Functional Groups 23 1 23 3 Functional Groups




- Slides: 22

Functional Groups 23. 1 -23. 3

Functional Groups Functional Group: a specific arrangement of atoms in an organic compound that is capable of characteristic chemical reactions. Organic compounds are classified based on their functional groups.

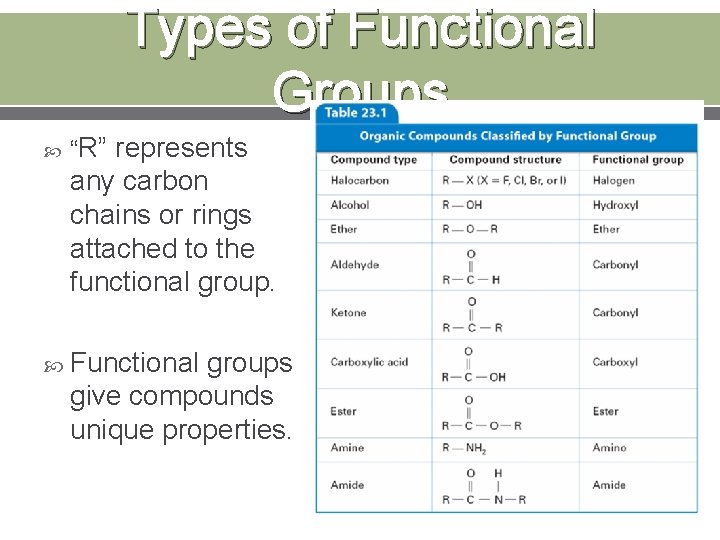
Types of Functional Groups “R” represents any carbon chains or rings attached to the functional group. Functional groups give compounds unique properties.

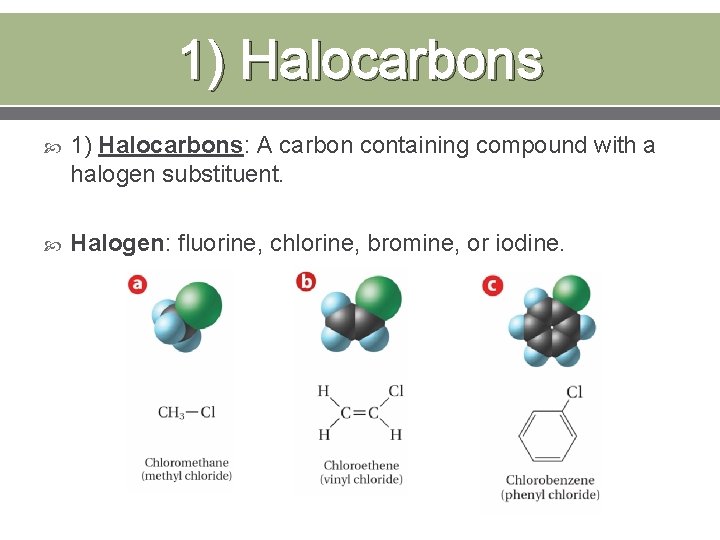
1) Halocarbons 1) Halocarbons: A carbon containing compound with a halogen substituent. Halogen: fluorine, chlorine, bromine, or iodine.

