Chapter 2 Alkanes Nomenclature and an Introduction to

- Slides: 28

Chapter 2 Alkanes: Nomenclature and an Introduction to Synthesis

t Functional Groups èFunctional group families are characterized by the presence of a certain arrangement of atoms called a functional group èA functional group is the site of most chemical reactivity of a molecule H The functional group is responsible for many of the physical properties of a molecule èAlkanes do not have a functional groups H Carbon-carbon single bonds and carbon-hydrogen bonds are generally very unreactive Chapter 4 2

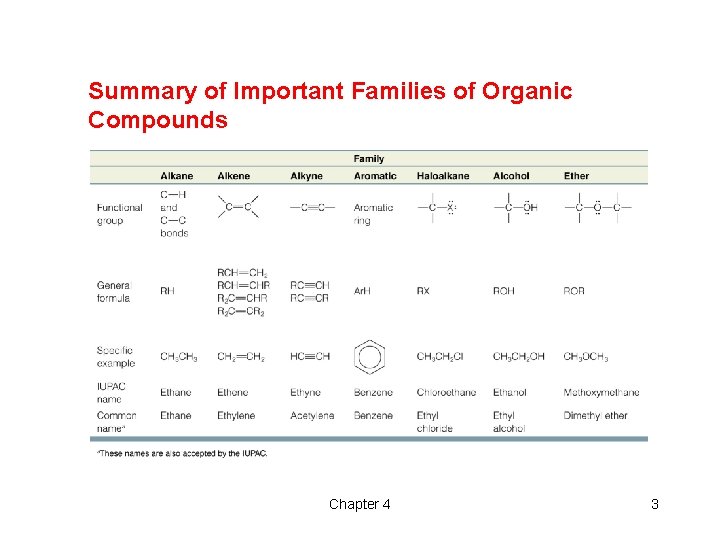
Summary of Important Families of Organic Compounds Chapter 4 3

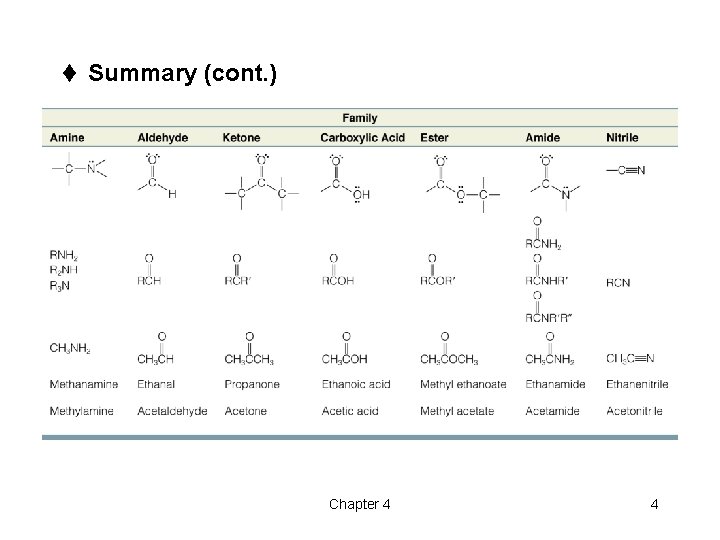
t Summary (cont. ) Chapter 4 4

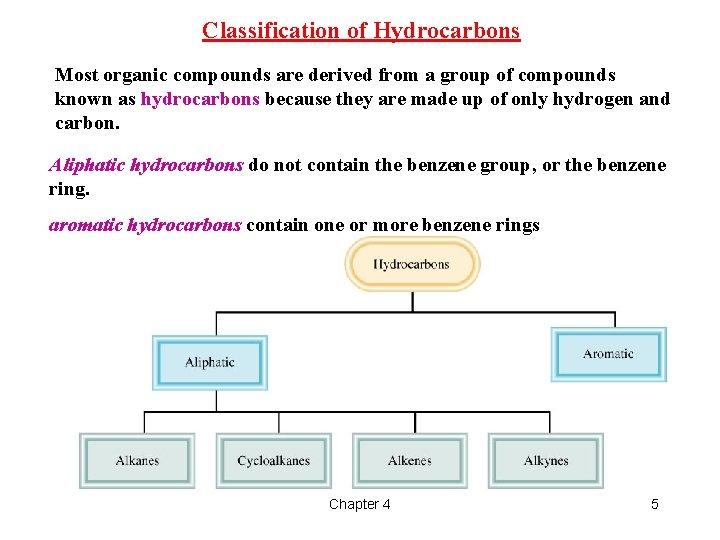
Classification of Hydrocarbons Most organic compounds are derived from a group of compounds known as hydrocarbons because they are made up of only hydrogen and carbon. Aliphatic hydrocarbons do not contain the benzene group, or the benzene ring. aromatic hydrocarbons contain one or more benzene rings Chapter 4 5

