Chapter 4 Phase changes and Phase diagrams 1

















- Slides: 51

Chapter 4 Phase changes and Phase diagrams 1

4. 1 Phase changes 4. 2 Energy of phase changes 4. 3 Phase diagram 4. 4 Phase rule 2

4. 5 Phase diagram for two component systems 4. 6 The lever rule 3

4. 1 Phase changes 4

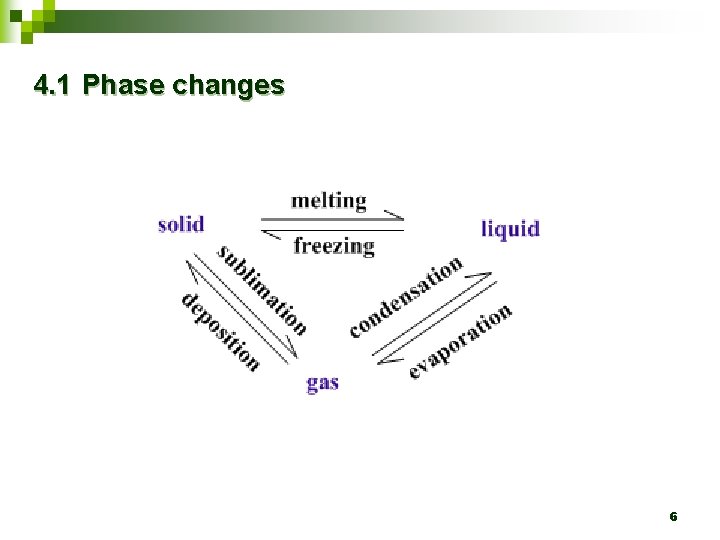
4. 1 Phase changes Ø Ø Phase refers to a single homogeneous physical state : solid, liquid or gas. The change from one phase to another is called a a phase change. 5

4. 1 Phase changes 6

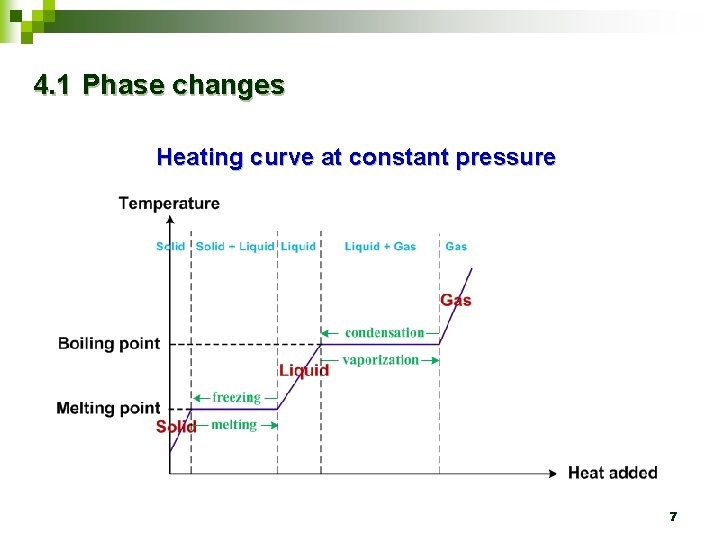
4. 1 Phase changes Heating curve at constant pressure 7

Phase Change Material (PCM)

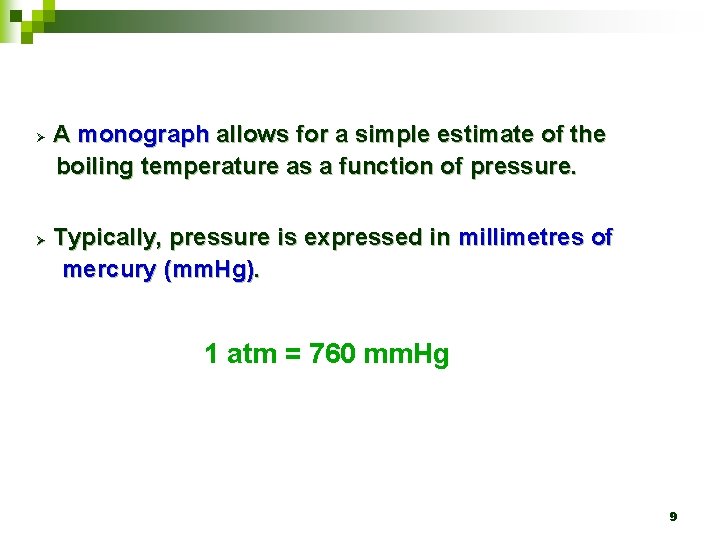
Ø Ø A monograph allows for a simple estimate of the boiling temperature as a function of pressure. Typically, pressure is expressed in millimetres of mercury (mm. Hg). 1 atm = 760 mm. Hg 9

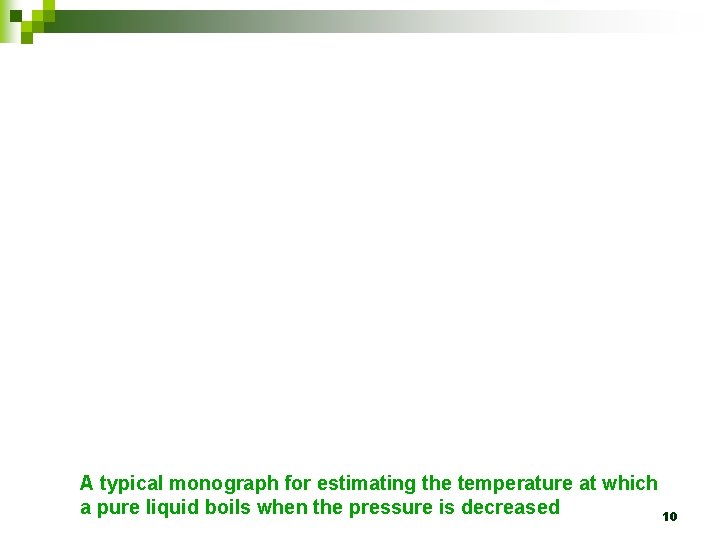
A typical monograph for estimating the temperature at which a pure liquid boils when the pressure is decreased 10