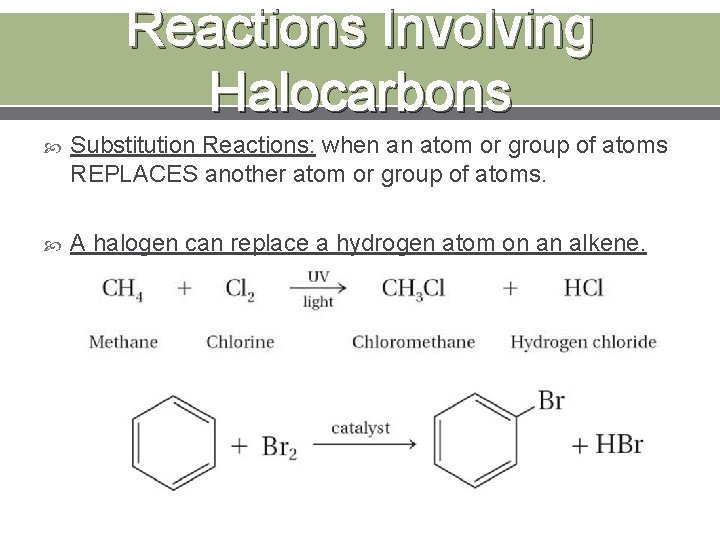
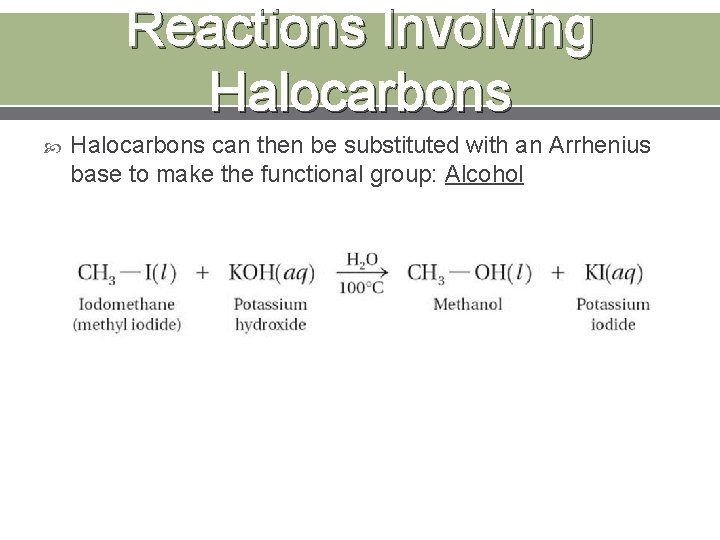
Reactions Involving Halocarbons Substitution Reactions: when an atom or group of atoms REPLACES another atom or group of atoms. A halogen can replace a hydrogen atom on an alkene.

Reactions Involving Halocarbons can then be substituted with an Arrhenius base to make the functional group: Alcohol

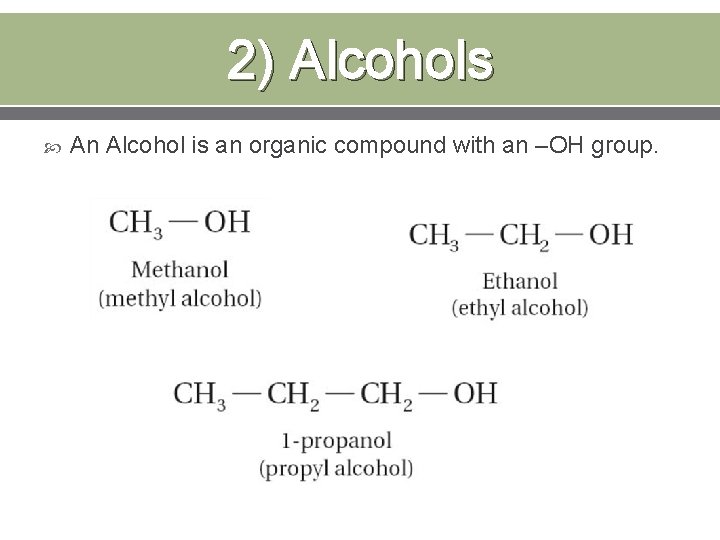
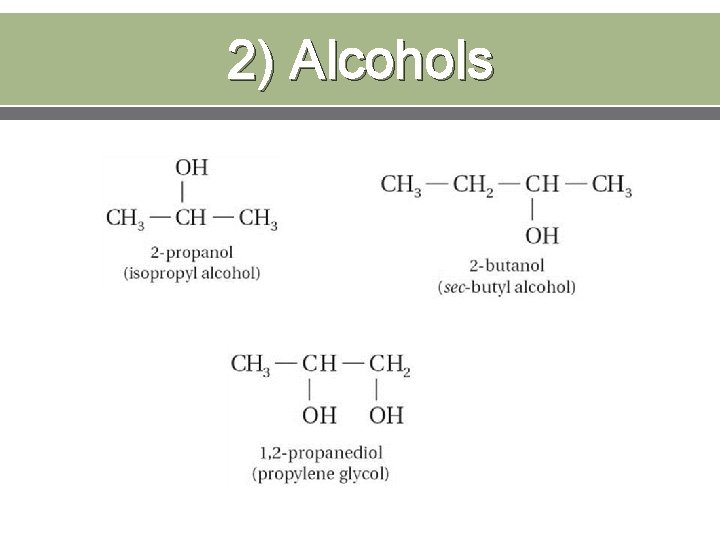
2) Alcohols An Alcohol is an organic compound with an –OH group.

