Functional Groups Learning Goals 1 Identify functional groups


- Slides: 26

Functional Groups

Learning Goals 1. Identify functional groups: (alkane, alkene, alkyne, alcohol, carbonyl, aldehyde, ketone, carboxylic acid, ether, ester, amine, amide, halogenoalkane ) 2. Identify primary alcohols, secondary alcohols, tertiary alcohol, primary amine, secondary amine, tertiary amine

Functional Groups • As has already been indicated, alkanes are relatively unreactive. • For an organic molecule to be reactive it needs something additional. • A site of reactivity in an organic molecule is called a functional group. • C=C double is a functional group. • Other functional groups contain elements other than C or H, notably O, N and Cl.

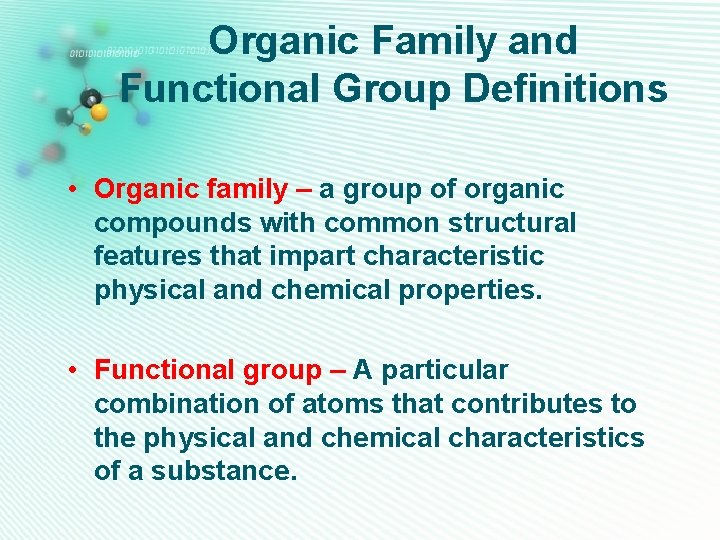
Organic Family and Functional Group Definitions • Organic family – a group of organic compounds with common structural features that impart characteristic physical and chemical properties. • Functional group – A particular combination of atoms that contributes to the physical and chemical characteristics of a substance.

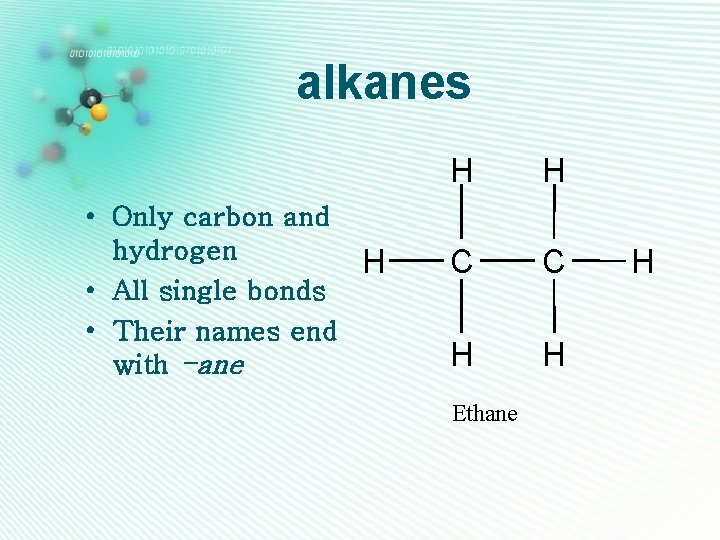
alkanes • Only carbon and hydrogen H • All single bonds • Their names end with -ane H H C C H H Ethane H

