Chapter 24 Organic Chemistry Classes of Organic compounds


- Slides: 27

Chapter 24 Organic Chemistry Classes of Organic compounds � 24. 2 Alphatic hydrocarbons v Alkane v cycloalkane v Alkenes v Alkynes Ø Aromatic hydrocarbon Ø Chemistry of functional groups v Alcohol , ethers , aldhyde and ketones , carboxylic acid , esters � 24. 1 Dr. Laila Al-Harbi

24. 1 Classes of Organic compounds � The chemistry of carbon compounds. � Carbon has the ability to form long chains and ring structure. Dr. Laila Al-Harbi

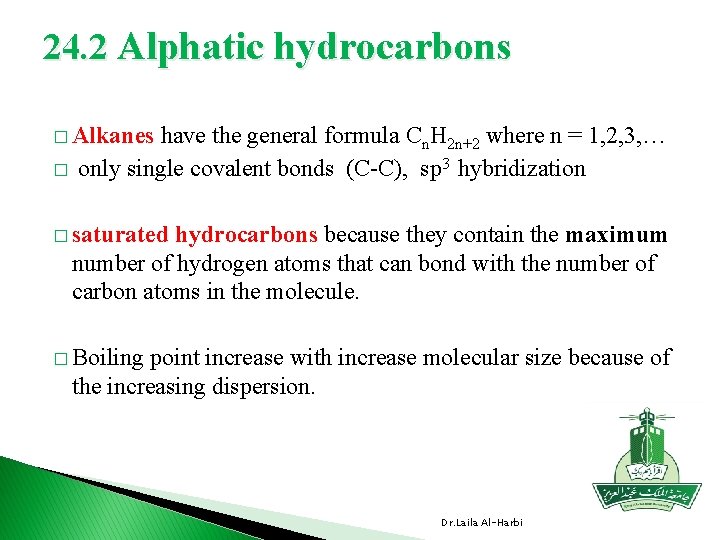
24. 2 Alphatic hydrocarbons � Alkanes � have the general formula Cn. H 2 n+2 where n = 1, 2, 3, … only single covalent bonds (C-C), sp 3 hybridization � saturated hydrocarbons because they contain the maximum number of hydrogen atoms that can bond with the number of carbon atoms in the molecule. � Boiling point increase with increase molecular size because of the increasing dispersion. Dr. Laila Al-Harbi

