Nucleic Acids We have studied three other sets
- Slides: 21

Nucleic Acids

We have studied three other sets of Macromolecules: Carbohydrates, lipids, & proteins The 4 th macromolecule used by organisms: Nucleic Acids

Two main types: DNA (deoxyribonucleic acid) - found in the nucleus RNA (ribonucleic acid) – found all over the cell DNA functions: • Store genetic information “blueprint” • Transfer genetic blueprint to other generations • Controls many cellular functions RNA functions: • Carries genetic information out of nucleus • Builds proteins

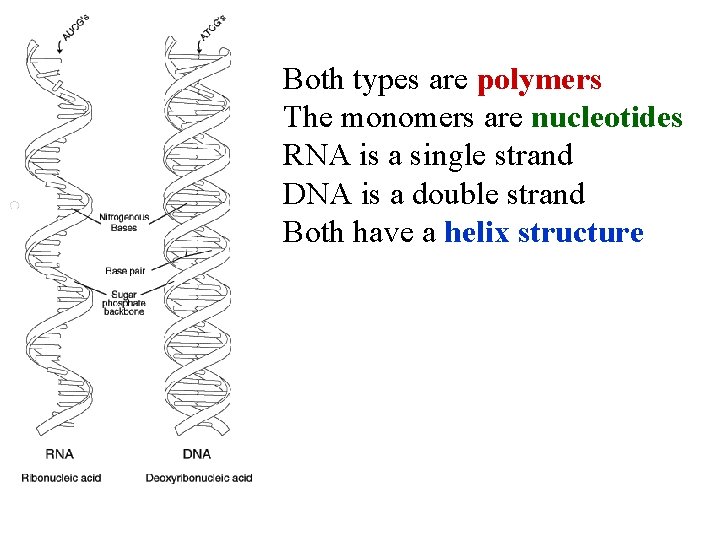
Both types are polymers The monomers are nucleotides RNA is a single strand DNA is a double strand Both have a helix structure

The basic unit for DNA and RNA is a “nucleotide” Composed of 3 parts: 1. a nitrogenous base (1 of 5 types) 2. a pentose sugar (1 of 2 types) 3. a phosphate

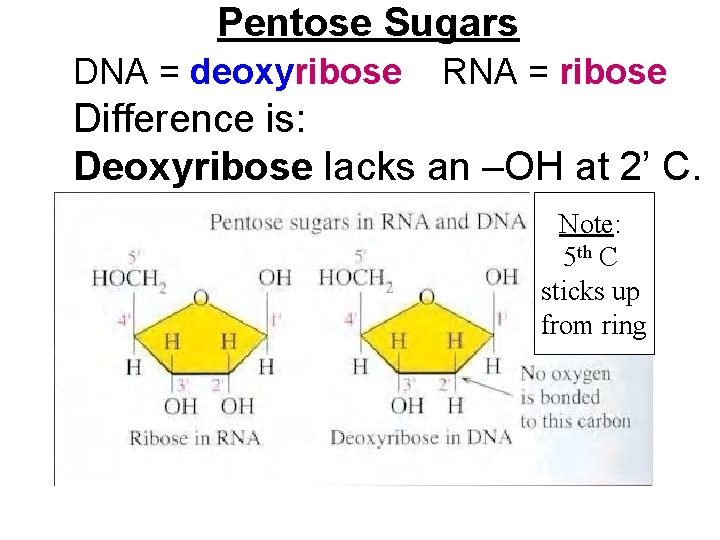
Pentose Sugars DNA = deoxyribose RNA = ribose Difference is: Deoxyribose lacks an –OH at 2’ C. Note: 5 th C sticks up from ring