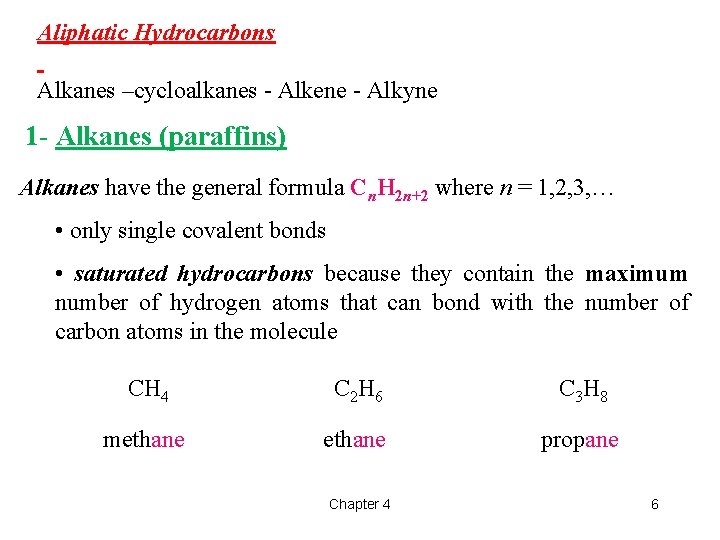
Aliphatic Hydrocarbons Alkanes –cycloalkanes - Alkene - Alkyne 1 - Alkanes (paraffins) Alkanes have the general formula Cn. H 2 n+2 where n = 1, 2, 3, … • only single covalent bonds • saturated hydrocarbons because they contain the maximum number of hydrogen atoms that can bond with the number of carbon atoms in the molecule CH 4 C 2 H 6 C 3 H 8 methane propane Chapter 4 6

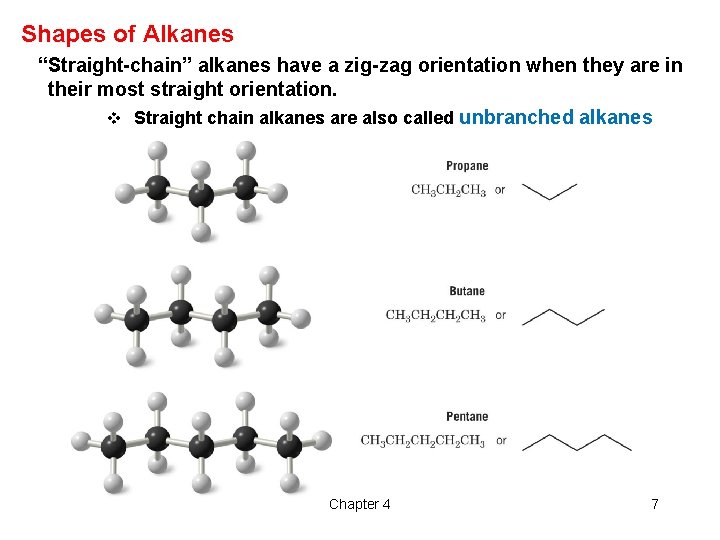
Shapes of Alkanes “Straight-chain” alkanes have a zig-zag orientation when they are in their most straight orientation. v Straight chain alkanes are also called unbranched alkanes Chapter 4 7

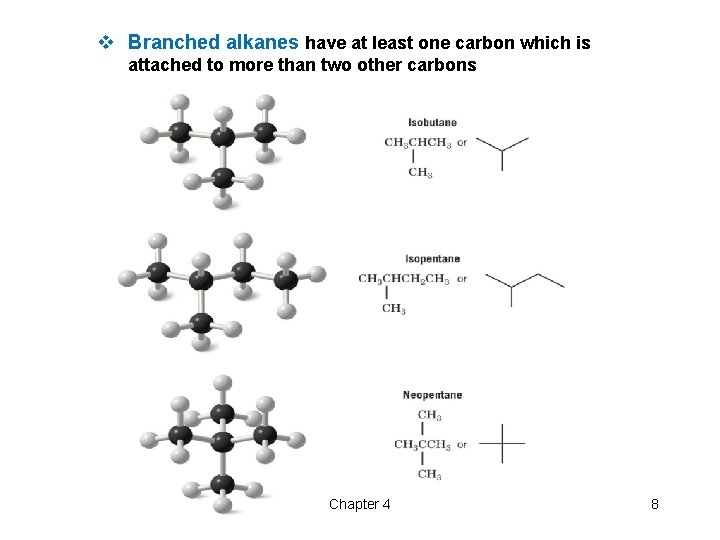
v Branched alkanes have at least one carbon which is attached to more than two other carbons Chapter 4 8