2) Alcohols

2) Alcohols Ethanol (ethyl alcohol) is a common component of many household products. Fermentation is the production of ethanol and carbon dioxide from sugars by the action of yeast or bacteria.

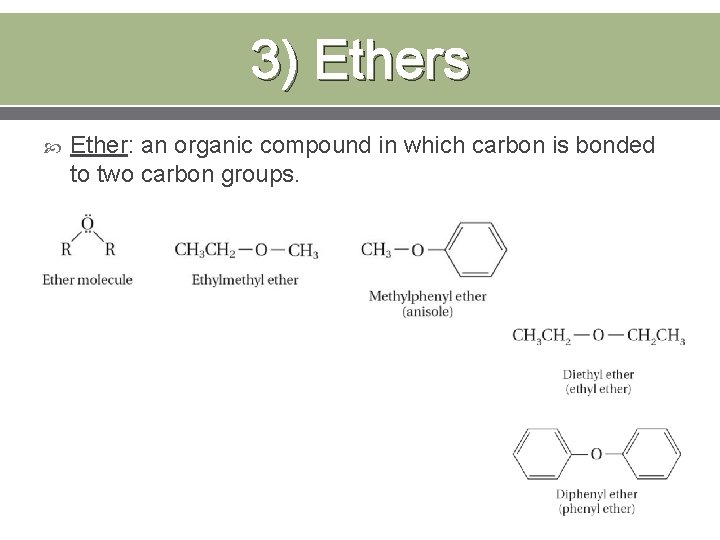
3) Ethers Ether: an organic compound in which carbon is bonded to two carbon groups.

3) Ethers The earliest anesthetics, used during the Civil War, belonged to a class of chemical compounds called ethers.

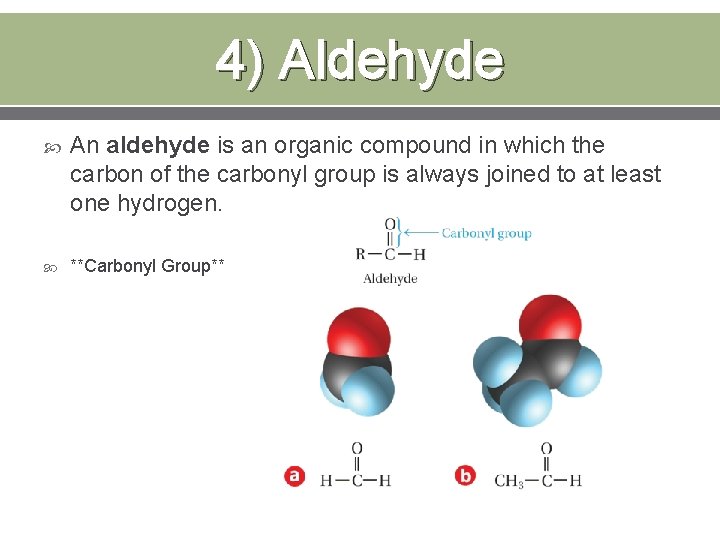
4) Aldehyde An aldehyde is an organic compound in which the carbon of the carbonyl group is always joined to at least one hydrogen. **Carbonyl Group**