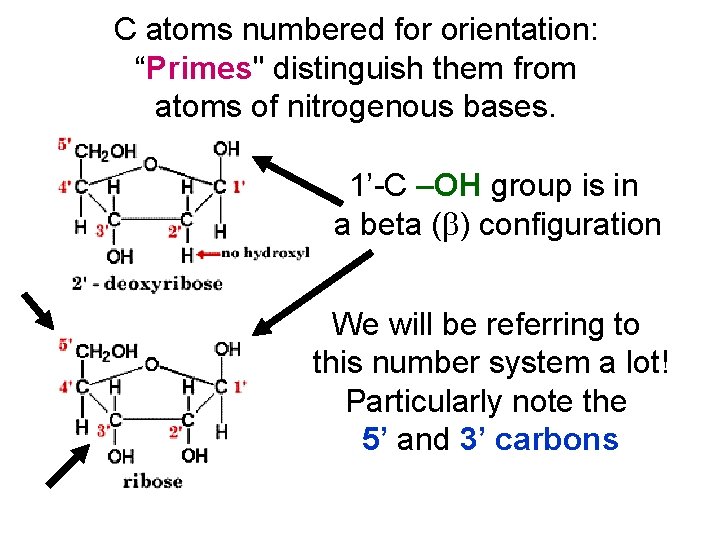
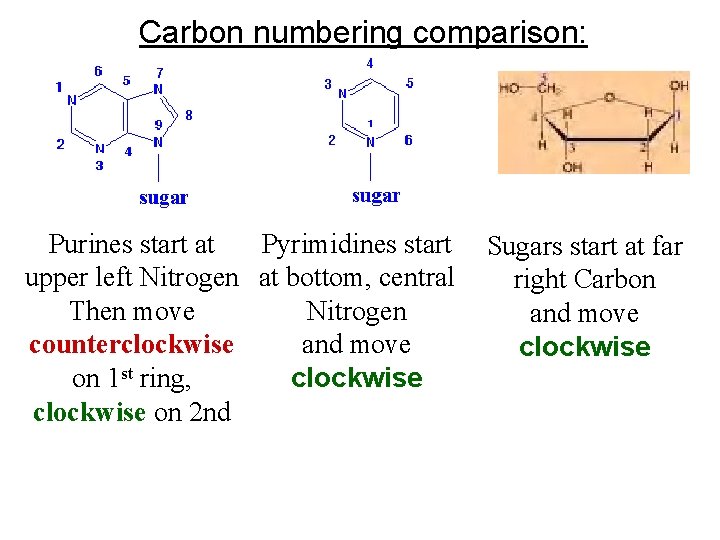
C atoms numbered for orientation: “Primes" distinguish them from atoms of nitrogenous bases. 1’-C –OH group is in a beta ( ) configuration We will be referring to this number system a lot! Particularly note the 5’ and 3’ carbons

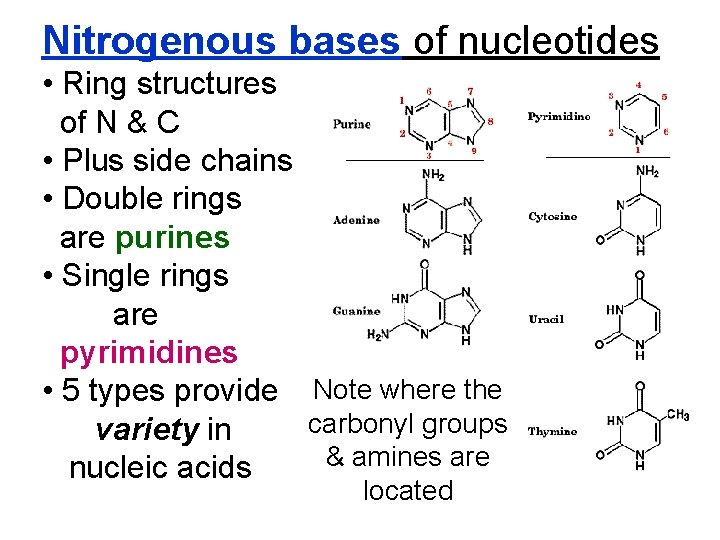
Nitrogenous bases of nucleotides • Ring structures of N & C • Plus side chains • Double rings are purines • Single rings are pyrimidines • 5 types provide Note where the carbonyl groups variety in & amines are nucleic acids located