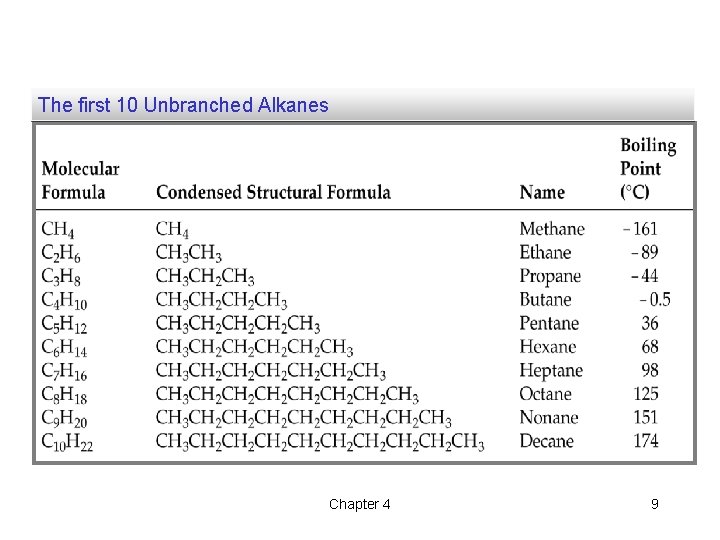
The first 10 Unbranched Alkanes Chapter 4 9

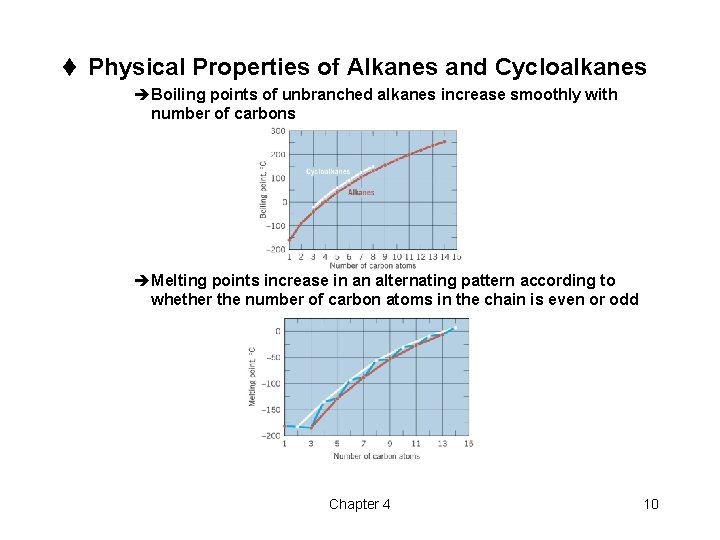
t Physical Properties of Alkanes and Cycloalkanes èBoiling points of unbranched alkanes increase smoothly with number of carbons èMelting points increase in an alternating pattern according to whether the number of carbon atoms in the chain is even or odd Chapter 4 10

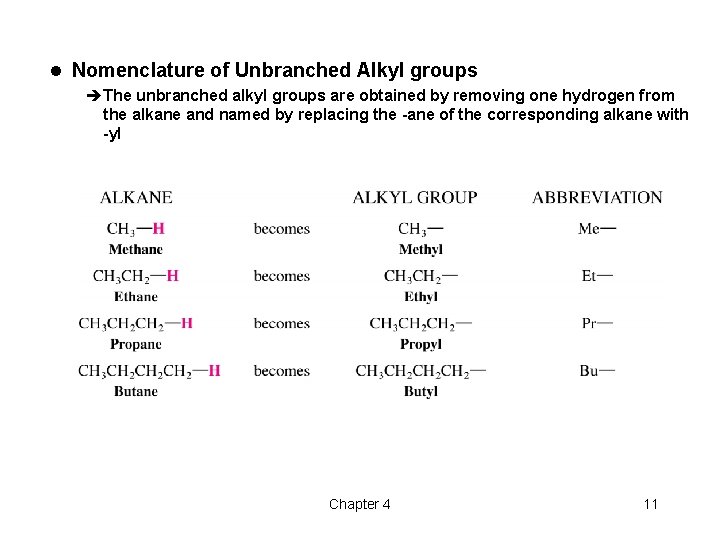
l Nomenclature of Unbranched Alkyl groups èThe unbranched alkyl groups are obtained by removing one hydrogen from the alkane and named by replacing the -ane of the corresponding alkane with -yl Chapter 4 11

