Organic functional groups Organic functional groups l What










- Slides: 11

Organic functional groups

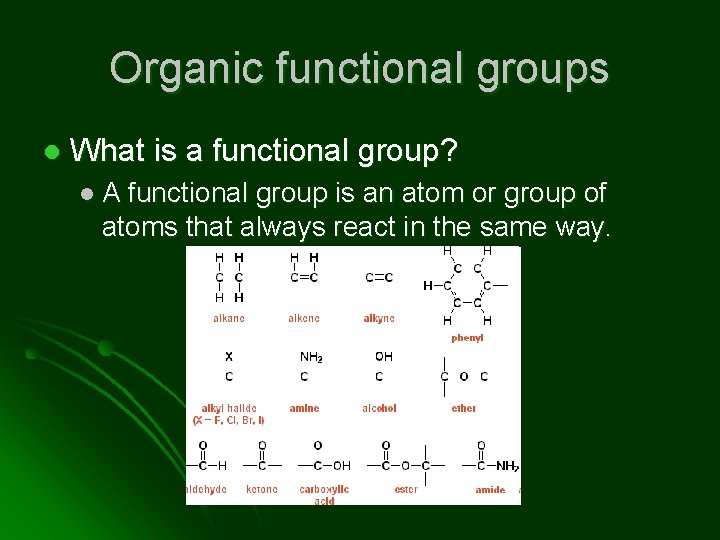
Organic functional groups l What is a functional group? l. A functional group is an atom or group of atoms that always react in the same way.

Organic functional groups l What are halocarbons? l This is when the functional group is a halogen. l Alkyl halide is halogen attached to aliphatic carbon chain. l Aryl halide is halogen attached to aromatic carbon ring. l Naming is IUPAC rules using fluoro- chlorobromo- prefixes. l What is 1 -bromo-5 -chloropentane?

Organic functional groups l What is a hydroxyl group? l Hydroxyl is –OH. It changes a hydrocarbon to an alcohol. l The naming rules follow IUPAC rules with the ending of the hydrocarbon becoming –ol. l What is 1, 2, 3 -propantriol?

Organic functional groups l What is the ether functional group? l Ethers have an oxygen in the carbon chain. l When naming an ether use the names of the 2 chains attached to the oxygen. Use alphabetical rules for first hydrocarbon named. l If they are the same, just use the name. l What is ethylmethyl ether? l What is butyl ether?

Organic functional groups l What is the amine functional group? l This group is NH 2. l The amine is a derivative of ammonia. l Naming rules use the suffix –amine. l Di, tri and tetra are used to give numbers if there are more than one. l What is 1, 1, 4, 4 -butantetraamine?

Organic functional groups l What is a carbonyl group? l Carbonyl groups are oxygen double bonded to a carbon. l An aldehyde is an organic compound with the carbonyl group at the end of a chain. l Naming is done by changing the ending to –al, or adding aldehyde to the end. l A ketone has the carbonyl group in the middle of the chain. l Endings of ketones are changed to -one.

Organic functional groups l What is the carboxyl group? l This group has carbonyl group with a hydroxyl group attached. l Compounds with this group are called carboxylic acids. l The formal names change the ending to –anoic acid.

Organic functional groups l What is an ester group? l This is an organic compound with the hydrogen of the hydroxyl has been replaced with an alkyl group. l Naming is done by writing the name of the alkyl group followed by the name of the acid with the –ic acid ending replaced with –ate. l What does methyl hexanoate look like?

Organic functional groups l What is an amide functional group? l An amide is an ester group with the single bonded oxygen replaced with a nitrogen atom bonded to other atoms. l Naming is the name of the alkane with the final e changed to –amide. l What does ethanamide look like?

Organic functional groups l What are the properties of each of the functional groups listed here?