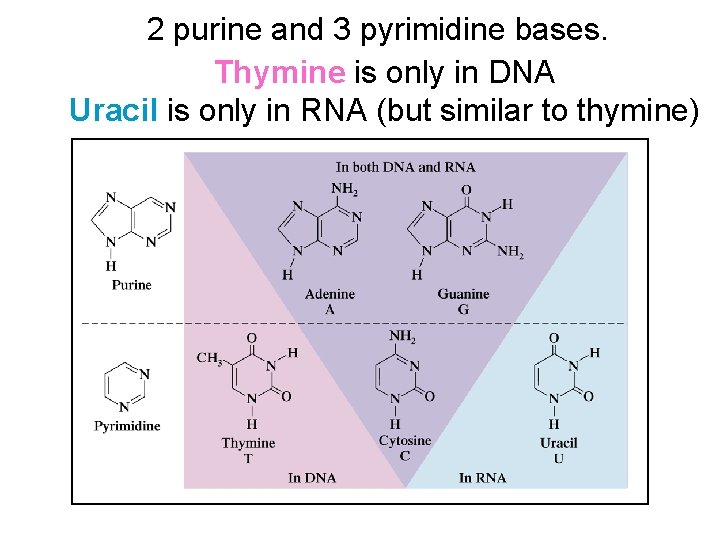
2 purine and 3 pyrimidine bases. Thymine is only in DNA Uracil is only in RNA (but similar to thymine)

Carbon numbering comparison: Purines start at Pyrimidines start upper left Nitrogen at bottom, central Then move Nitrogen counterclockwise and move on 1 st ring, clockwise on 2 nd Sugars start at far right Carbon and move clockwise

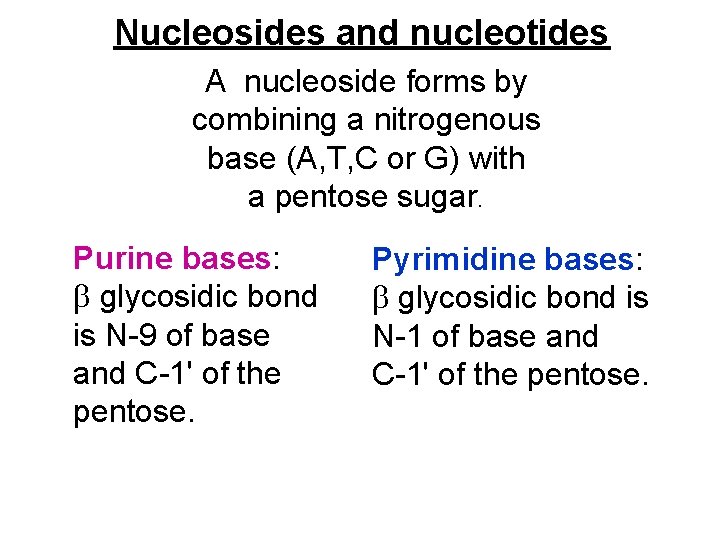
Nucleosides and nucleotides A nucleoside forms by combining a nitrogenous base (A, T, C or G) with a pentose sugar. Purine bases: glycosidic bond is N-9 of base and C-1' of the pentose. Pyrimidine bases: glycosidic bond is N-1 of base and C-1' of the pentose.

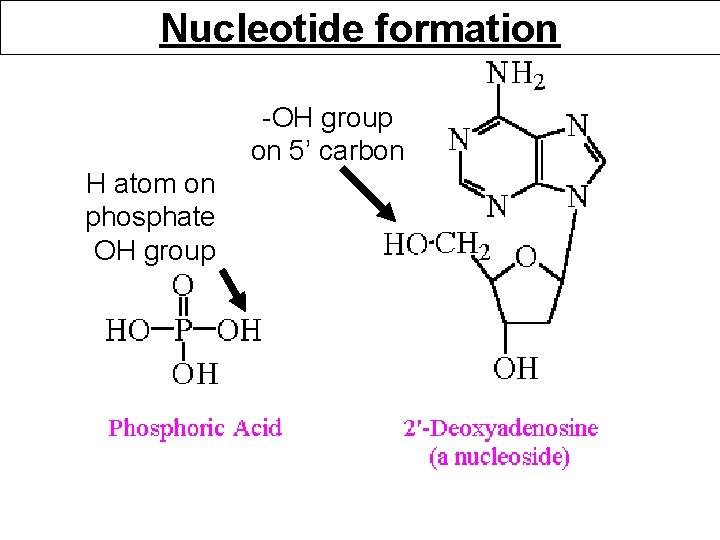
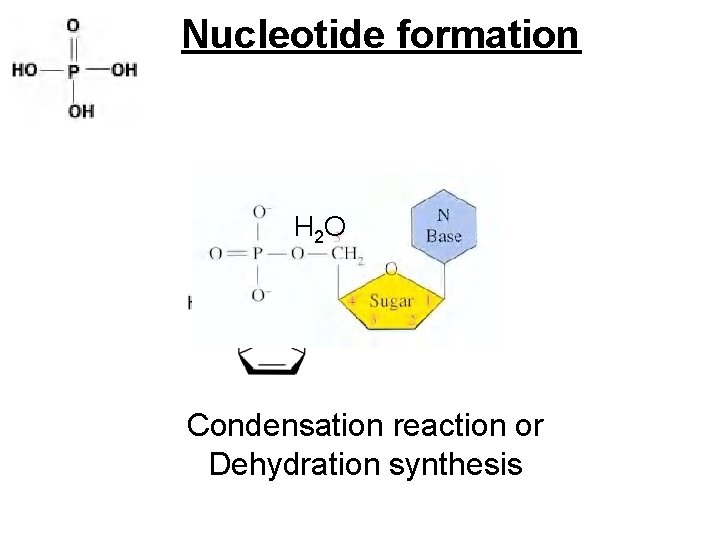
Nucleotide formation -OH group on 5’ carbon H atom on phosphate OH group

