Organic Chemistry Functional Groups Nomenclature Functional groups Functional








- Slides: 27

Organic Chemistry: Functional Groups Nomenclature

Functional groups • Functional groups are parts of molecules that result in characteristic features • Note the chemical and physical properties of a compound e. g. properties such as boiling and melting point change due to functional groups • About 100 functional groups exist, we will focus on about 10 • Useful to group the infinite number of possible organic compounds

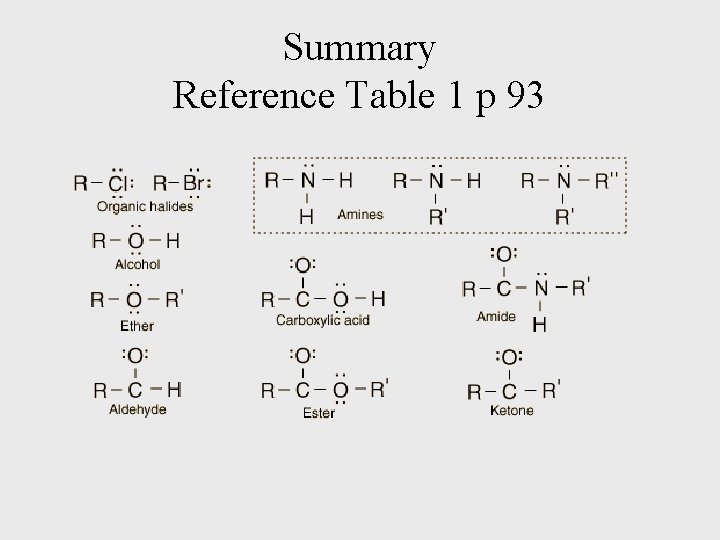
Summary Reference Table 1 p 93

Organic Halides • Halogens attached to a carbon chain are named as a side chain. • The following prefixes are assigned • F-flouro Cl- chloro Br-bromo I-iodo • The location and number is identified • E. g. 1, 2 -dichloro-ethane • HW: Organic halides Q 1, 2 p. 33

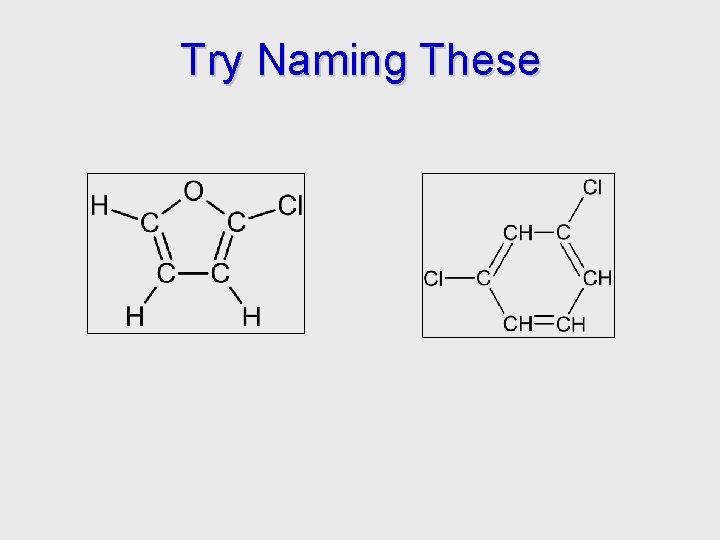
Try Naming These

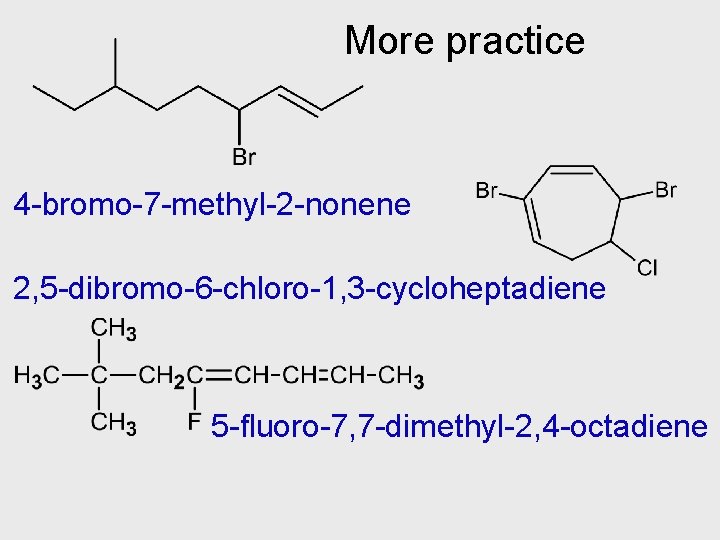
More practice 4 -bromo-7 -methyl-2 -nonene 2, 5 -dibromo-6 -chloro-1, 3 -cycloheptadiene 5 -fluoro-7, 7 -dimethyl-2, 4 -octadiene