Ø This is how a boiling temperature at reduced pressure is estimated with a monograph: Place a straight ruler against the applied pressure as indicated on the curved right-hand scale (c). The ruler must also pass through the normal boiling temperature on the middle scale (b). The reduced-pressure boiling temperature is then read off the left-hand scale (a). 11

Ø As an example, if the normal boiling temperature is 200 o. C, then the reduced boiling temperature may be halved to 100 o. C if the applied pressure is approximately 15 mm. Hg. 12

Rotary evaporator 13

4. 2 Energy of phase changes 14

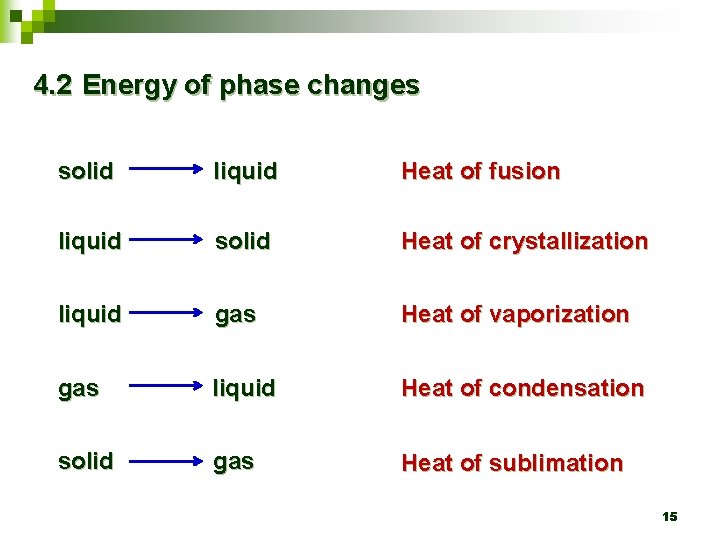
4. 2 Energy of phase changes solid liquid Heat of fusion liquid solid Heat of crystallization liquid gas Heat of vaporization gas liquid Heat of condensation solid gas Heat of sublimation 15

4. 2 Energy of phase changes Example How much heat energy is required to melt 100 g of ice to liquid water at 0 o. C? The molar heat of fusion for water is 6. 01 k. J/mol. 16

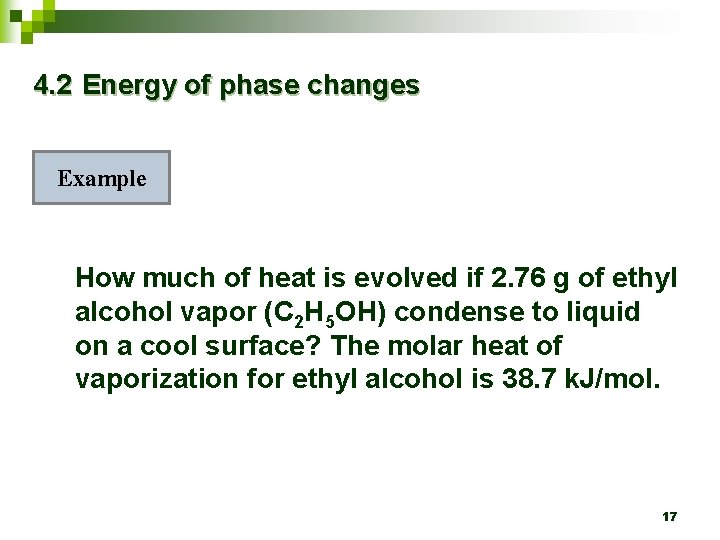
4. 2 Energy of phase changes Example How much of heat is evolved if 2. 76 g of ethyl alcohol vapor (C 2 H 5 OH) condense to liquid on a cool surface? The molar heat of vaporization for ethyl alcohol is 38. 7 k. J/mol. 17

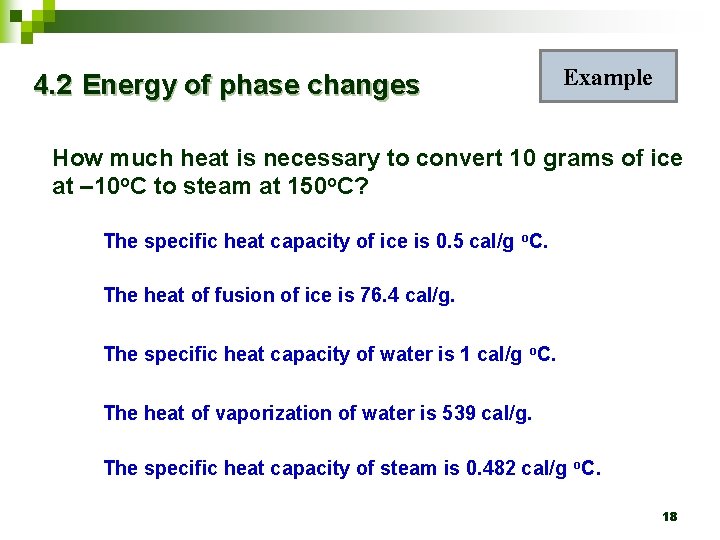
4. 2 Energy of phase changes Example How much heat is necessary to convert 10 grams of ice at – 10 o. C to steam at 150 o. C? The specific heat capacity of ice is 0. 5 cal/g o. C. The heat of fusion of ice is 76. 4 cal/g. The specific heat capacity of water is 1 cal/g o. C. The heat of vaporization of water is 539 cal/g. The specific heat capacity of steam is 0. 482 cal/g o. C. 18