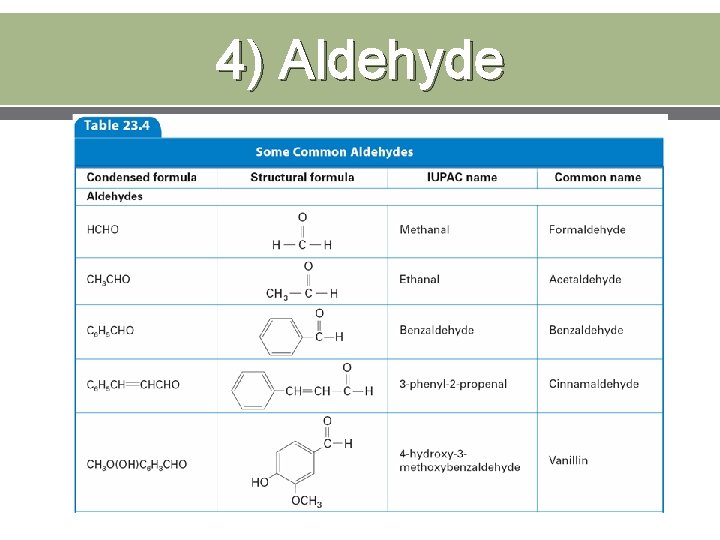
4) Aldehyde

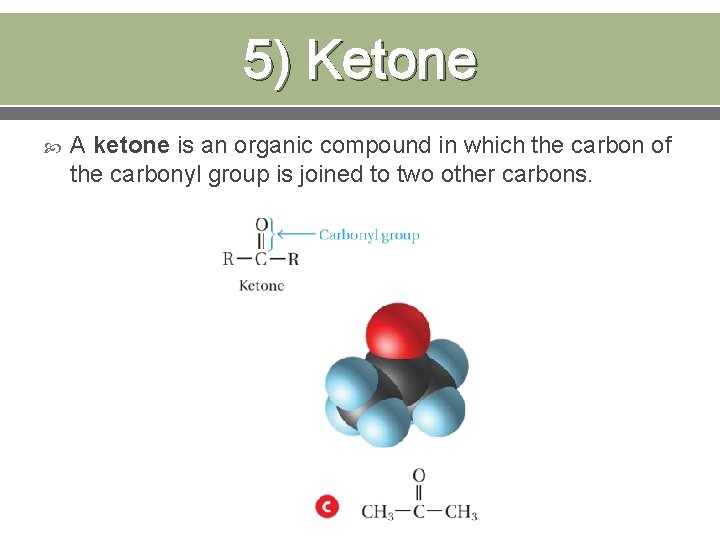
5) Ketone A ketone is an organic compound in which the carbon of the carbonyl group is joined to two other carbons.

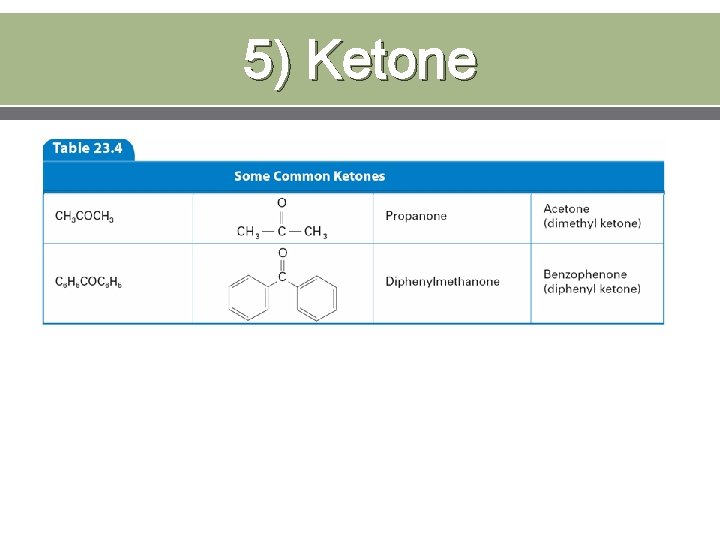
5) Ketone

Aldehyde and Ketone o Uses of Aldehydes and Ketones • Many aldehydes and ketones have distinctive odors. • Aromatic aldehydes are often used as flavoring agents. • Benzaldehyde is known as oil of bitter almond. • Cinnamaldehyde is the source of the odor of oil of cinnamon. Vanillin, an aldehyde, comes from vanilla beans. A solvent used to remove nail polish is acetone, a ketone.