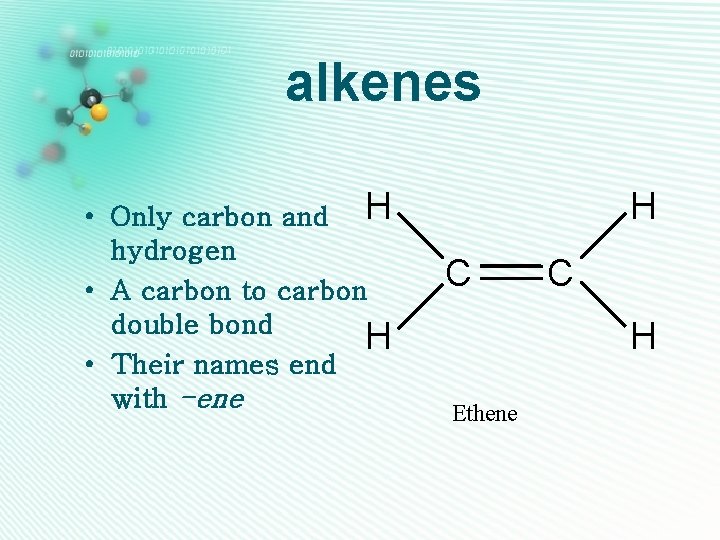
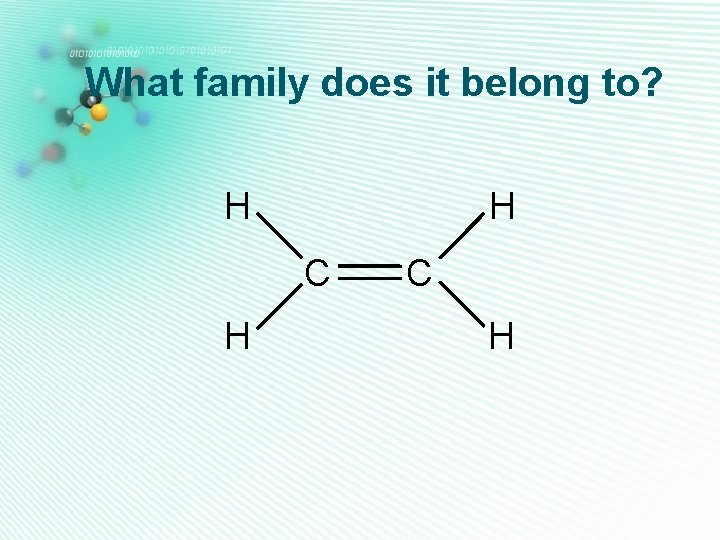
alkenes • Only carbon and H hydrogen • A carbon to carbon double bond H • Their names end with -ene H C C H Ethene

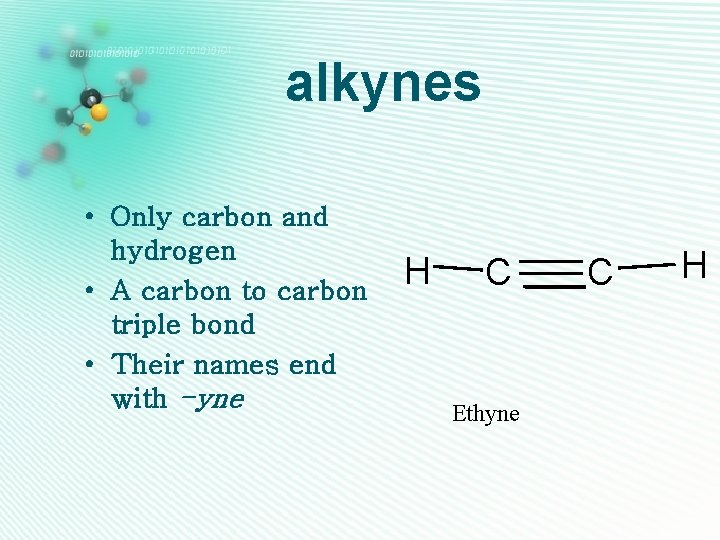
alkynes • Only carbon and hydrogen • A carbon to carbon triple bond • Their names end with -yne H C Ethyne C H