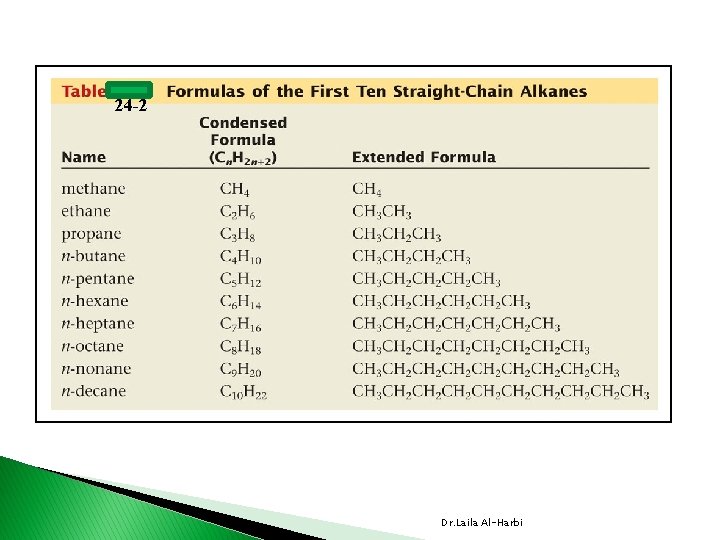
24 -2 Dr. Laila Al-Harbi

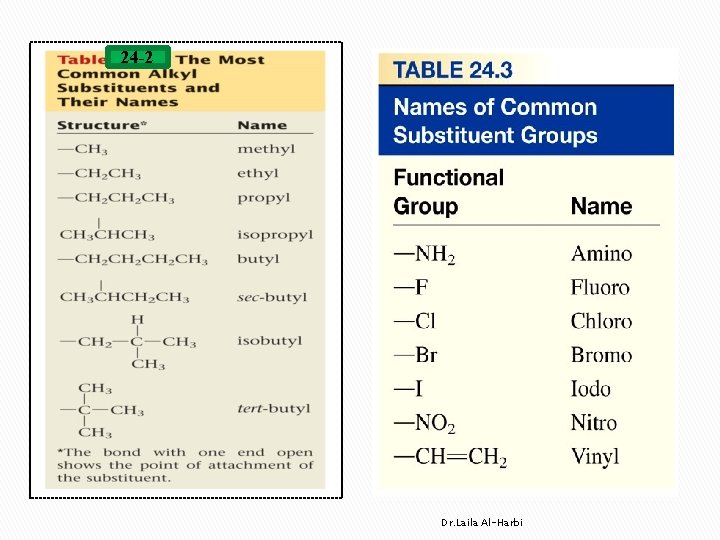
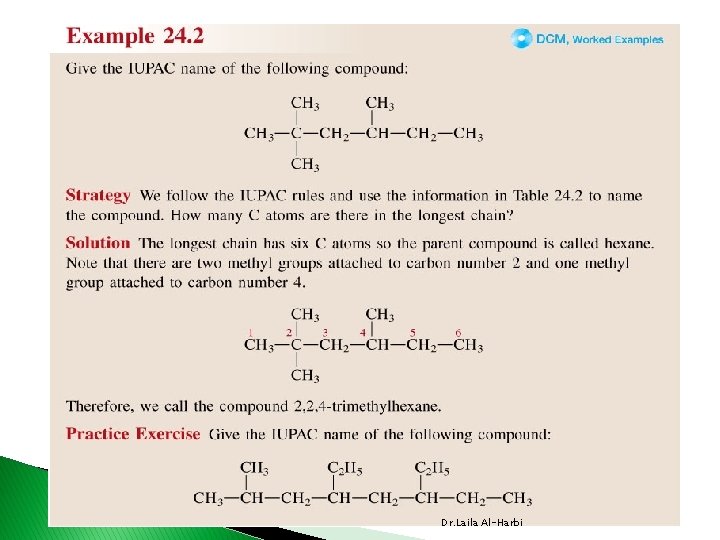
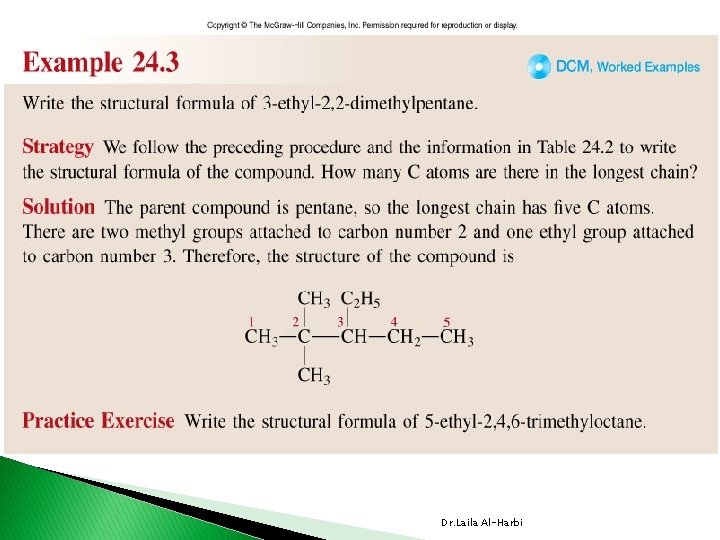
Alkanes Nomenclature � IUPAC International Union of Pure and Applied Chemistry. � each name consist of 3 parts v prefix indicates position (1, 3, …etc. ) , number (di, tri, tetra …etc. ) and type of branches (alkyl or substituent groups table 24 -3) vparent �indicates the length of the longest carbon chain or ring v suffix �indicates the type of hydrocarbon �ane, ene, yne Dr. Laila Al-Harbi