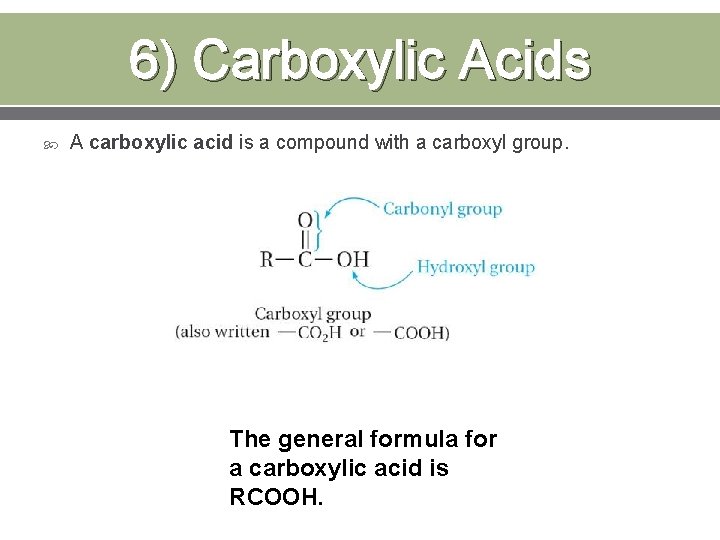
6) Carboxylic Acids A carboxylic acid is a compound with a carboxyl group. The general formula for a carboxylic acid is RCOOH.

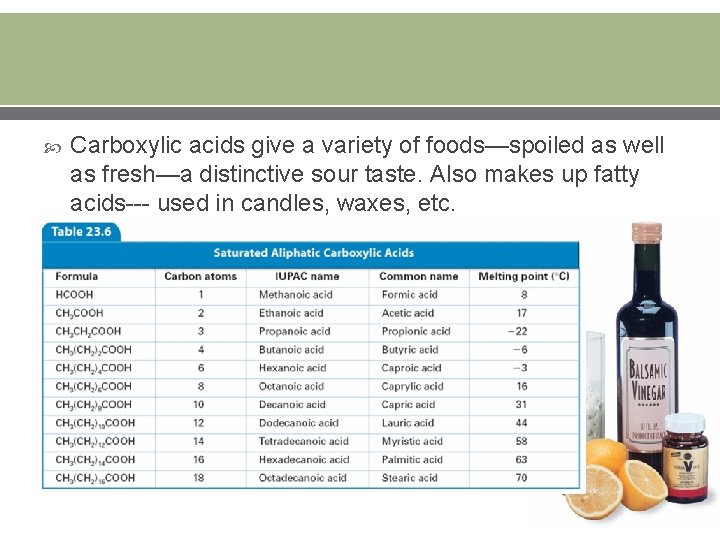
Carboxylic acids give a variety of foods—spoiled as well as fresh—a distinctive sour taste. Also makes up fatty acids--- used in candles, waxes, etc.

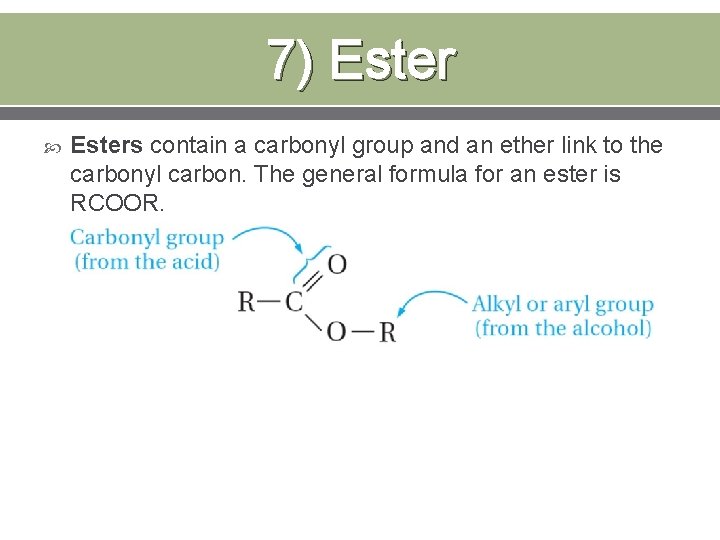
7) Esters contain a carbonyl group and an ether link to the carbonyl carbon. The general formula for an ester is RCOOR.