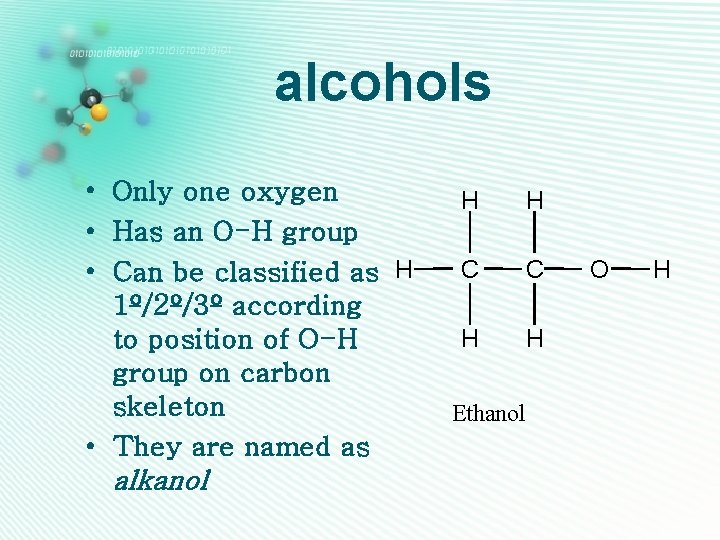
alcohols • Only one oxygen • Has an O-H group • Can be classified as H 1º/2º/3º according to position of O-H group on carbon skeleton • They are named as alkanol H H C C H H Ethanol O H

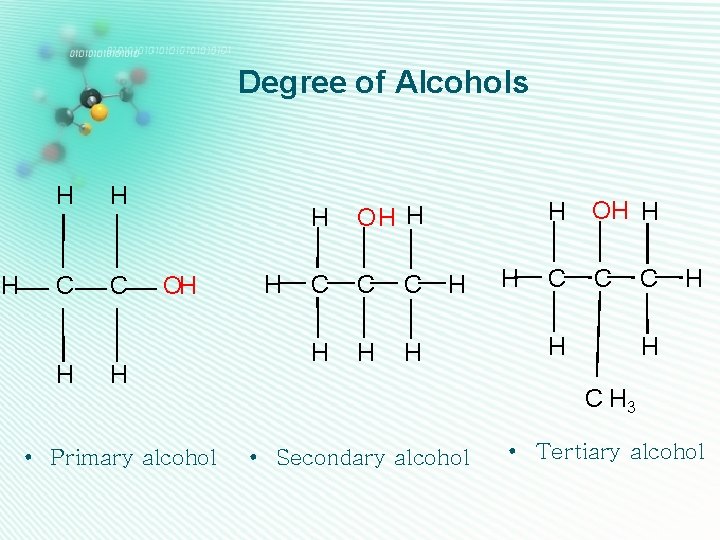
Degree of Alcohols H H H C C H OH H • Primary alcohol H H OH H C C C H H H OH H C C H H C H 3 • Secondary alcohol • Tertiary alcohol

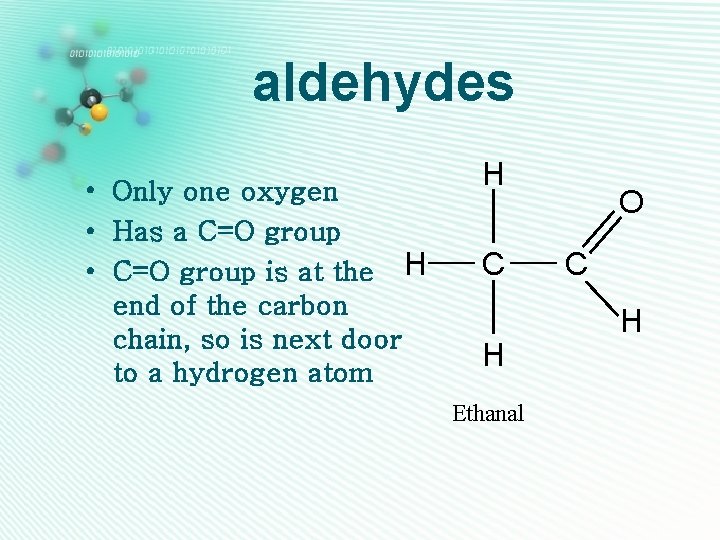
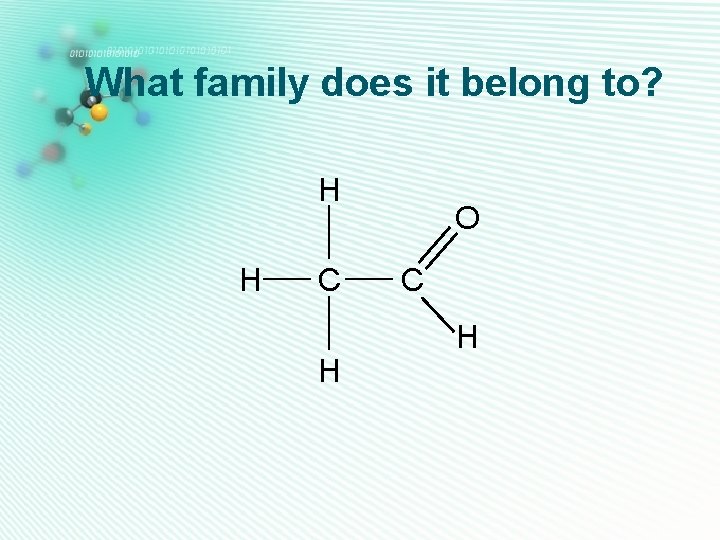
aldehydes • Only one oxygen • Has a C=O group • C=O group is at the H end of the carbon chain, so is next door to a hydrogen atom H C H Ethanal O C H