4. 3 Phase diagram 19

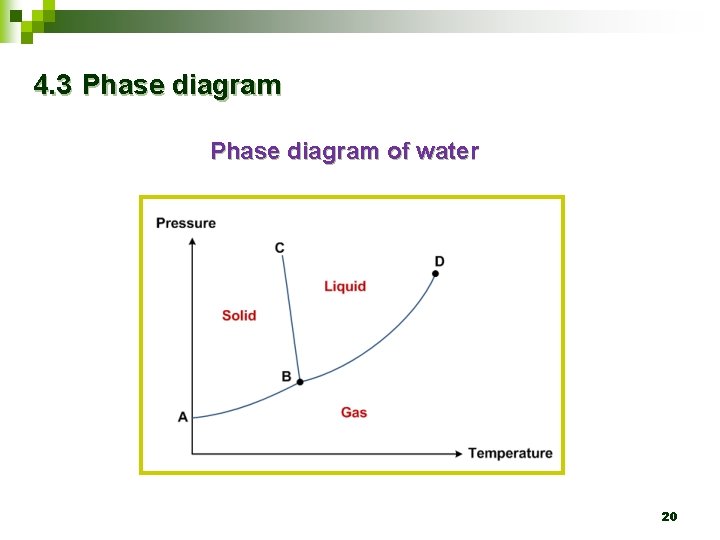
4. 3 Phase diagram of water 20

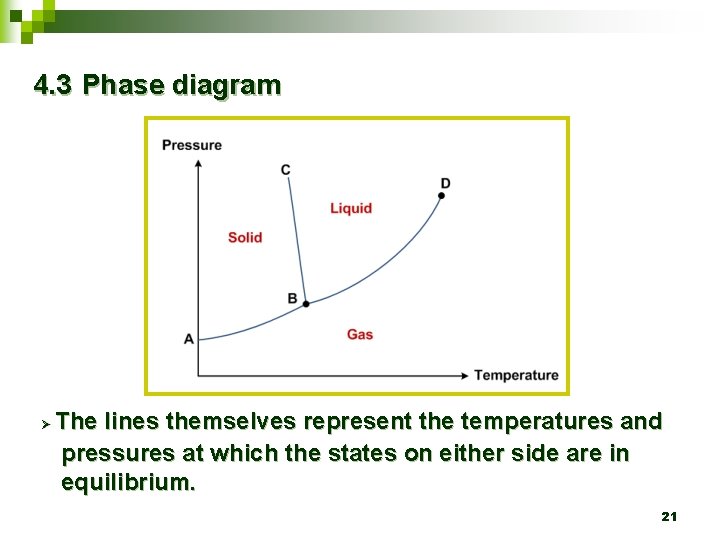
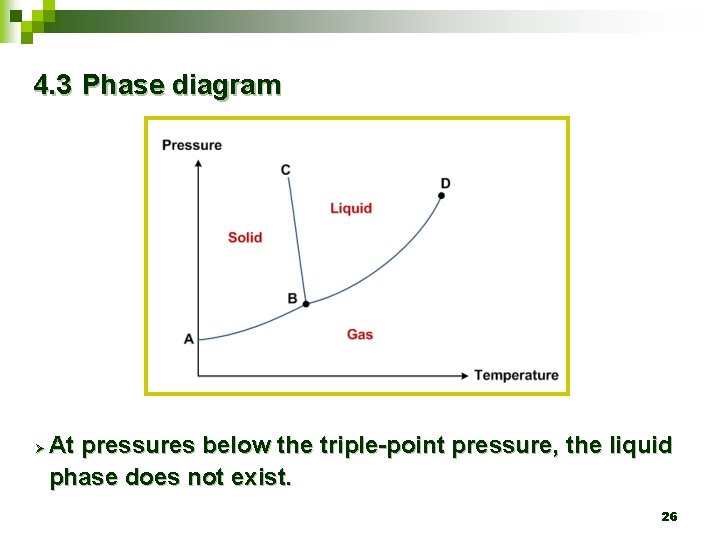
4. 3 Phase diagram Ø The lines themselves represent the temperatures and pressures at which the states on either side are in equilibrium. 21