Alcohols • • Alcohols contain polar hydroxyl groups -OH Named by using the ending –ol The location is given e. g. 2 -propanol • HW: Alcohols: Q 1 -3 p. 41

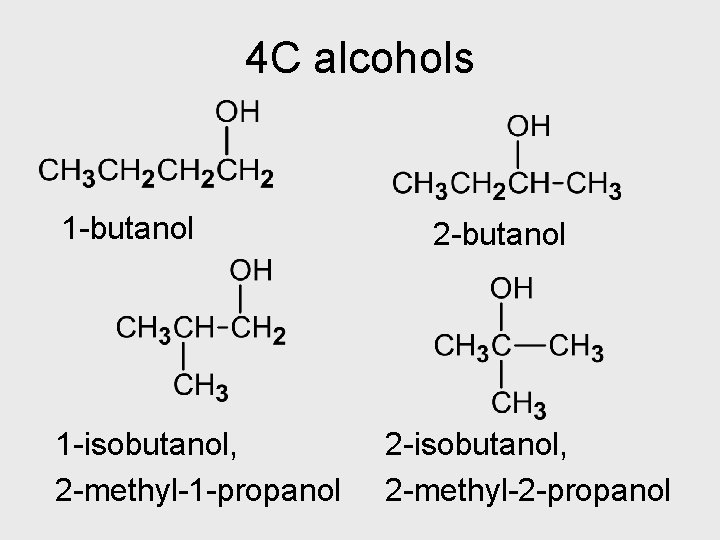
4 C alcohols 1 -butanol 1 -isobutanol, 2 -methyl-1 -propanol 2 -butanol 2 -isobutanol, 2 -methyl-2 -propanol

Ethers • Contain a C-O-C • Named by identify the carbon groups as side chains • E. g. methyl ether • HW: Ethers: Q 11 p 46

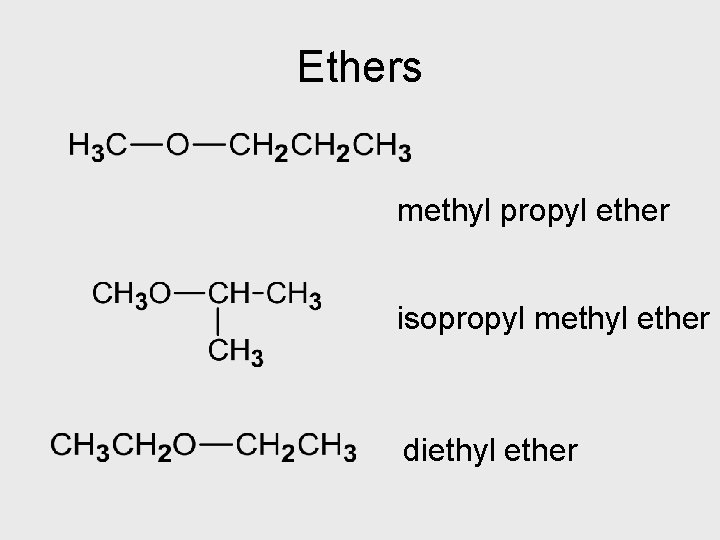
Ethers methyl propyl ether isopropyl methyl ether diethyl ether