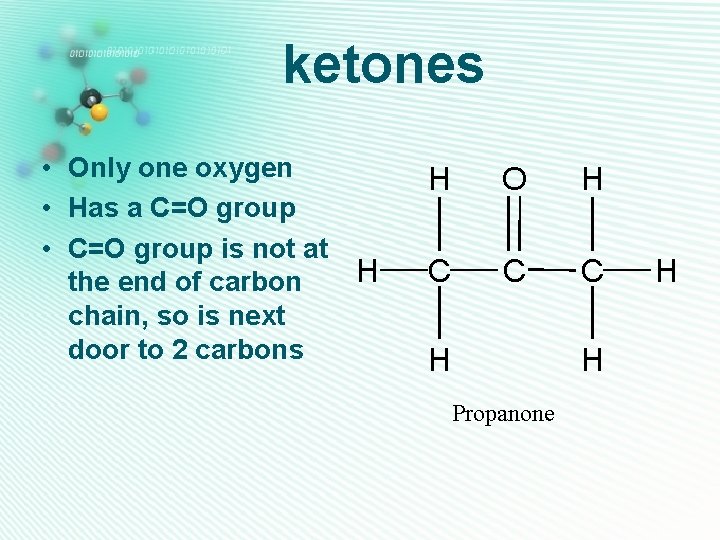
ketones • Only one oxygen • Has a C=O group • C=O group is not at H the end of carbon chain, so is next door to 2 carbons H O H C C C H H Propanone H

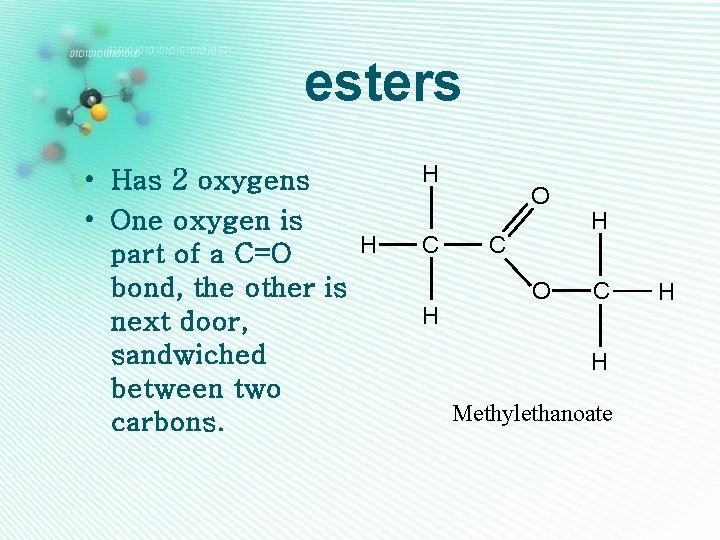
esters • Has 2 oxygens • One oxygen is H part of a C=O bond, the other is next door, sandwiched between two carbons. H C H O C O H C H Methylethanoate H