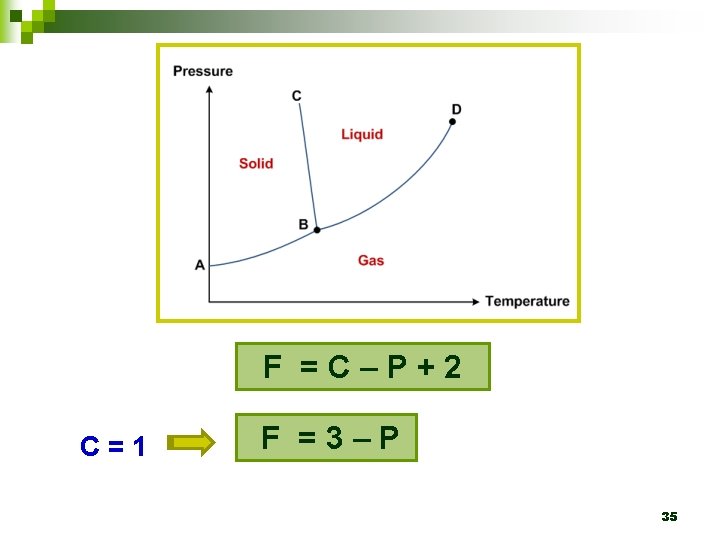
Ø The line A-B represents the temperatures and pressures where the gas state and the solid state are in equilibrium. Sublimation curve 22

Ø The line B-C represents the temperatures and pressures at which the solid and liquid states are at equilibrium. Melting curve 23

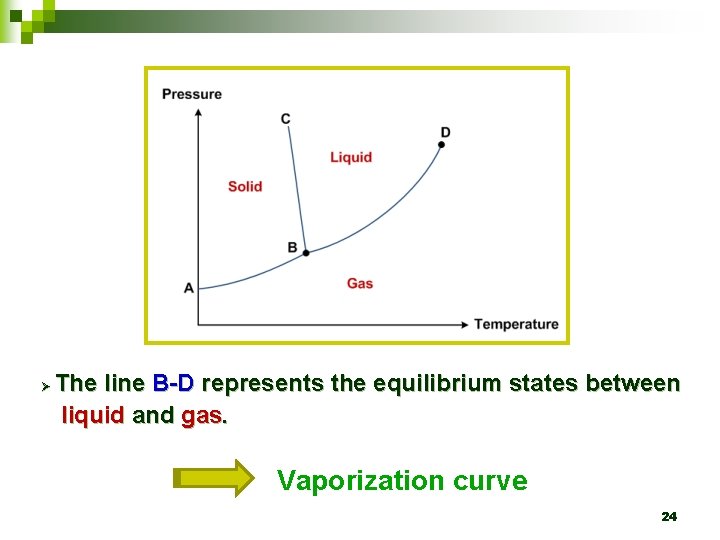
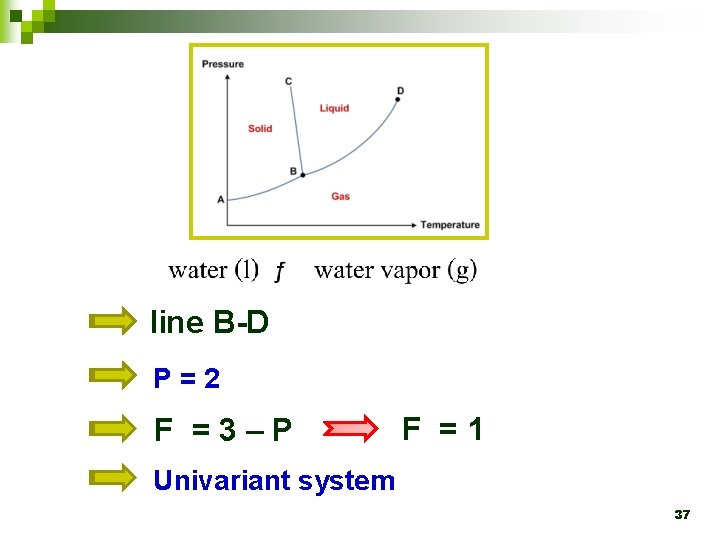
Ø The line B-D represents the equilibrium states between liquid and gas. Vaporization curve 24

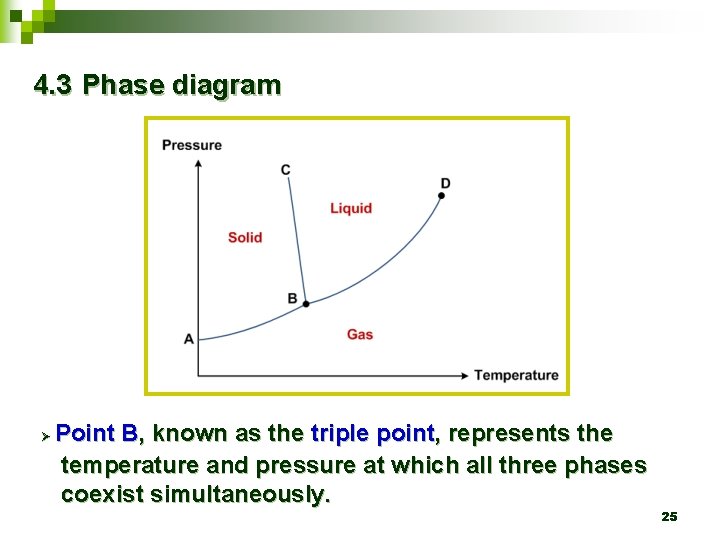
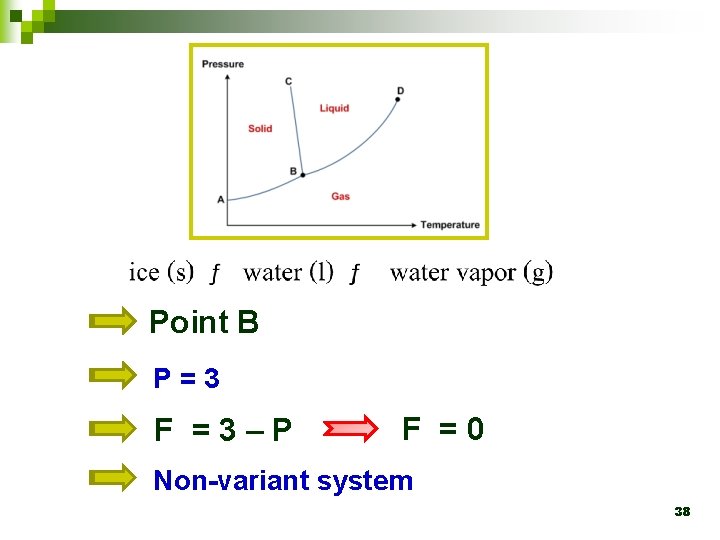
4. 3 Phase diagram Ø Point B, known as the triple point, represents the temperature and pressure at which all three phases coexist simultaneously. 25

