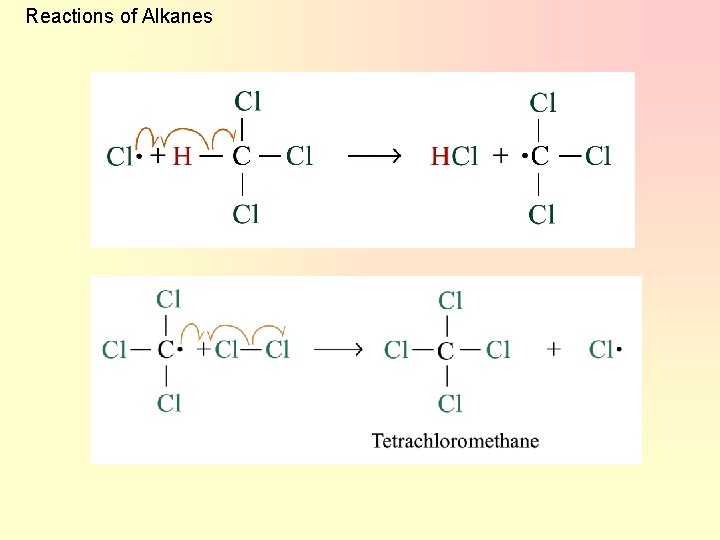
Reactions of Alkanes 2. Chain propagation Step 2: Step 3: • steps 2 and 3 repeat hundreds or thousands of time due to formation of the reactive intermediate in each step chain reaction

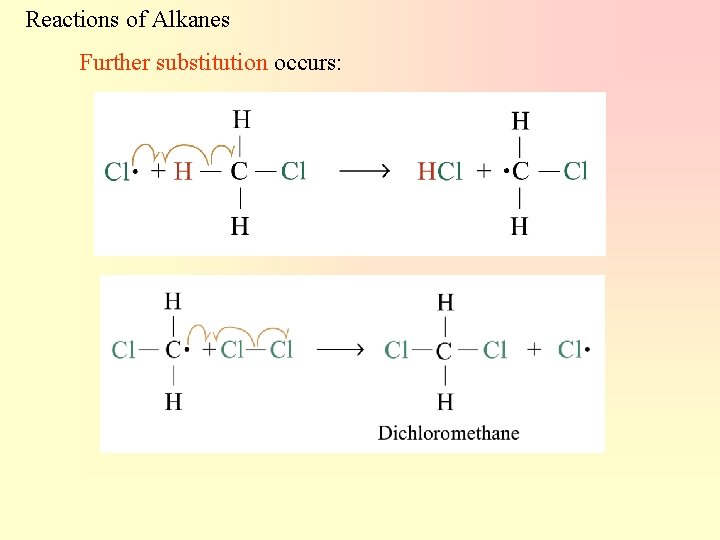
Reactions of Alkanes Further substitution occurs:

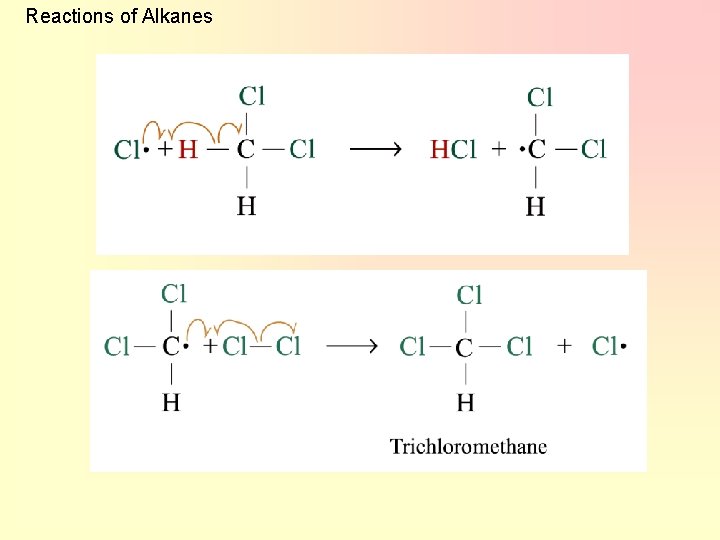
Reactions of Alkanes

Reactions of Alkanes

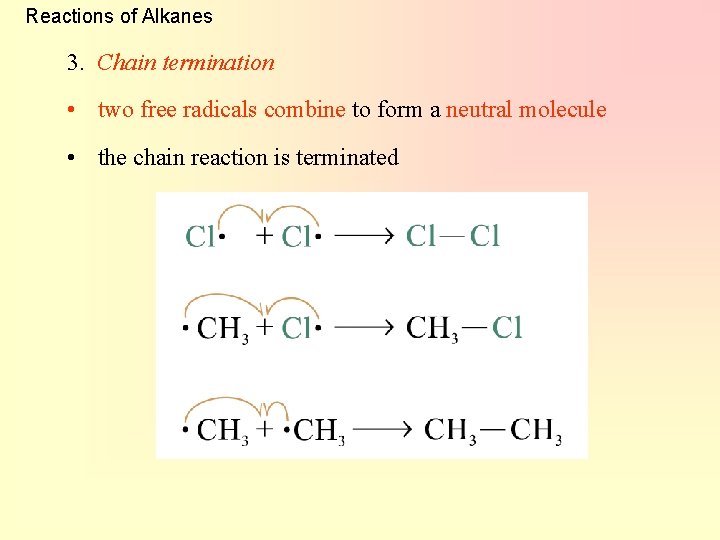
Reactions of Alkanes 3. Chain termination • two free radicals combine to form a neutral molecule • the chain reaction is terminated

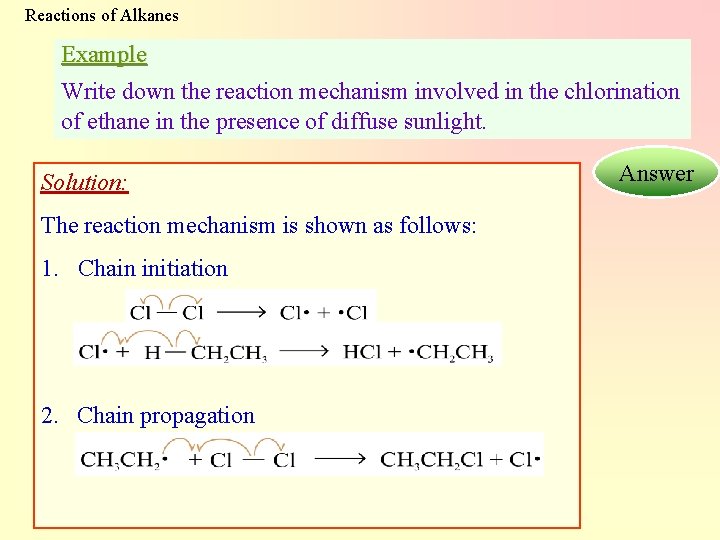
Reactions of Alkanes Example Write down the reaction mechanism involved in the chlorination of ethane in the presence of diffuse sunlight. Solution: The reaction mechanism is shown as follows: 1. Chain initiation 2. Chain propagation Answer

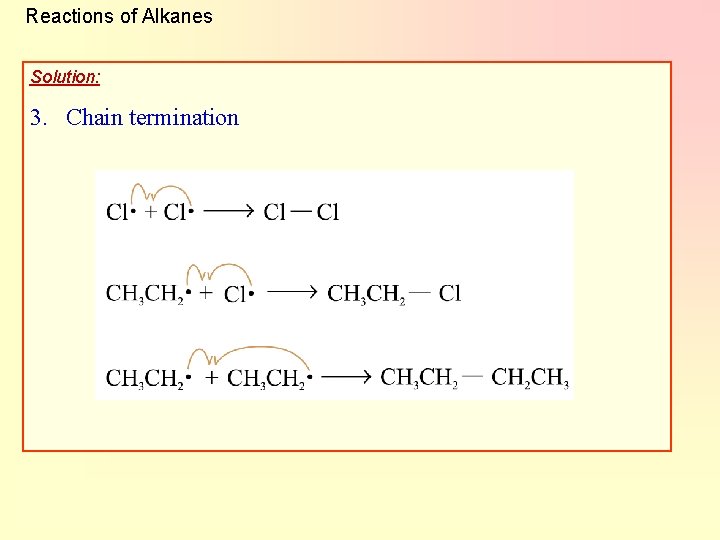
Reactions of Alkanes Solution: 3. Chain termination