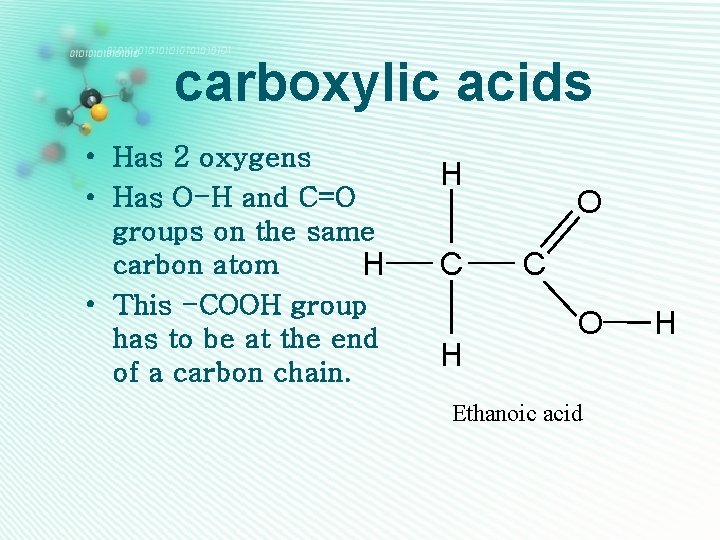
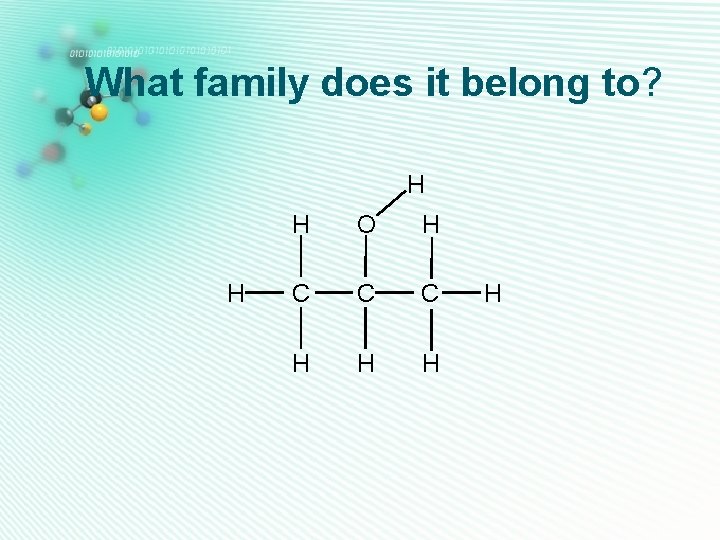
carboxylic acids • Has 2 oxygens • Has O-H and C=O groups on the same carbon atom H • This -COOH group has to be at the end of a carbon chain. H C H O C O Ethanoic acid H

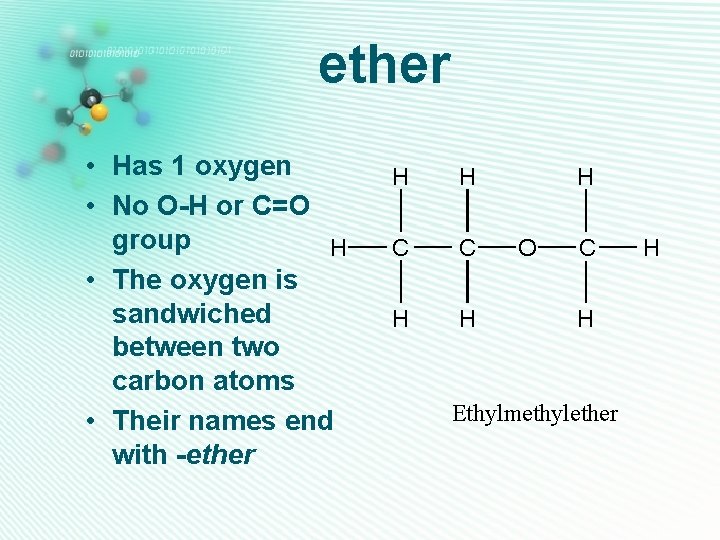
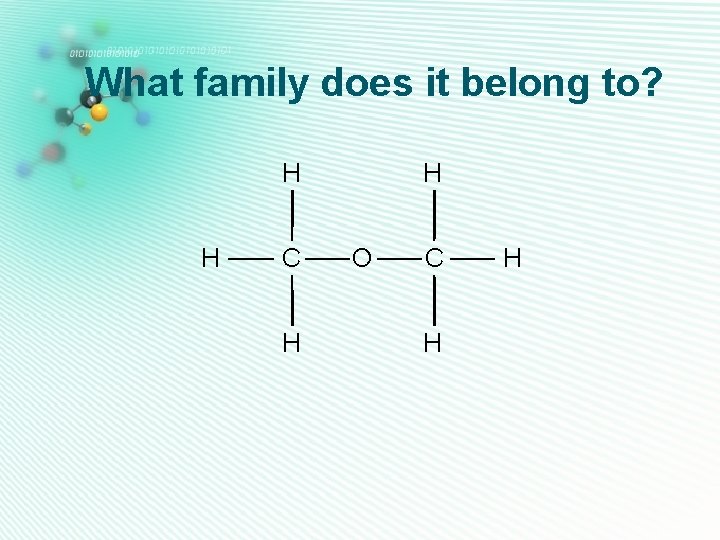
ether • Has 1 oxygen • No O-H or C=O group H • The oxygen is sandwiched between two carbon atoms • Their names end with -ether H H C C H H H O C H Ethylmethylether H