4. 3 Phase diagram Ø At pressures below the triple-point pressure, the liquid phase does not exist. 26

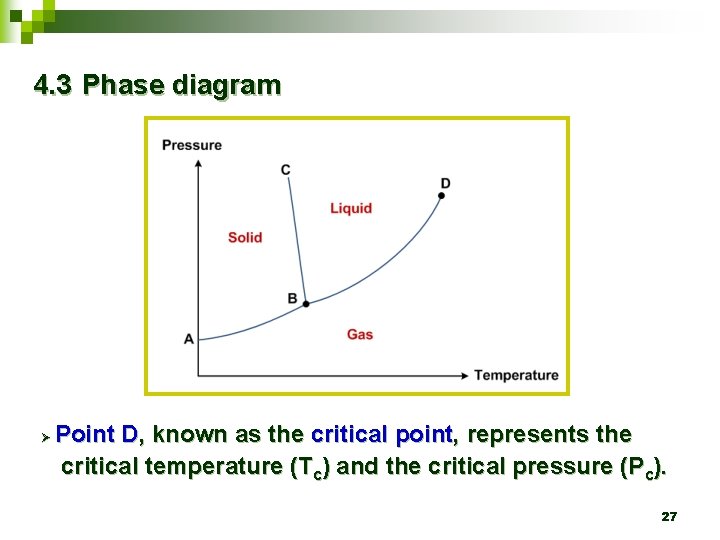
4. 3 Phase diagram Ø Point D, known as the critical point, represents the critical temperature (Tc) and the critical pressure (Pc). 27

4. 3 Phase diagram Ø Ø This critical point is the highest temperature and highest pressure at which there is a difference between liquid and gas states. At either a temperature or a pressure over the critical point, only a single fluid state exists. 28

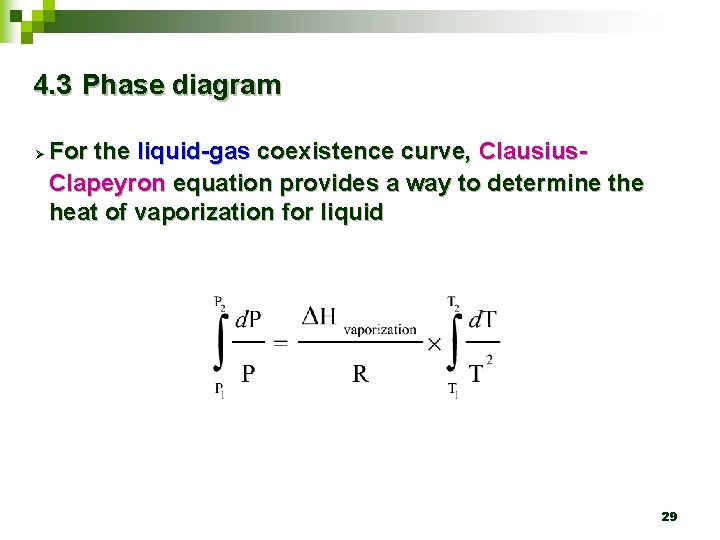
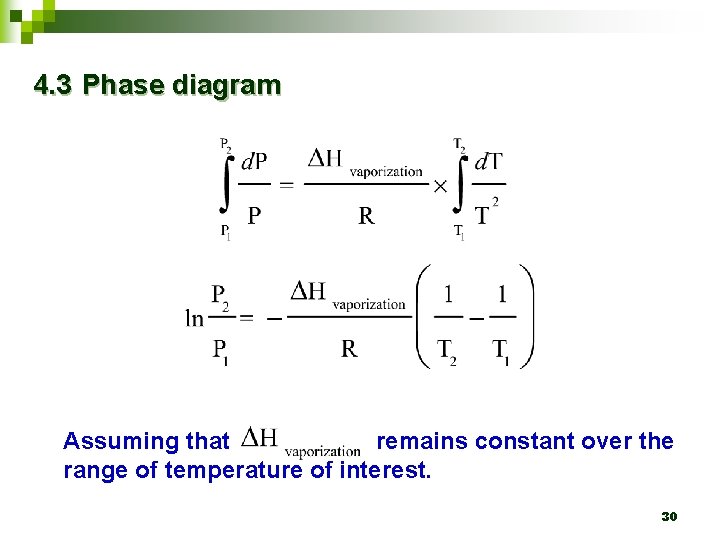
4. 3 Phase diagram Ø For the liquid-gas coexistence curve, Clausius. Clapeyron equation provides a way to determine the heat of vaporization for liquid 29

4. 3 Phase diagram Assuming that remains constant over the range of temperature of interest. 30