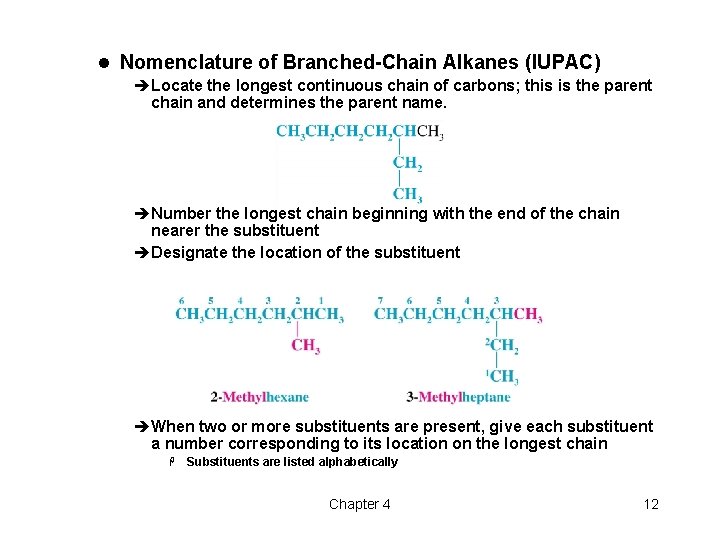
l Nomenclature of Branched-Chain Alkanes (IUPAC) èLocate the longest continuous chain of carbons; this is the parent chain and determines the parent name. èNumber the longest chain beginning with the end of the chain nearer the substituent èDesignate the location of the substituent èWhen two or more substituents are present, give each substituent a number corresponding to its location on the longest chain H Substituents are listed alphabetically Chapter 4 12

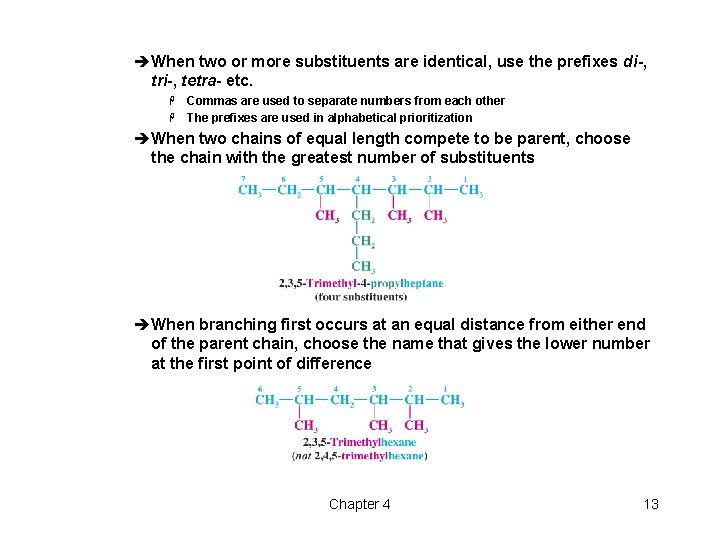
èWhen two or more substituents are identical, use the prefixes di-, tri-, tetra- etc. Commas are used to separate numbers from each other H The prefixes are used in alphabetical prioritization H èWhen two chains of equal length compete to be parent, choose the chain with the greatest number of substituents èWhen branching first occurs at an equal distance from either end of the parent chain, choose the name that gives the lower number at the first point of difference Chapter 4 13

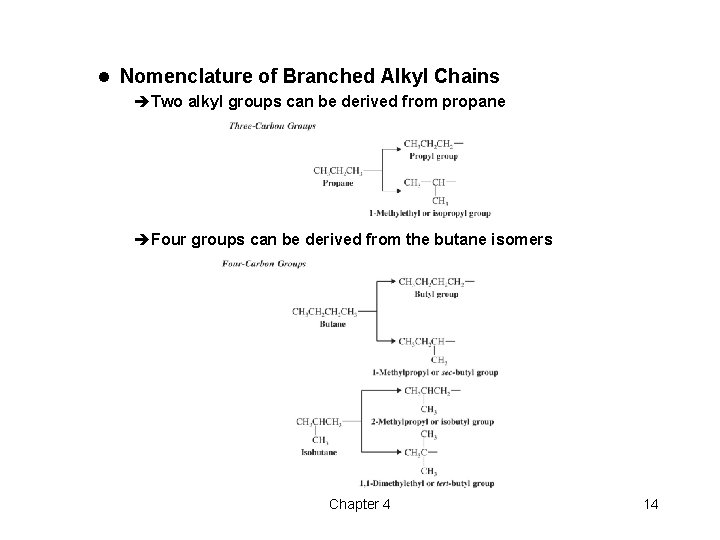
l Nomenclature of Branched Alkyl Chains èTwo alkyl groups can be derived from propane èFour groups can be derived from the butane isomers Chapter 4 14

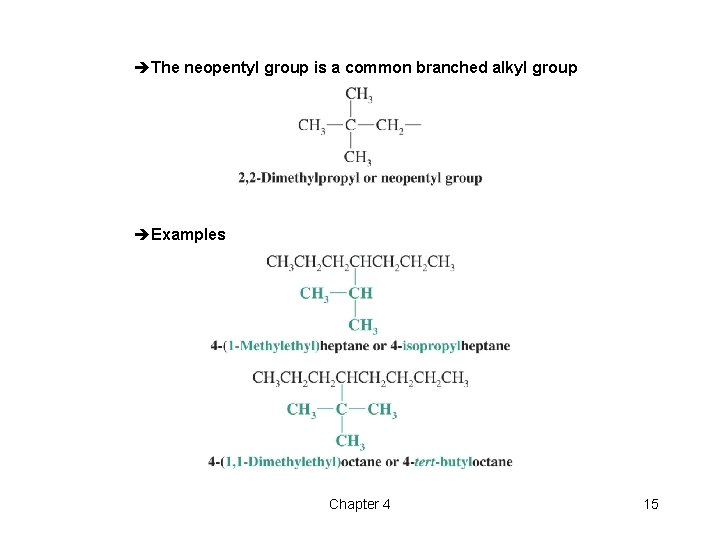
èThe neopentyl group is a common branched alkyl group èExamples Chapter 4 15