Nucleotide formation H 2 O Condensation reaction or Dehydration synthesis

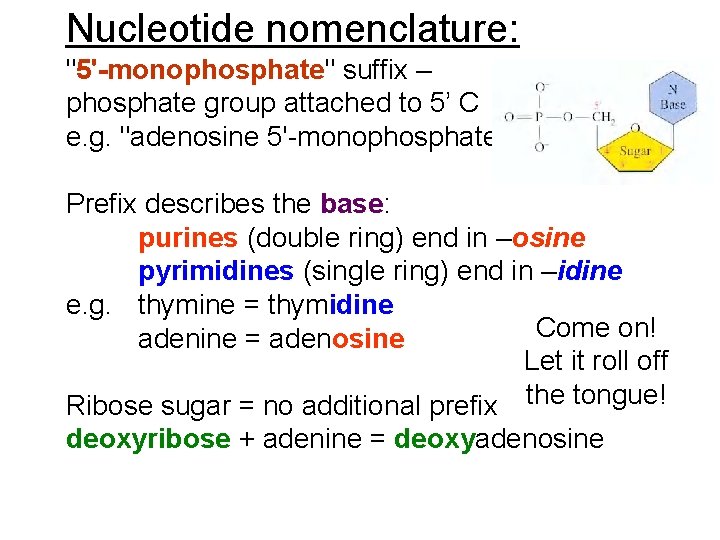
Nucleotide nomenclature: "5'-monophosphate" suffix – phosphate group attached to 5’ C e. g. "adenosine 5'-monophosphate". Prefix describes the base: purines (double ring) end in –osine pyrimidines (single ring) end in –idine e. g. thymine = thymidine Come on! adenine = adenosine Let it roll off Ribose sugar = no additional prefix the tongue! deoxyribose + adenine = deoxyadenosine

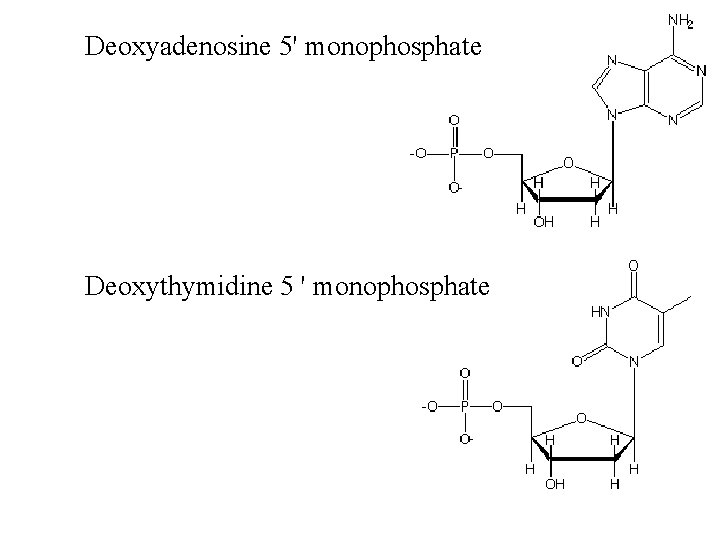
Deoxyadenosine 5' monophosphate Deoxythymidine 5 ' monophosphate