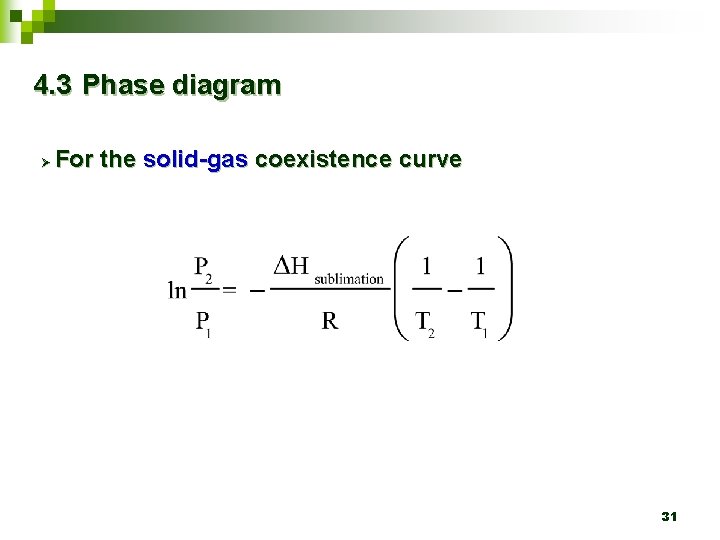
4. 3 Phase diagram Ø For the solid-gas coexistence curve 31

4. 3 Phase diagram Example The normal boiling temperature of benzene is 353. 24 K and the vapor pressure of liquid benzene is 1 104 Pa at 20 o. C. Calculate 32

4. 4 Phase rule 33

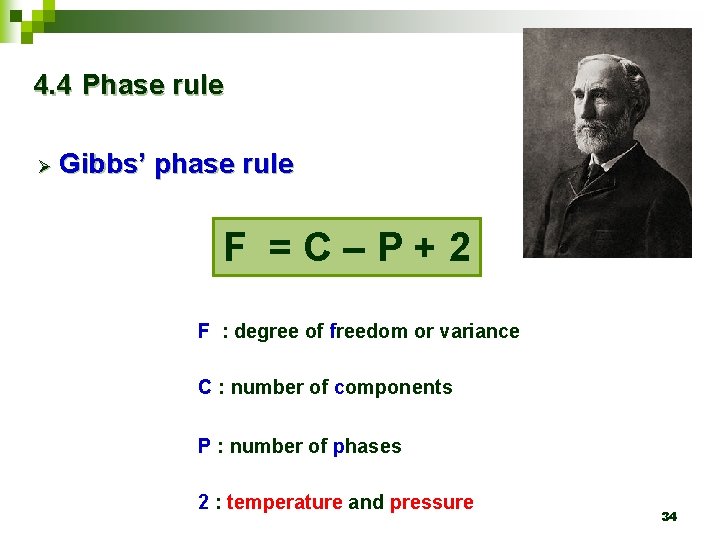
4. 4 Phase rule Ø Gibbs’ phase rule F =C–P+2 F : degree of freedom or variance C : number of components P : number of phases 2 : temperature and pressure 34

F =C–P+2 C=1 F =3–P 35

Water (l) P=1 F =3–P F =2 Bivariant system 36

line B-D P=2 F =3–P F =1 Univariant system 37

Point B P=3 F =3–P F =0 Non-variant system 38

4. 5 Phase diagram for two component systems 39

4. 5 Phase diagram for two component systems Ø A binary system consisting of two substances which do not react chemically in solid phase but is miscible in all proportions in liquid phase is called eutectic system. 40

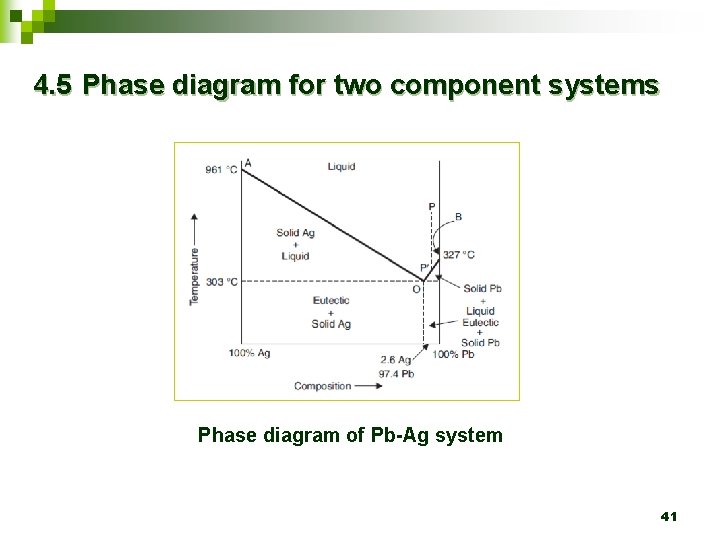
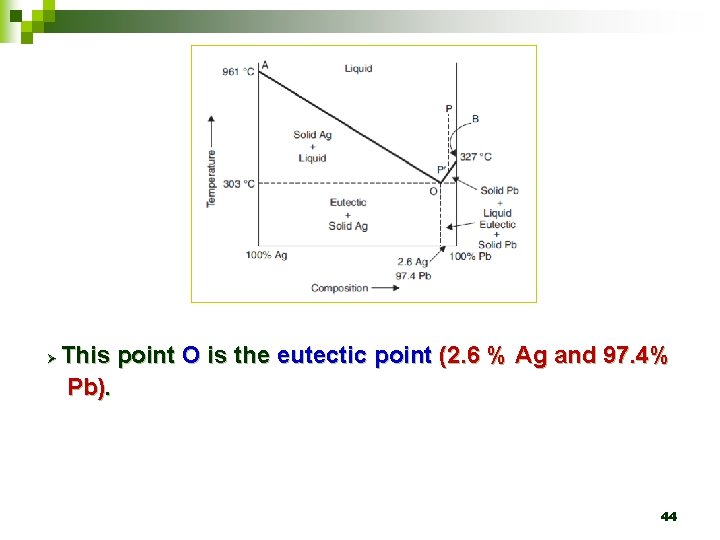
4. 5 Phase diagram for two component systems Phase diagram of Pb-Ag system 41