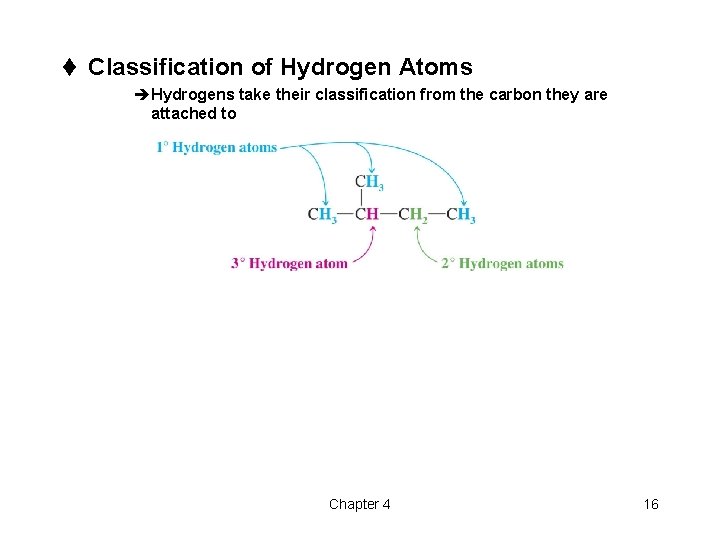
t Classification of Hydrogen Atoms èHydrogens take their classification from the carbon they are attached to Chapter 4 16

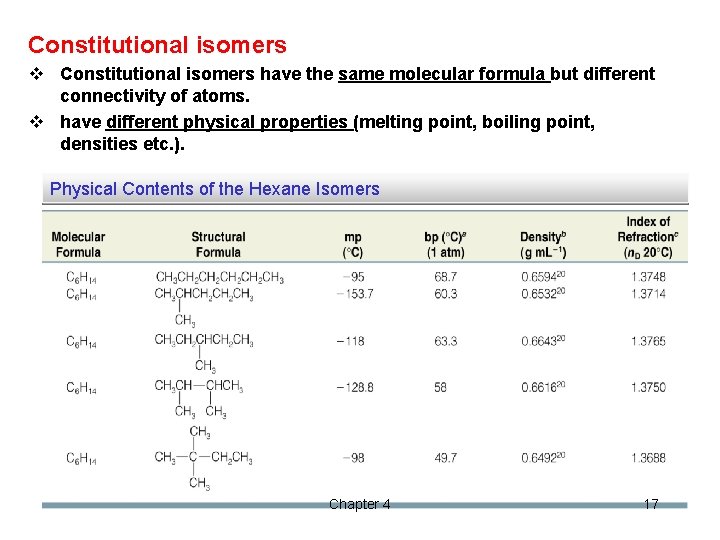
Constitutional isomers v Constitutional isomers have the same molecular formula but different connectivity of atoms. v have different physical properties (melting point, boiling point, densities etc. ). Physical Contents of the Hexane Isomers Chapter 4 17

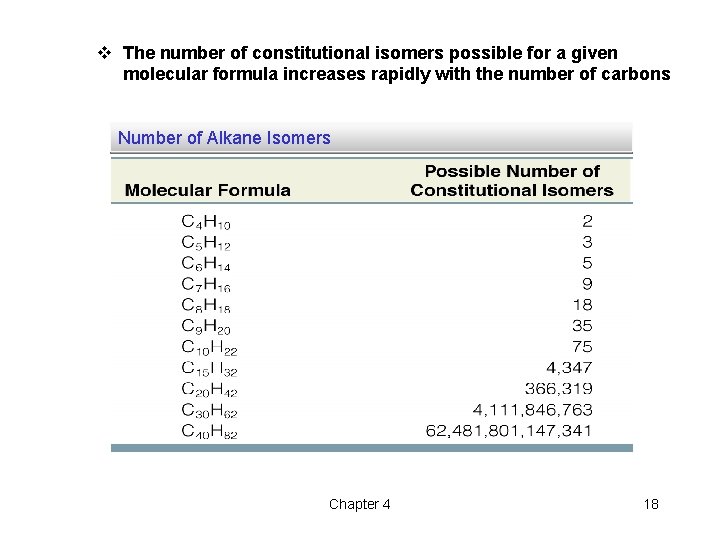
v The number of constitutional isomers possible for a given molecular formula increases rapidly with the number of carbons Number of Alkane Isomers Chapter 4 18