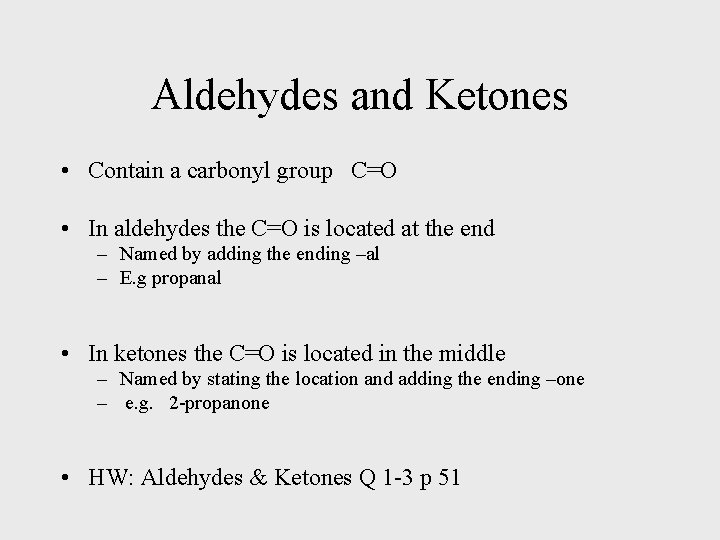
Aldehydes and Ketones • Contain a carbonyl group C=O • In aldehydes the C=O is located at the end – Named by adding the ending –al – E. g propanal • In ketones the C=O is located in the middle – Named by stating the location and adding the ending –one – e. g. 2 -propanone • HW: Aldehydes & Ketones Q 1 -3 p 51

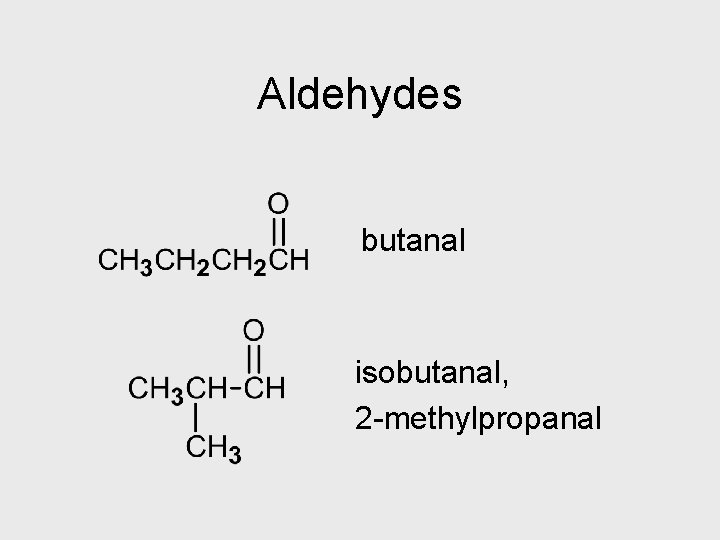
Aldehydes butanal isobutanal, 2 -methylpropanal

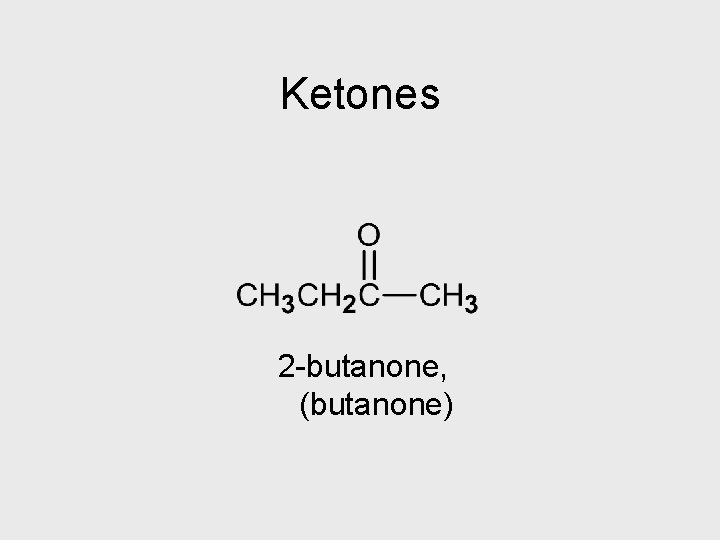
Ketones 2 -butanone, (butanone)

Carboxylic Acids • Contains a carboxyl group • The group is polar and can have a negative charge • The hydrogen on the hydroxyl group can be released to make the substance acidic • Named with the ending oic acid • HW: Carboxylic Acid Q 1&2 p 60