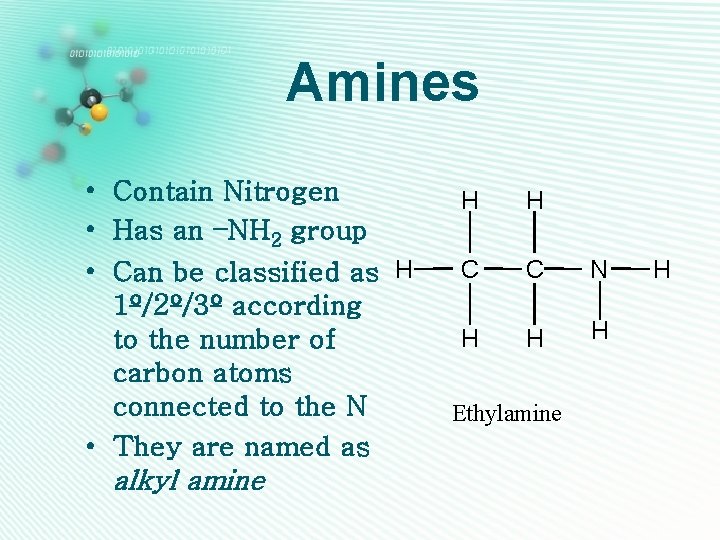
Amines • Contain Nitrogen • Has an –NH 2 group • Can be classified as H 1º/2º/3º according to the number of carbon atoms connected to the N • They are named as alkyl amine H H C C N H H H Ethylamine H