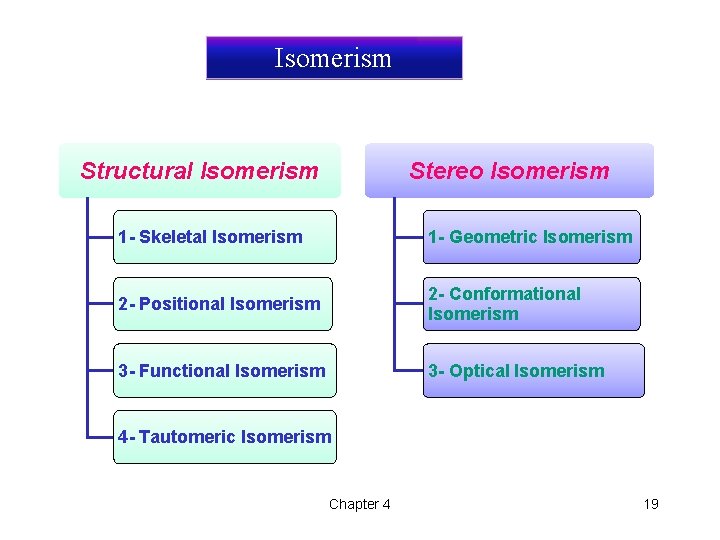
Isomerism Structural Isomerism Stereo Isomerism 1 - Skeletal Isomerism 1 - Geometric Isomerism 2 - Positional Isomerism 2 - Conformational Isomerism 3 - Functional Isomerism 3 - Optical Isomerism 4 - Tautomeric Isomerism Chapter 4 19

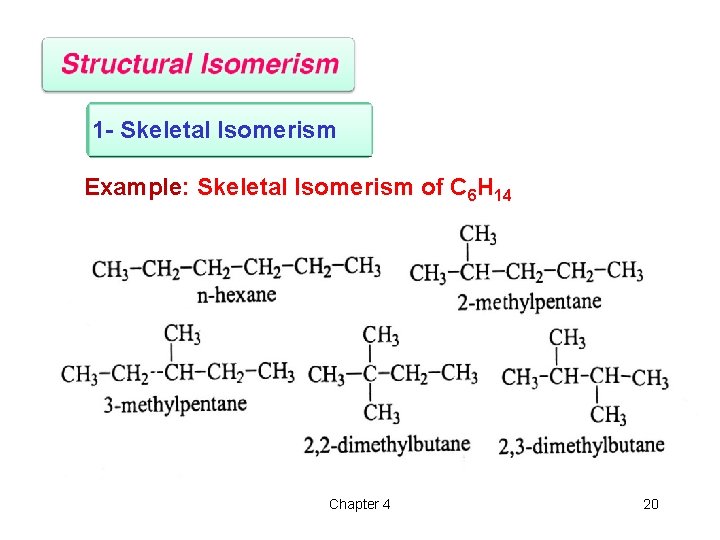
1 - Skeletal Isomerism Example: Skeletal Isomerism of C 6 H 14 Chapter 4 20

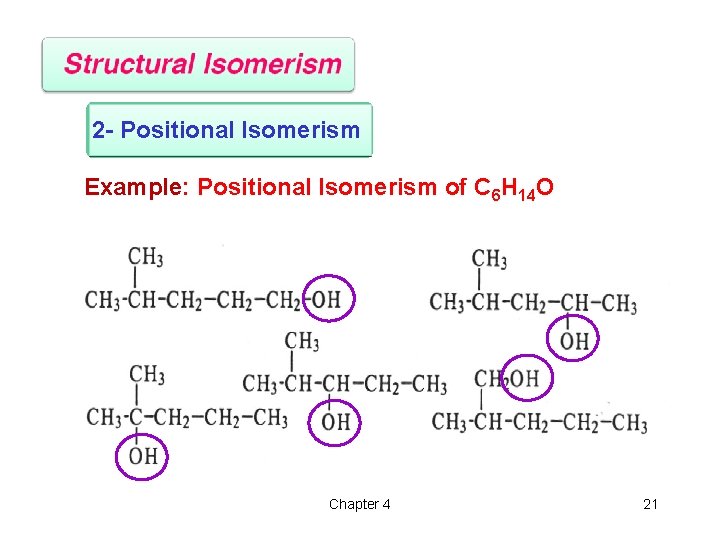
2 - Positional Isomerism Example: Positional Isomerism of C 6 H 14 O Chapter 4 21