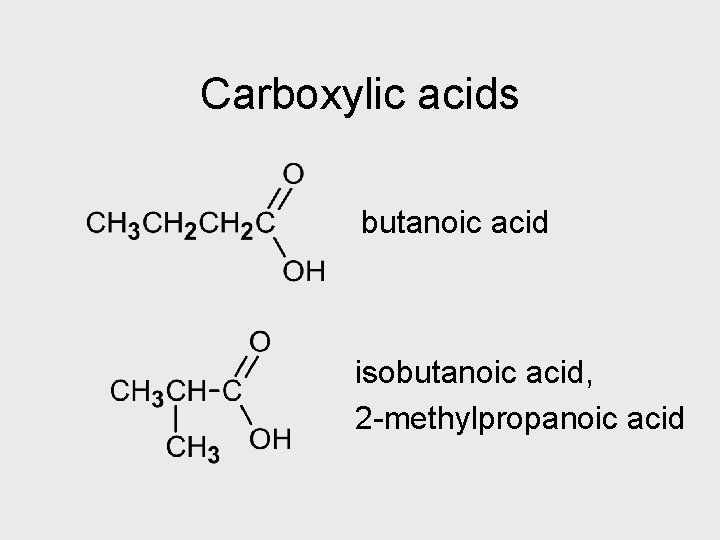
Carboxylic acids butanoic acid isobutanoic acid, 2 -methylpropanoic acid

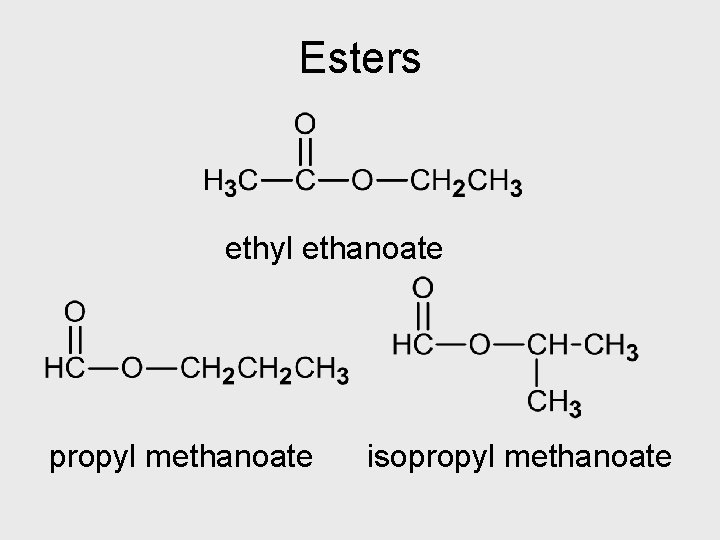
Esters • Esters form from a reaction between an alcohol and a carboxylic acid • Triglicerides contain the ester functional group HW: Esters: Q 11 -12 p 66

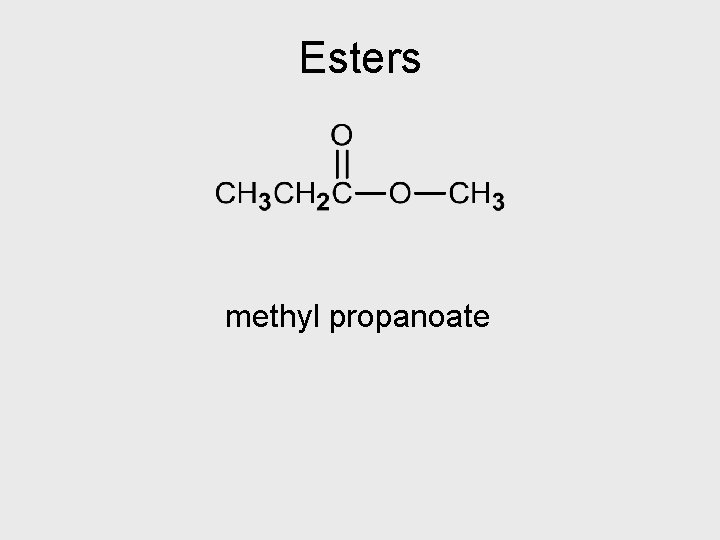
Esters methyl propanoate

Esters ethyl ethanoate propyl methanoate isopropyl methanoate

Hydroxyl, carbonyl, carboxyl • Q: which functional groups contain a hydroxyl group? • A carbonyl group? • A carboxyl group?