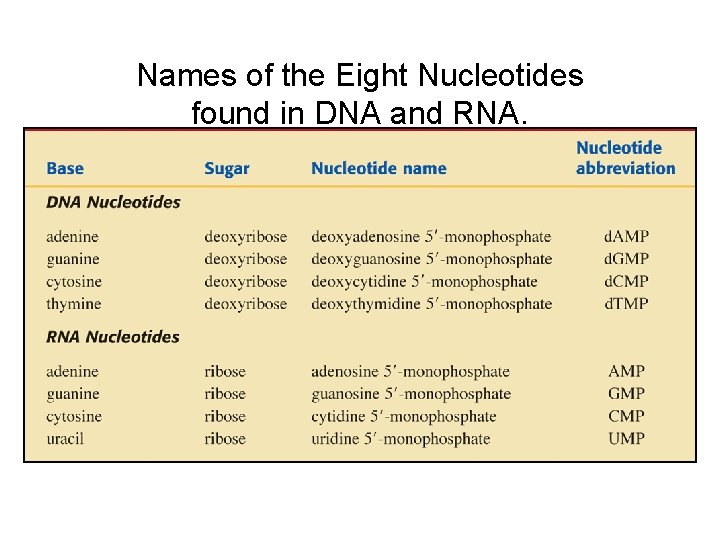
Names of the Eight Nucleotides found in DNA and RNA.

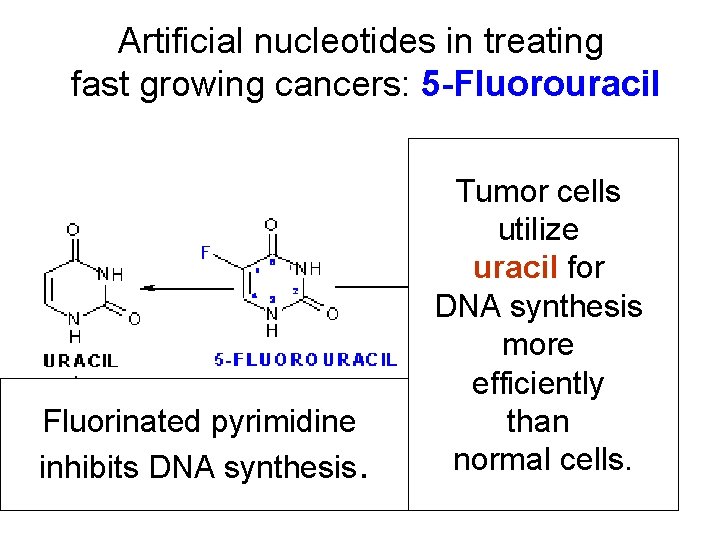
Artificial nucleotides in treating fast growing cancers: 5 -Fluorouracil Fluorinated pyrimidine inhibits DNA synthesis. Tumor cells utilize uracil for DNA synthesis more efficiently than normal cells.

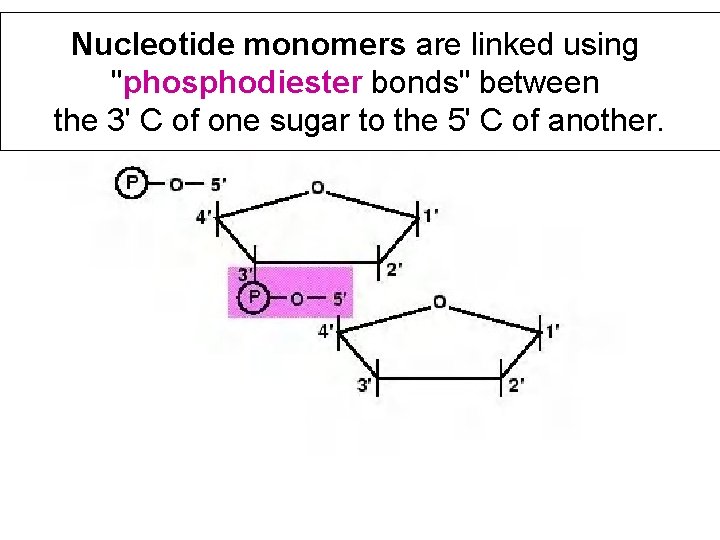
Nucleotide monomers are linked using "phosphodiester bonds" between the 3' C of one sugar to the 5' C of another.

