Summary from Organic Chemistry Packet Introduction to organic



- Slides: 14

Summary from Organic Chemistry Packet: Introduction to organic chemistry

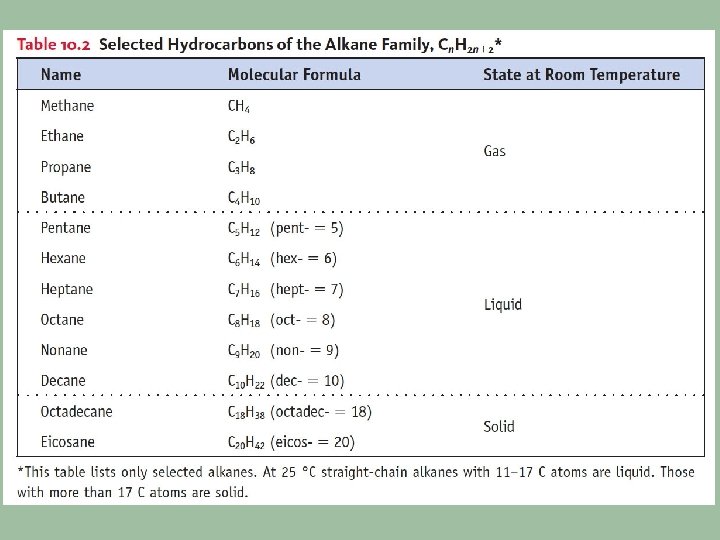
1. Nomenclature based on alkanes • Know alkanes for C 1 -C 10 • Recognize the formula/name of – Alkenes (double bond) – Alkynes (triple bond) – Cycloalkanes (ring structure) Ex: Write the chemical formula and structural formula for: Octane 2 - Butyne 1, 3 - Heptadiene

Hydrocarbons • Compounds of C and H and often a third or more additional elements • Subgroups: – Alkanes: Containing only C-C single bonds – Alkenes: C=C double bonds as well as single bonds – Alkynes: carbon-carbon triple bonds as well as single bonds. – Aromatic: hydrocarbons containing benzene rings.


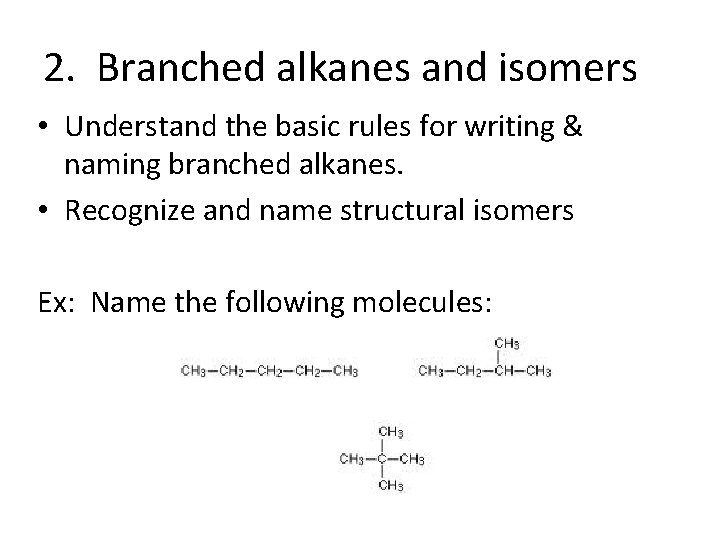
2. Branched alkanes and isomers • Understand the basic rules for writing & naming branched alkanes. • Recognize and name structural isomers Ex: Name the following molecules:

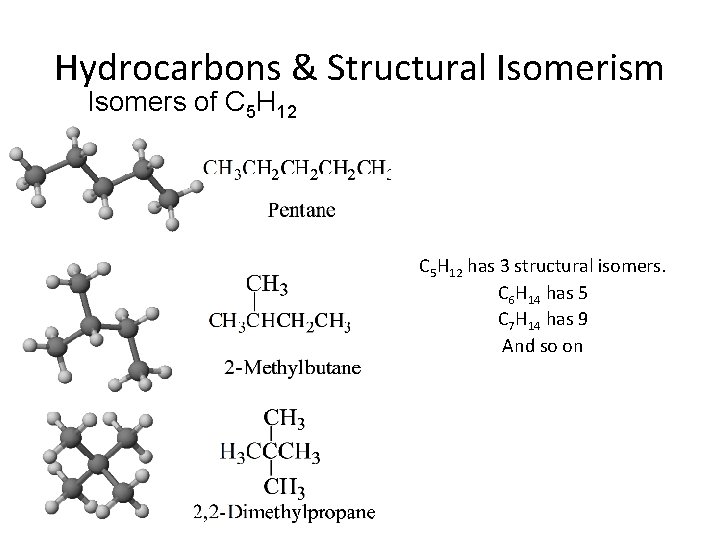
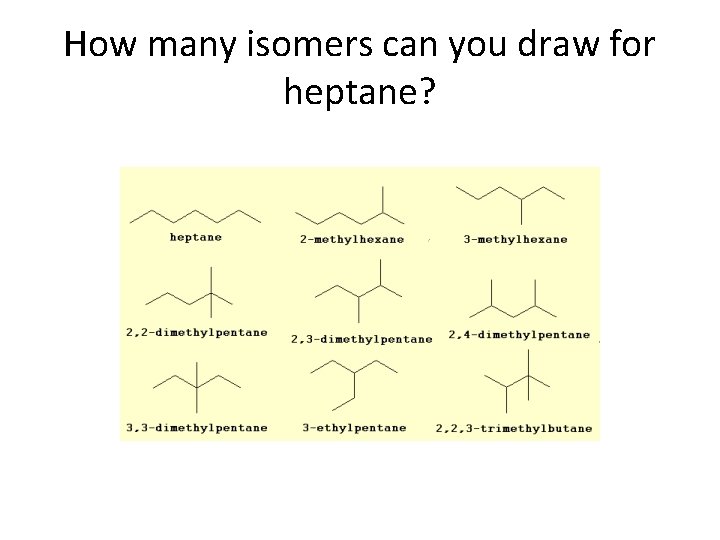
Hydrocarbons & Structural Isomerism Isomers of C 5 H 12 has 3 structural isomers. C 6 H 14 has 5 C 7 H 14 has 9 And so on