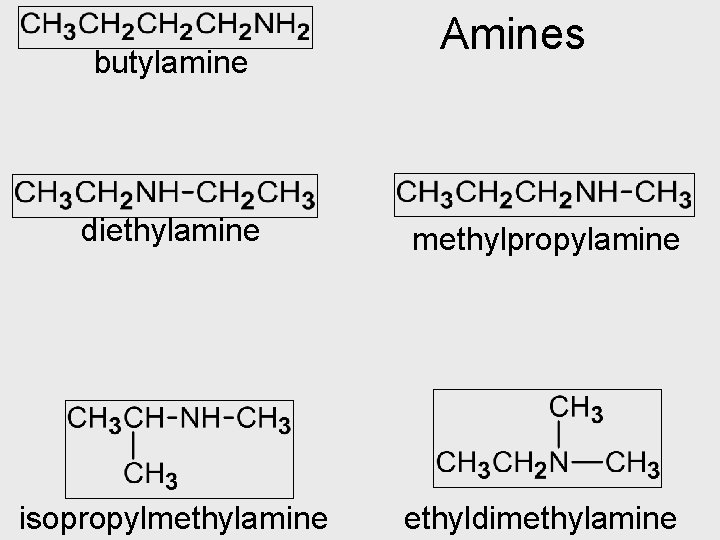
Amines • Contain an amino group -NH 2 • Can be at the end of the chain or have up to 3 chains bonded to it • HW: Amines Q 2, 3 p 72

butylamine Amines diethylamine methylpropylamine isopropylmethylamine ethyldimethylamine

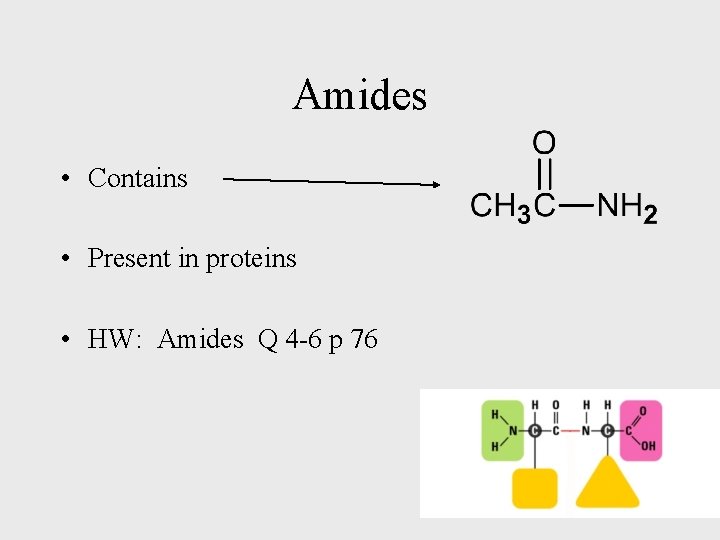
Amides • Contains • Present in proteins • HW: Amides Q 4 -6 p 76

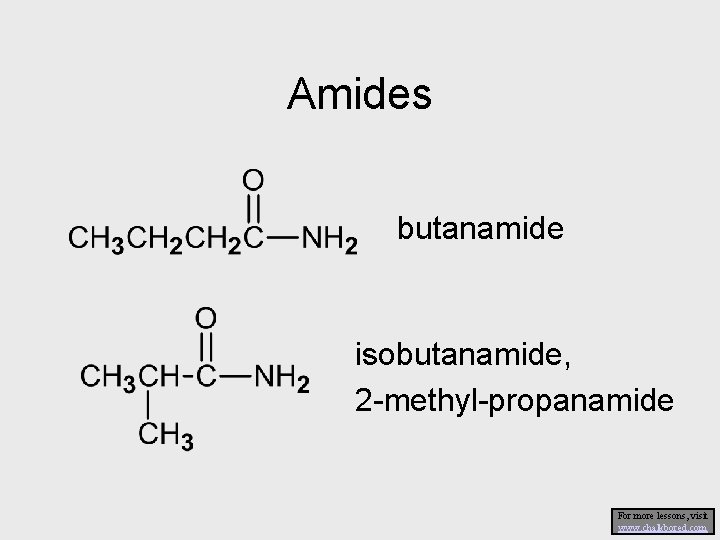
Amides butanamide isobutanamide, 2 -methyl-propanamide For more lessons, visit www. chalkbored. com