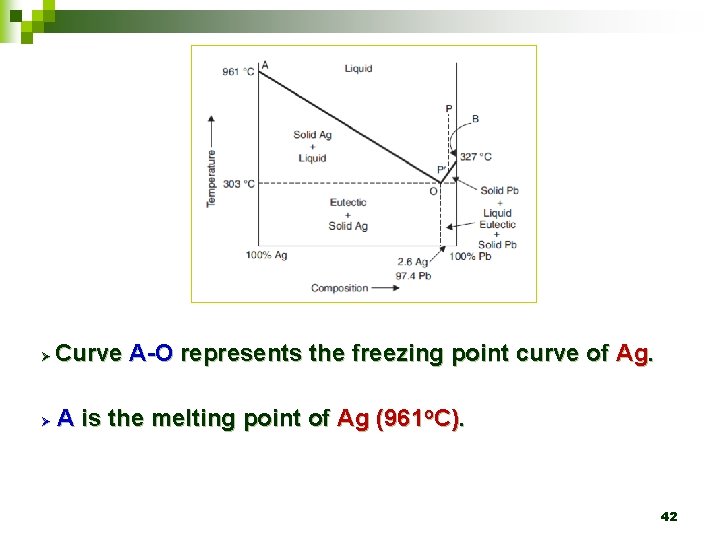
Ø Curve A-O represents the freezing point curve of Ag. Ø A is the melting point of Ag (961 o. C). 42

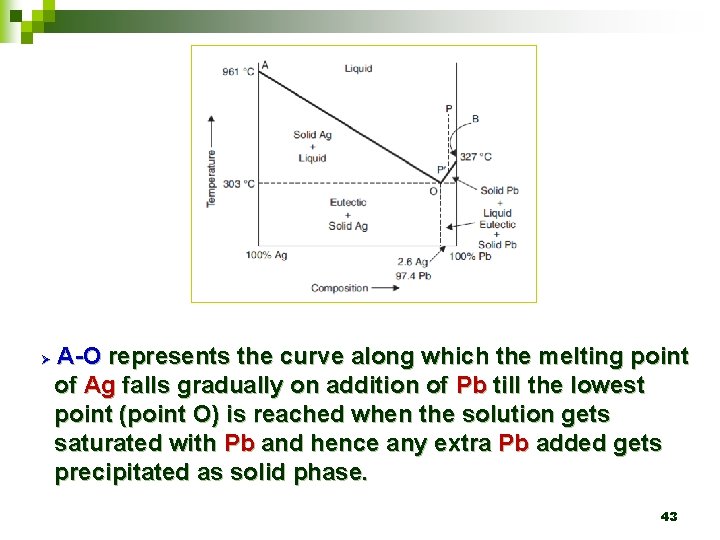
Ø A-O represents the curve along which the melting point of Ag falls gradually on addition of Pb till the lowest point (point O) is reached when the solution gets saturated with Pb and hence any extra Pb added gets precipitated as solid phase. 43

Ø This point O is the eutectic point (2. 6 % Ag and 97. 4% Pb). 44

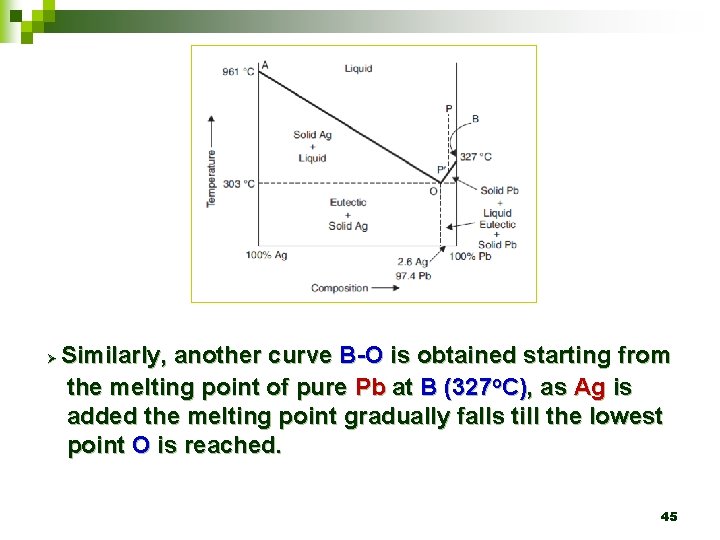
Ø Similarly, another curve B-O is obtained starting from the melting point of pure Pb at B (327 o. C), as Ag is added the melting point gradually falls till the lowest point O is reached. 45