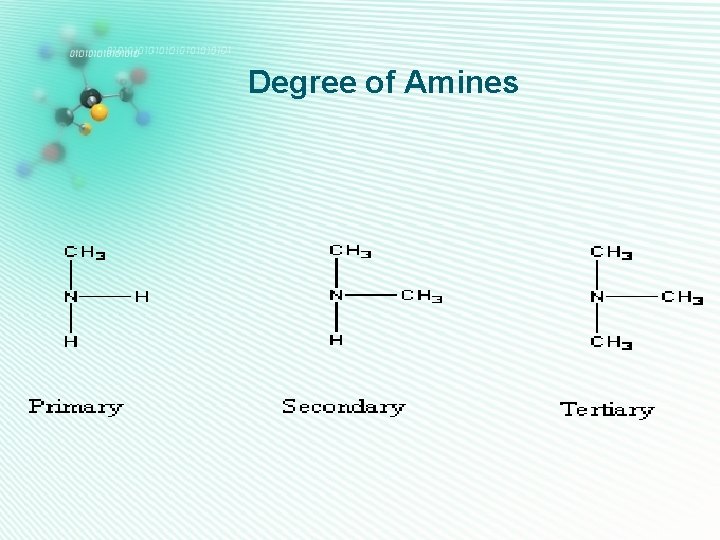
Degree of Amines

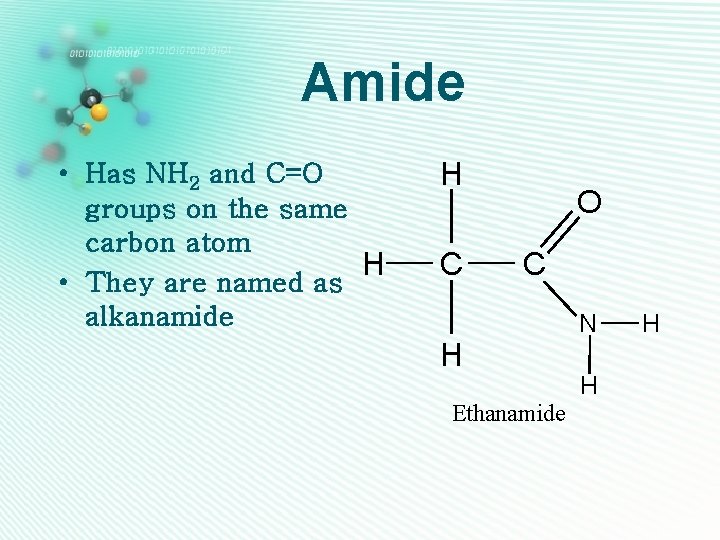
Amide • Has NH 2 and C=O groups on the same carbon atom H • They are named as alkanamide H C O C H Ethanamide N H H

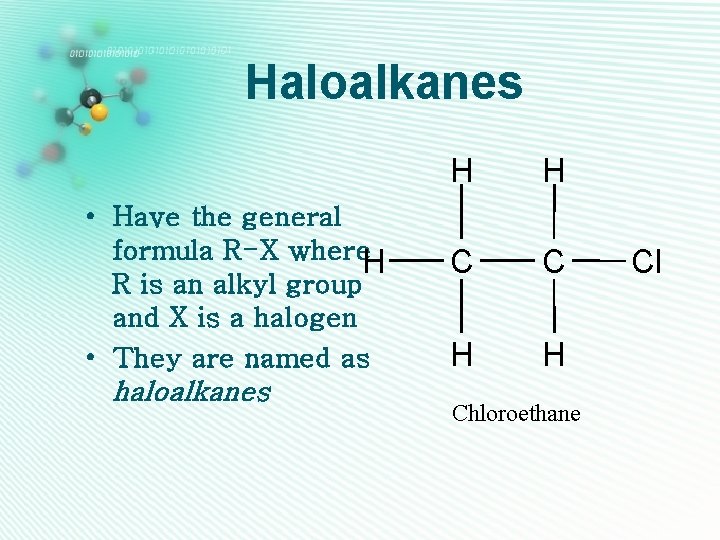
Haloalkanes • Have the general formula R-X where. H R is an alkyl group and X is a halogen • They are named as haloalkanes H H C C H H Chloroethane Cl