HW: Putting it all together: Q 3, 5, 6 p 96

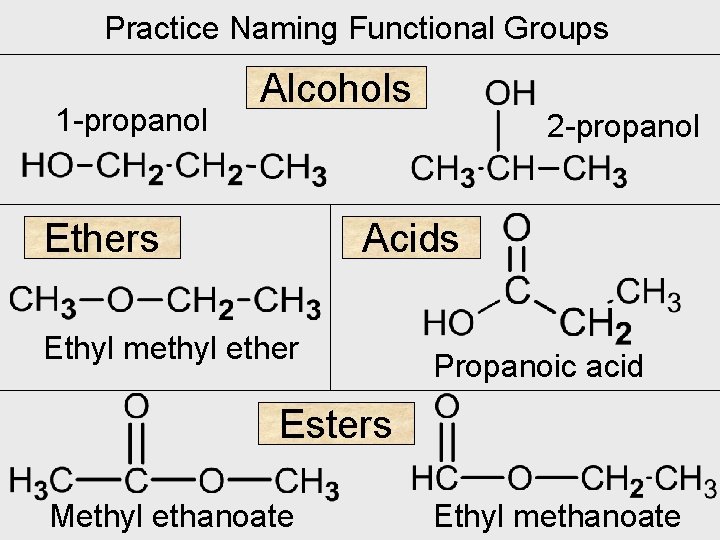
Practice Naming Functional Groups 1 -propanol Alcohols Ethers 2 -propanol Acids Ethyl methyl ether Propanoic acid Esters Methyl ethanoate Ethyl methanoate

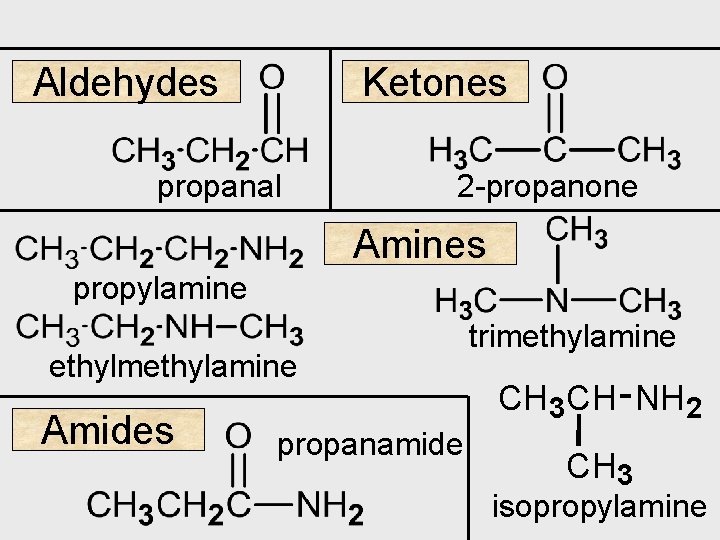
Aldehydes Ketones propanal 2 -propanone Amines propylamine ethylmethylamine Amides propanamide trimethylamine CH 3 CH NH 2 CH 3 isopropylamine

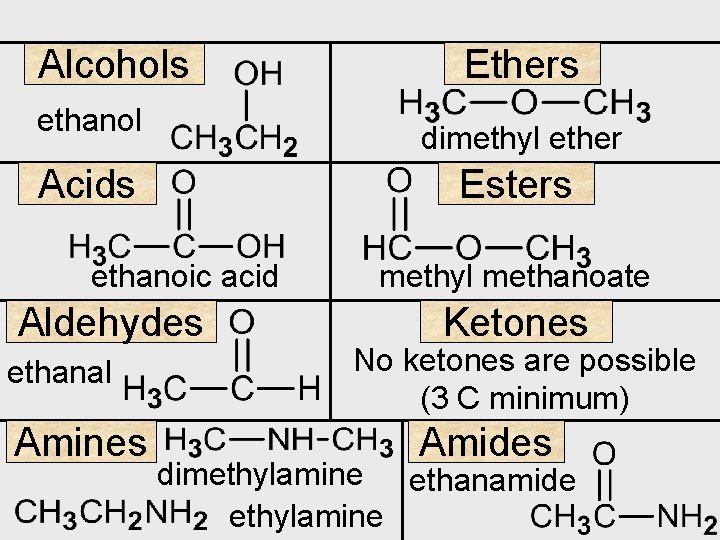
Alcohols ethanol dimethyl ether Acids Esters ethanoic acid Aldehydes ethanal Amines Ethers methyl methanoate Ketones No ketones are possible (3 C minimum) Amides dimethylamine ethanamide ethylamine