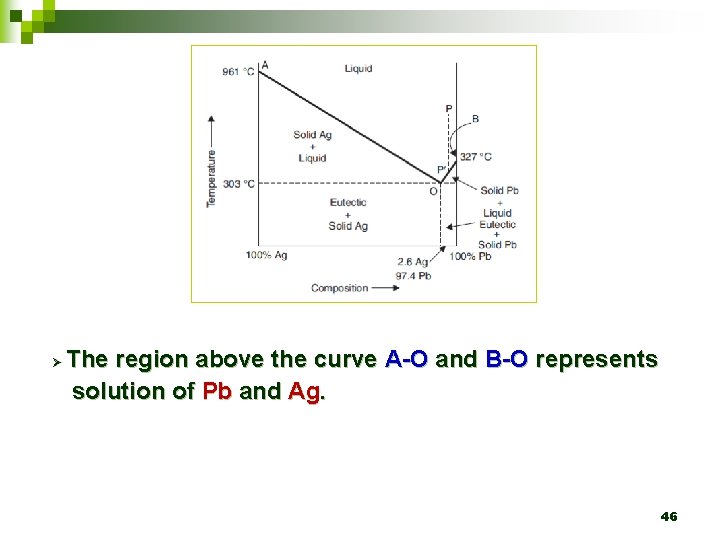
Ø The region above the curve A-O and B-O represents solution of Pb and Ag. 46

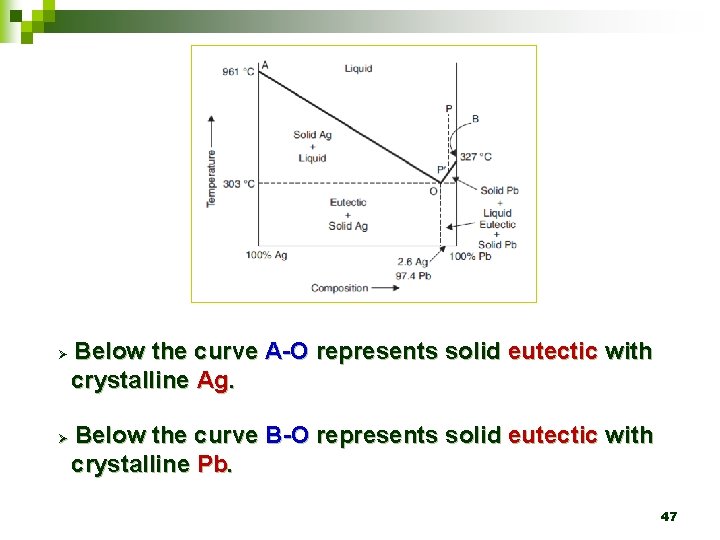
Ø Ø Below the curve A-O represents solid eutectic with crystalline Ag. Below the curve B-O represents solid eutectic with crystalline Pb. 47

4. 6 The lever rule 48

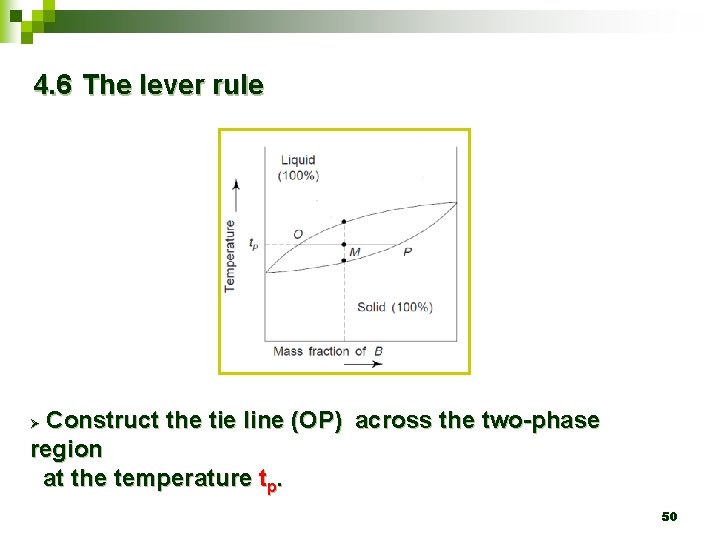
4. 6 The lever rule Ø This rule help to calculate the proportions of solid and liquid material present in the mixture at any given temperature. 49

4. 6 The lever rule Construct the tie line (OP) across the two-phase region at the temperature tp. Ø 50

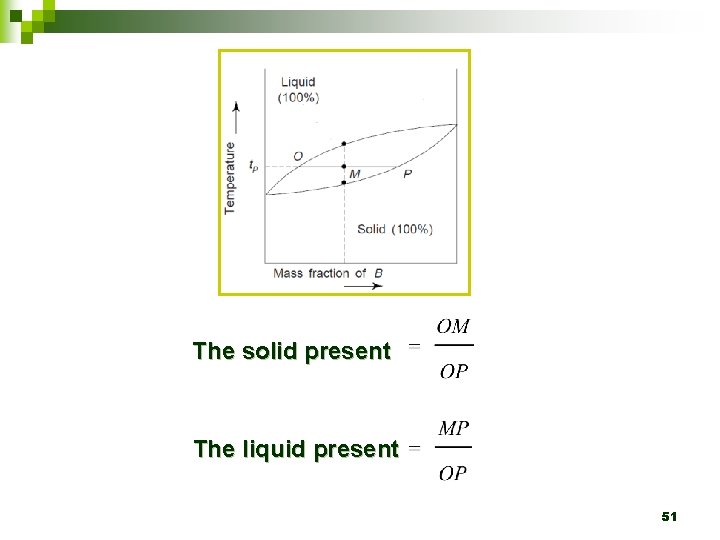
The solid present The liquid present 51