How many isomers can you draw for heptane?

Alkenes: Compounds with C=C Double Bonds • Result of loss of free rotation about the C=C brings about “Geometric Isomers” • Increase in reactivity over alkanes • X-Y addition reactions are possible • X 2, HX and H 2 O can add across the double bond.

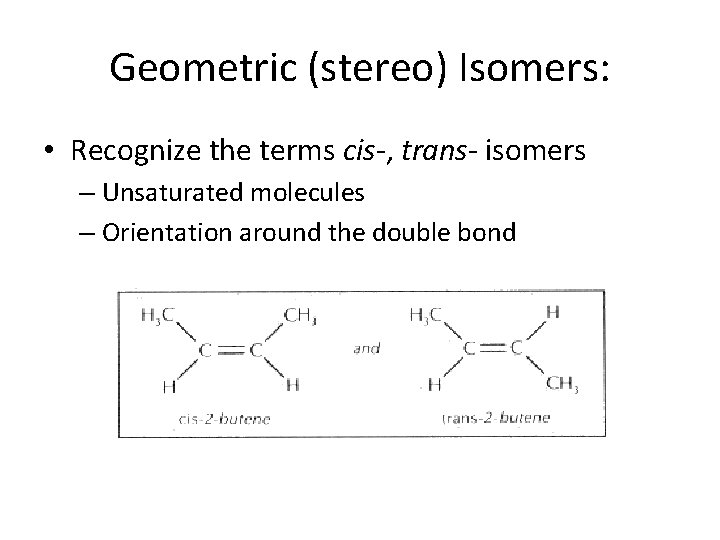
Geometric (stereo) Isomers: • Recognize the terms cis-, trans- isomers – Unsaturated molecules – Orientation around the double bond