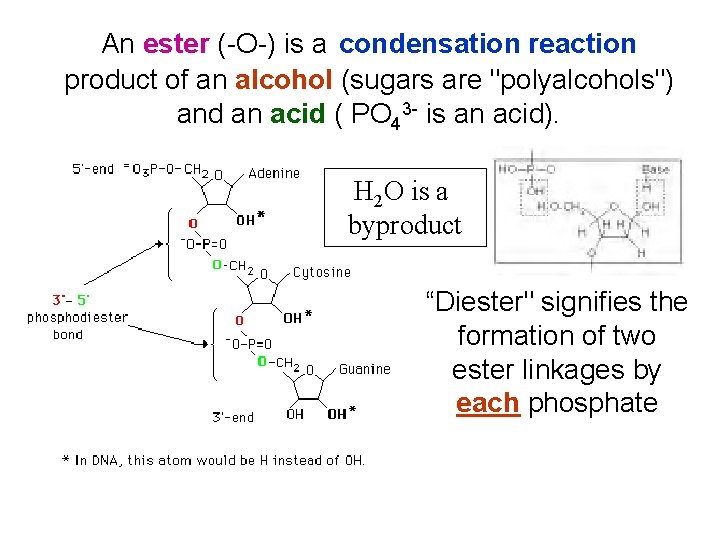
An ester (-O-) is a condensation reaction product of an alcohol (sugars are "polyalcohols") and an acid ( PO 43 - is an acid). H 2 O is a byproduct “Diester" signifies the formation of two ester linkages by each phosphate

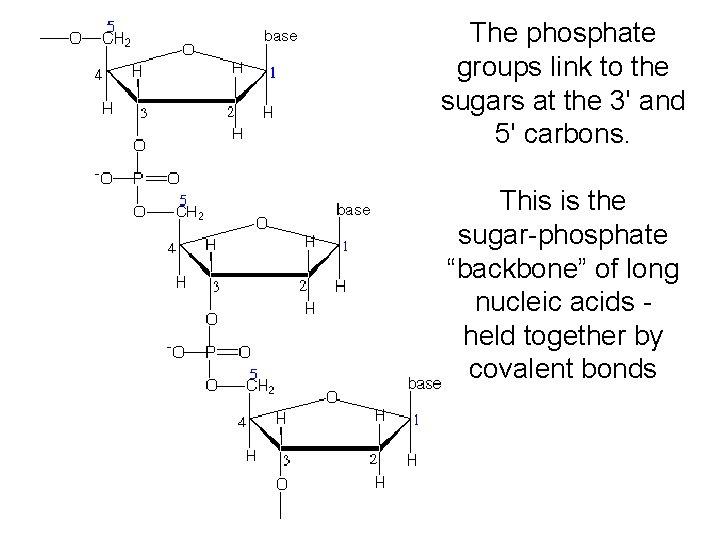
The phosphate groups link to the sugars at the 3' and 5' carbons. This is the sugar-phosphate “backbone” of long nucleic acids held together by covalent bonds

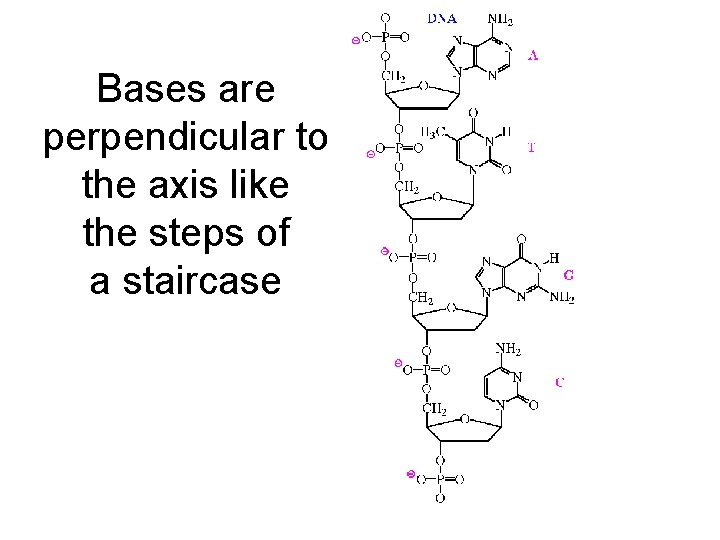
Bases are perpendicular to the axis like the steps of a staircase