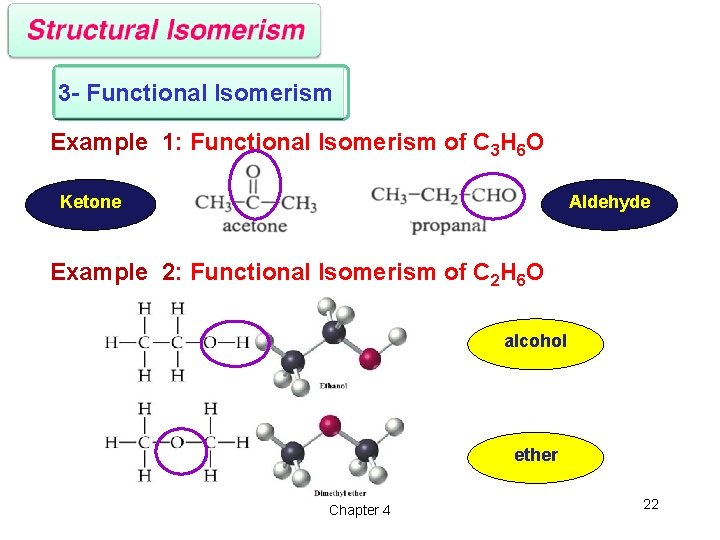
3 - Functional Isomerism Example 1: Functional Isomerism of C 3 H 6 O Ketone Aldehyde Example 2: Functional Isomerism of C 2 H 6 O alcohol ether Chapter 4 22

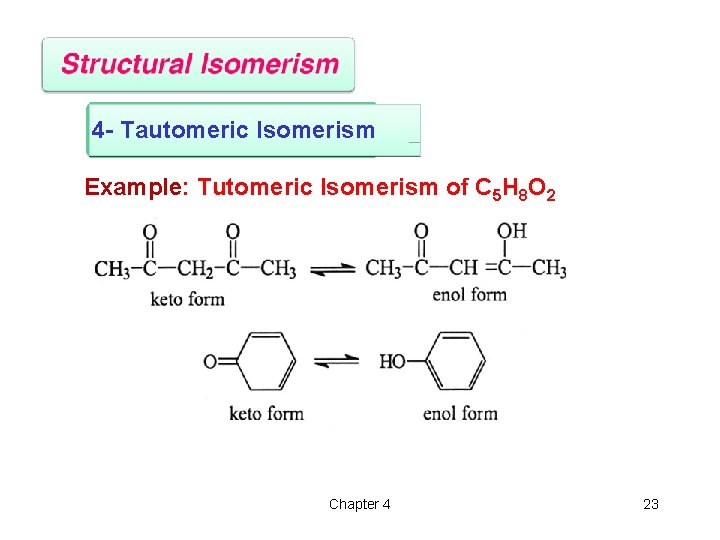
4 - Tautomeric Isomerism Example: Tutomeric Isomerism of C 5 H 8 O 2 Chapter 4 23

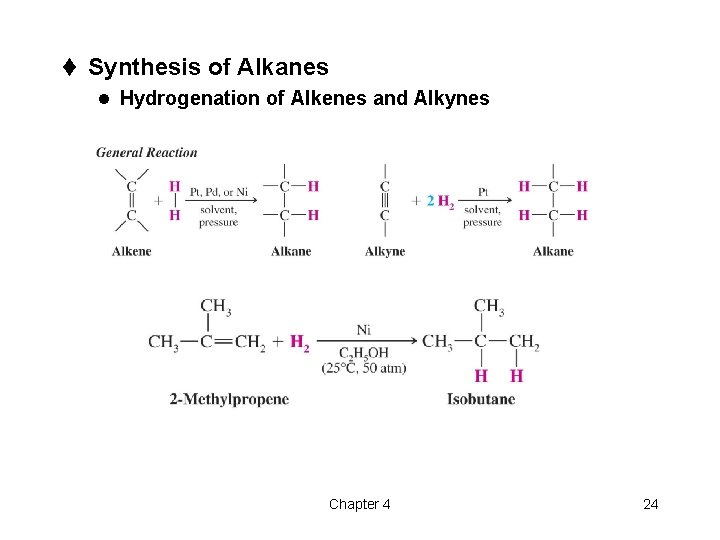
t Synthesis of Alkanes l Hydrogenation of Alkenes and Alkynes Chapter 4 24

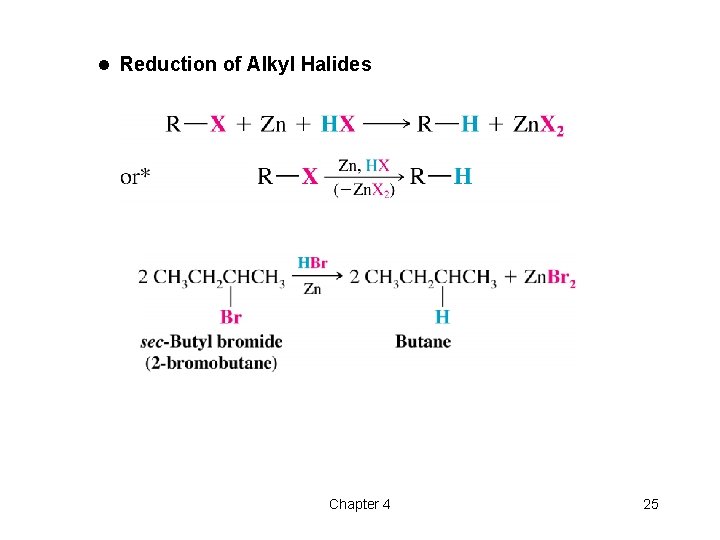
l Reduction of Alkyl Halides Chapter 4 25