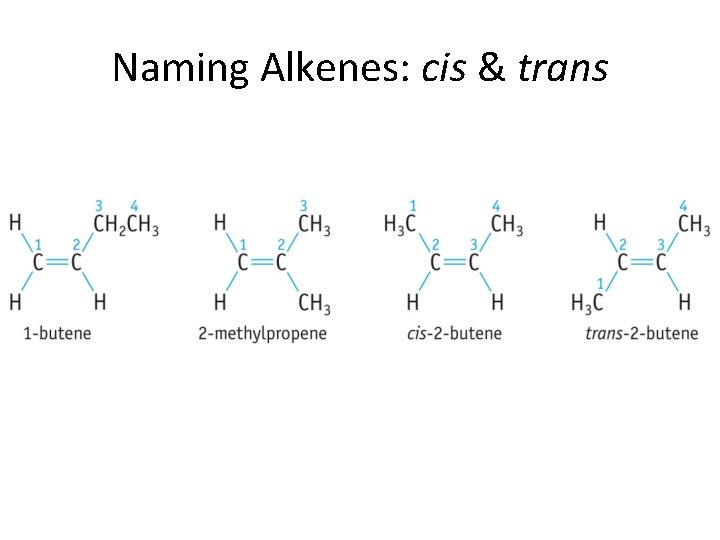
Naming Alkenes: cis & trans

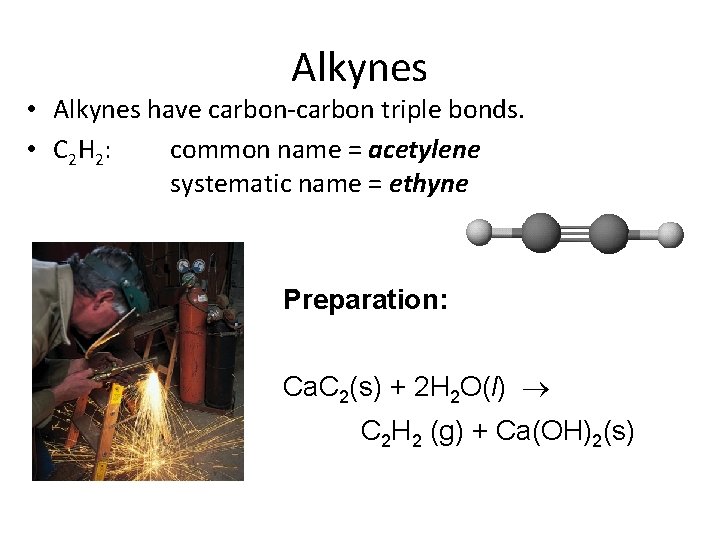
Alkynes • Alkynes have carbon-carbon triple bonds. • C 2 H 2 : common name = acetylene systematic name = ethyne Preparation: Ca. C 2(s) + 2 H 2 O(l) C 2 H 2 (g) + Ca(OH)2(s)

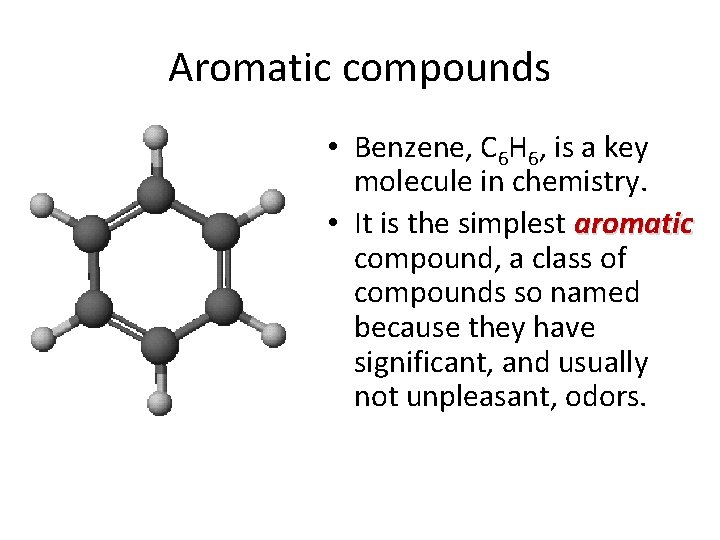
Aromatic compounds • Benzene, C 6 H 6, is a key molecule in chemistry. • It is the simplest aromatic compound, a class of compounds so named because they have significant, and usually not unpleasant, odors.

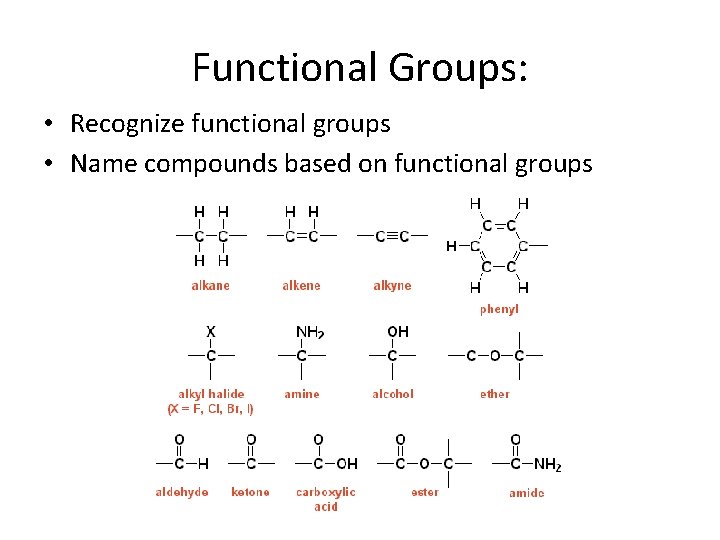
Functional Groups: • Recognize functional groups • Name compounds based on functional groups

Organic chemistry & reactions • Be familiar with some basic properties of an organic reaction: – – Combustion (know this for sure!) Condensation (H 2 O removed) Addition ( of a halogen to an unsaturated alkane) Substitution (of a halide)
 Ib chemistry organic chemistry
Ib chemistry organic chemistry Inorganic vs organic chemistry
Inorganic vs organic chemistry Importance of organic compounds
Importance of organic compounds Basic organic nomenclature packet
Basic organic nomenclature packet Geometry sol review packet
Geometry sol review packet Reference table n chemistry
Reference table n chemistry Numbering carbon chains
Numbering carbon chains Canola oil
Canola oil Ester organic chemistry
Ester organic chemistry Alkene formula
Alkene formula Rearranged most stable carbocation is
Rearranged most stable carbocation is Ee organic chemistry
Ee organic chemistry Ario organic chemistry
Ario organic chemistry Thermodynamic vs kinetic control
Thermodynamic vs kinetic control David klein organic chemistry 3rd edition
David klein organic chemistry 3rd edition


