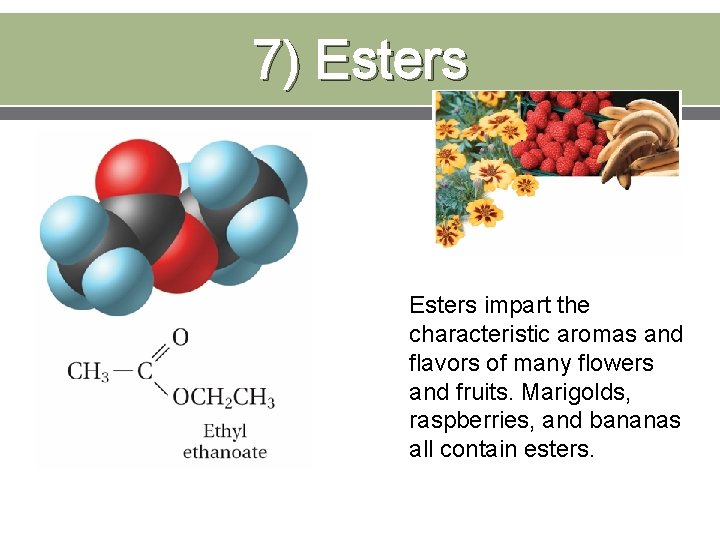
7) Esters impart the characteristic aromas and flavors of many flowers and fruits. Marigolds, raspberries, and bananas all contain esters.

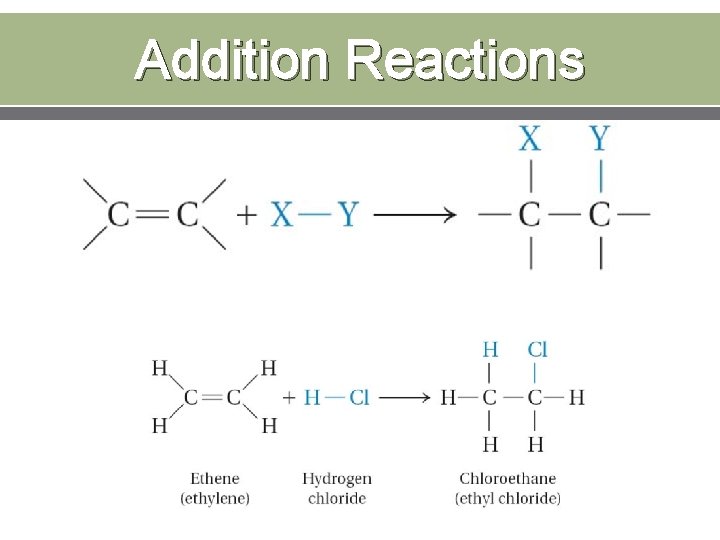
Addition Reactions

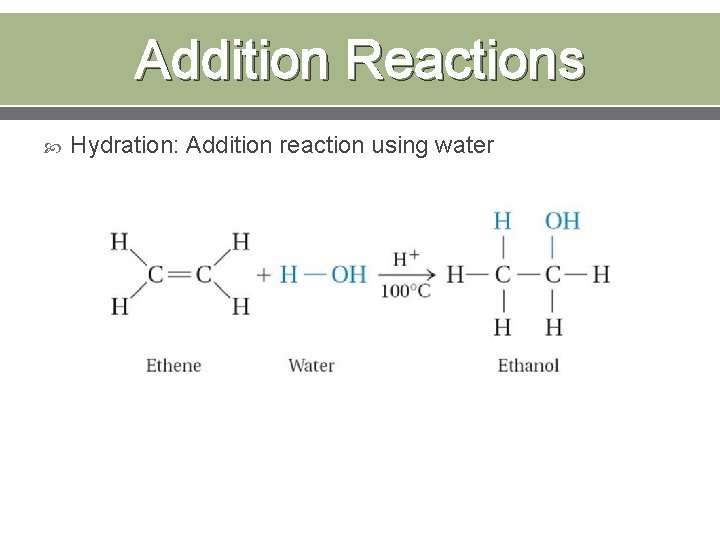
Addition Reactions Hydration: Addition reaction using water