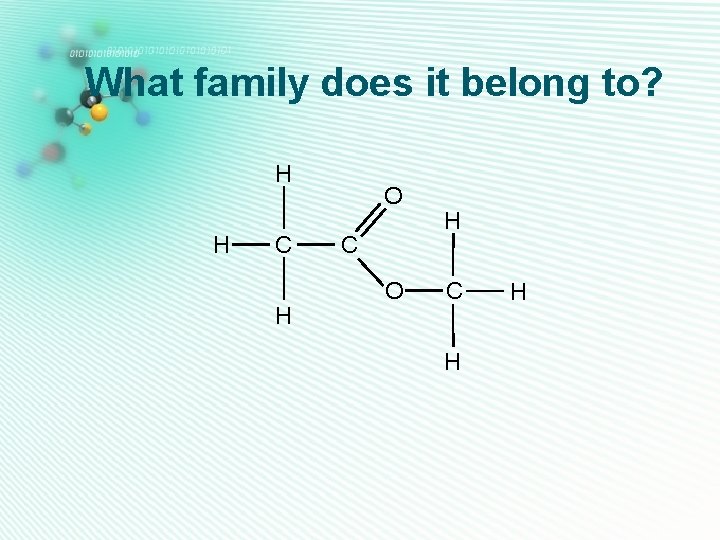
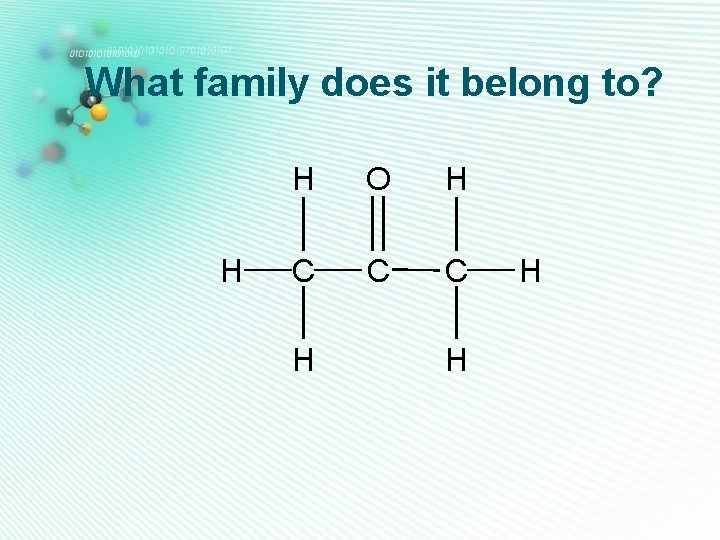
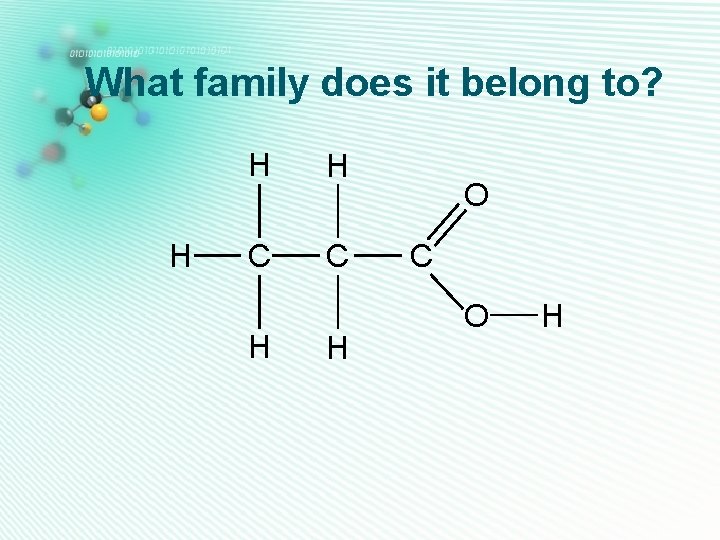
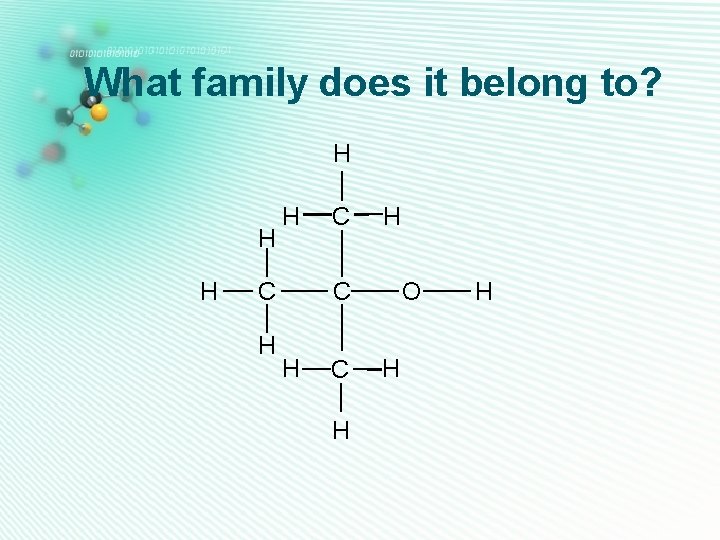
What family does it belong to? H H C H

What family does it belong to? H H C H O C H

What family does it belong to? H H H O H C C C H H

What family does it belong to? H H C H H O C H H

What family does it belong to? H H C H O C O H C H H

What family does it belong to? H H O H C C C H H H

What family does it belong to? H H H C C H H O C O H

What family does it belong to? H H H C C H H C O H H