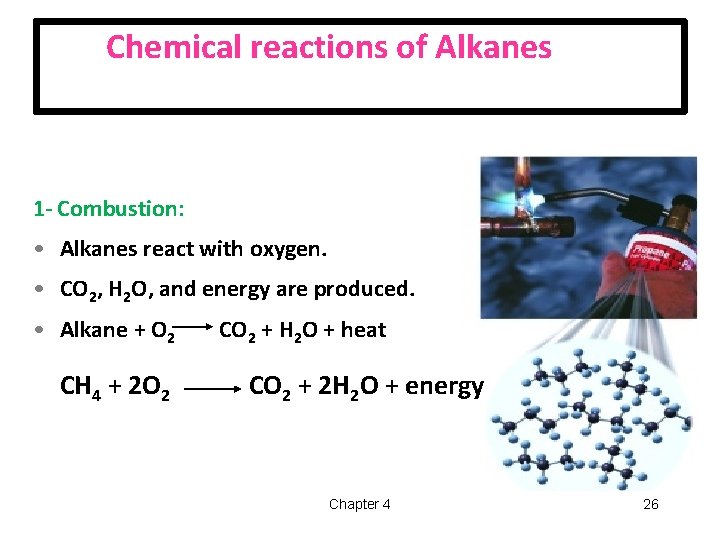
Chemical reactions of Alkanes 1 - Combustion: • Alkanes react with oxygen. • CO 2, H 2 O, and energy are produced. • Alkane + O 2 CH 4 + 2 O 2 CO 2 + H 2 O + heat CO 2 + 2 H 2 O + energy Chapter 4 26

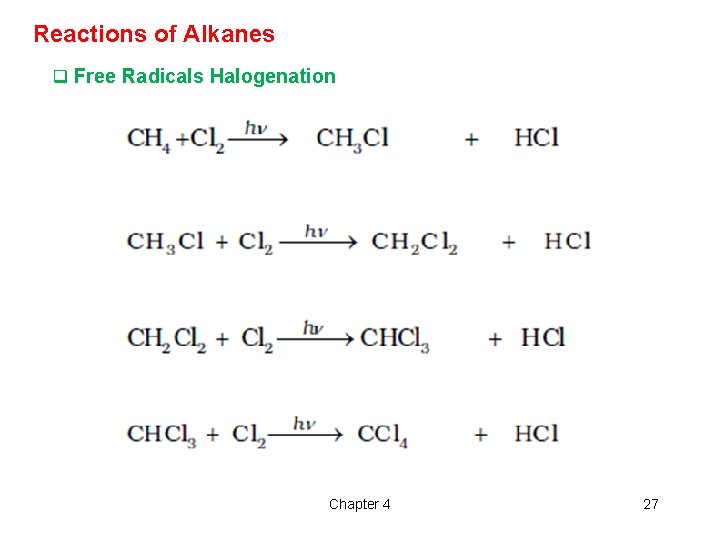
Reactions of Alkanes q Free Radicals Halogenation Chapter 4 27

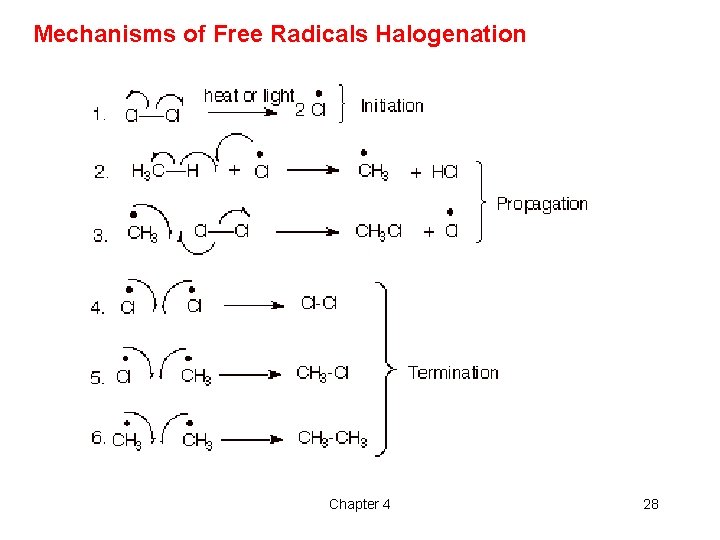
Mechanisms of Free Radicals Halogenation Chapter 4 28