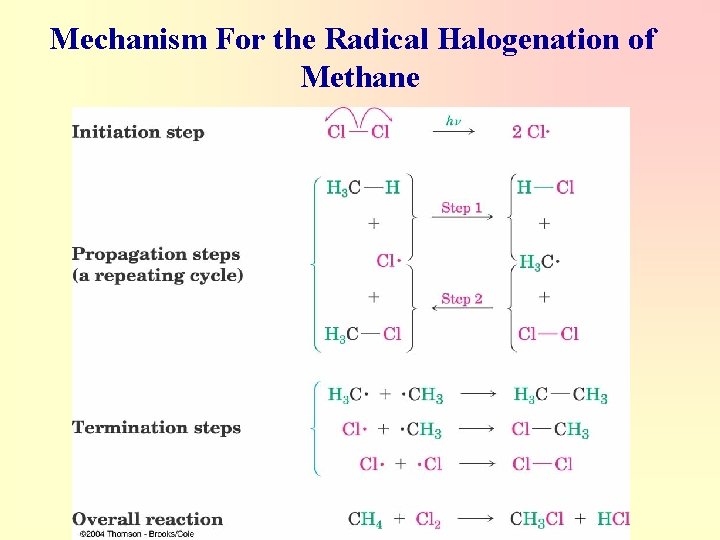
Mechanism For the Radical Halogenation of Methane
 Chemistry nomenclature
Chemistry nomenclature Alkanes solubility
Alkanes solubility Halogenation of alkanes mechanism
Halogenation of alkanes mechanism Alkane chemical formula
Alkane chemical formula Uses of alkanes
Uses of alkanes Alkanes list
Alkanes list General formula for alkyl group
General formula for alkyl group Alkanes alkenes alkynes
Alkanes alkenes alkynes 3-butyl-3-propyl-1-pentyne
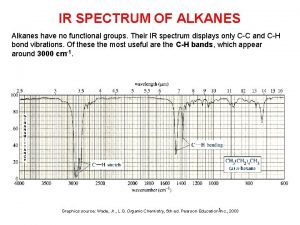
3-butyl-3-propyl-1-pentyne Terminal alkyne ir spectrum
Terminal alkyne ir spectrum Combustion of alkynes
Combustion of alkynes Alkane combustion
Alkane combustion Covalently def
Covalently def Alkylation of alkanes
Alkylation of alkanes Alkane viscosity
Alkane viscosity Cyclopentane uses
Cyclopentane uses Iupac stands for
Iupac stands for Bond angle in cyclohexane
Bond angle in cyclohexane Test for alkenes
Test for alkenes Alkane formula
Alkane formula Applications of alkanes
Applications of alkanes Example of criminal law
Example of criminal law Nomenclature of bolts
Nomenclature of bolts Single point cutting tool
Single point cutting tool Basic organic nomenclature packet
Basic organic nomenclature packet Nomenclature of saturated fatty acids
Nomenclature of saturated fatty acids Spring nomenclature
Spring nomenclature Tm 11-5810-410-13&p
Tm 11-5810-410-13&p Wire rope nomenclature
Wire rope nomenclature Remington 870 shotgun nomenclature
Remington 870 shotgun nomenclature Nucleotide nomenclature
Nucleotide nomenclature Atp ester bond
Atp ester bond Nomenclature of coordination compounds
Nomenclature of coordination compounds Lindlar pd
Lindlar pd Ester suffix
Ester suffix Nomenclature amines
Nomenclature amines End mill nomenclature
End mill nomenclature Down milling vs up milling
Down milling vs up milling échelle 3 plans pompier nomenclature
échelle 3 plans pompier nomenclature Spiral cam
Spiral cam Handcuffing positions
Handcuffing positions Nomenclature poteau incendie
Nomenclature poteau incendie Discrete igbts
Discrete igbts Rules of binomial nomenclature
Rules of binomial nomenclature Binomial nomenclature of humans
Binomial nomenclature of humans Binomial nomenclature examples
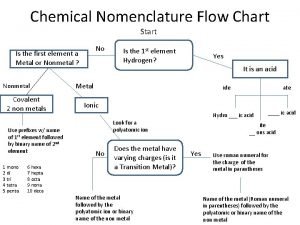
Binomial nomenclature examples Acid naming flow chart
Acid naming flow chart Naming ammonium salts
Naming ammonium salts