24 -2 Dr. Laila Al-Harbi

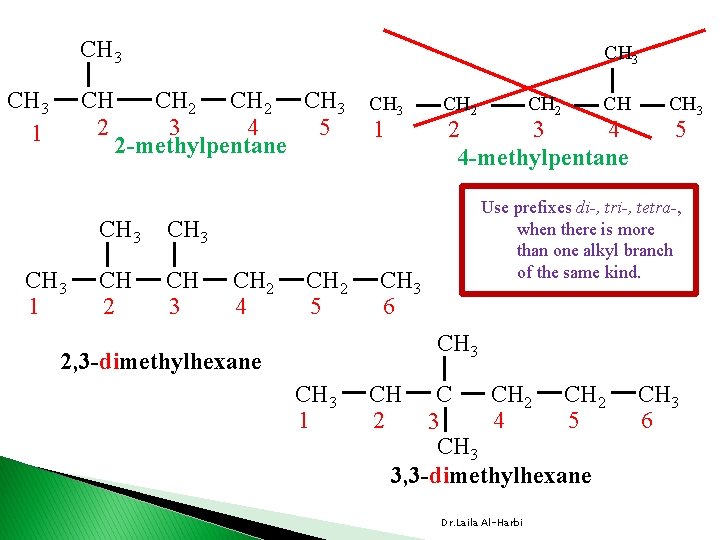
CH 3 CH 2 CH 3 1 CH 3 CH 2 CH 3 3 4 5 2 -methylpentane CH 3 CH 2 4 CH 2 5 CH 3 1 CH 2 3 4 4 -methylpentane CH 3 5 Use prefixes di-, tri-, tetra-, when there is more than one alkyl branch of the same kind. CH 3 6 CH 3 2, 3 -dimethylhexane CH 3 1 CH 2 4 5 3 CH 3 3, 3 -dimethylhexane Dr. Laila Al-Harbi CH 3 6

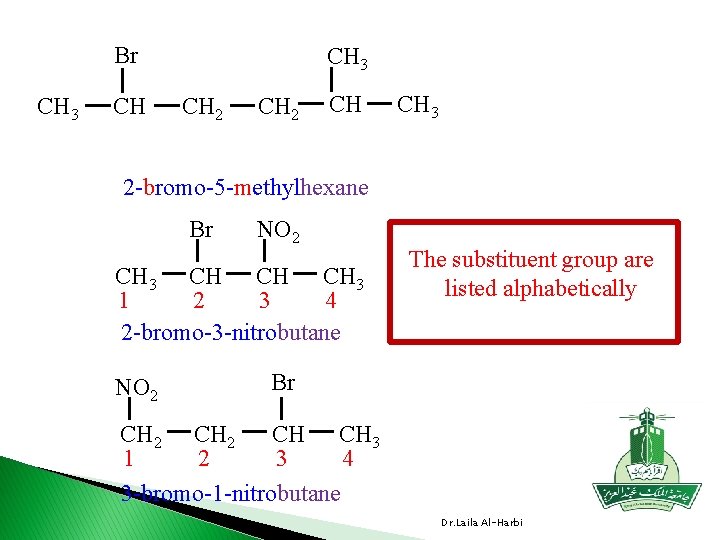
Br CH 3 CH 2 CH CH 3 2 -bromo-5 -methylhexane Br NO 2 CH 3 CH CH CH 3 1 2 3 4 2 -bromo-3 -nitrobutane NO 2 The substituent group are listed alphabetically Br CH CH 3 CH 2 1 2 3 4 3 -bromo-1 -nitrobutane Dr. Laila Al-Harbi

Dr. Laila Al-Harbi

Dr. Laila Al-Harbi

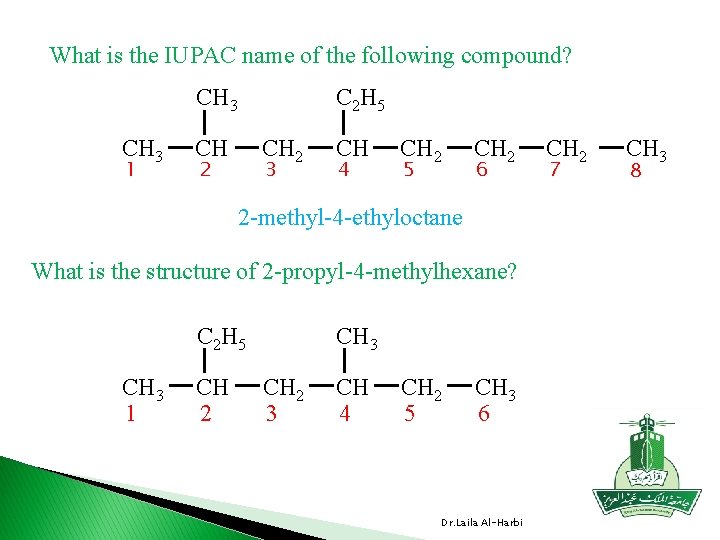
What is the IUPAC name of the following compound? CH 3 1 C 2 H 5 CH CH 2 2 3 CH 4 CH 2 5 CH 2 6 2 -methyl-4 -ethyloctane What is the structure of 2 -propyl-4 -methylhexane? C 2 H 5 CH 3 1 CH 2 CH 3 CH 2 3 CH 4 CH 2 5 CH 3 6 Dr. Laila Al-Harbi CH 2 7 CH 3 8

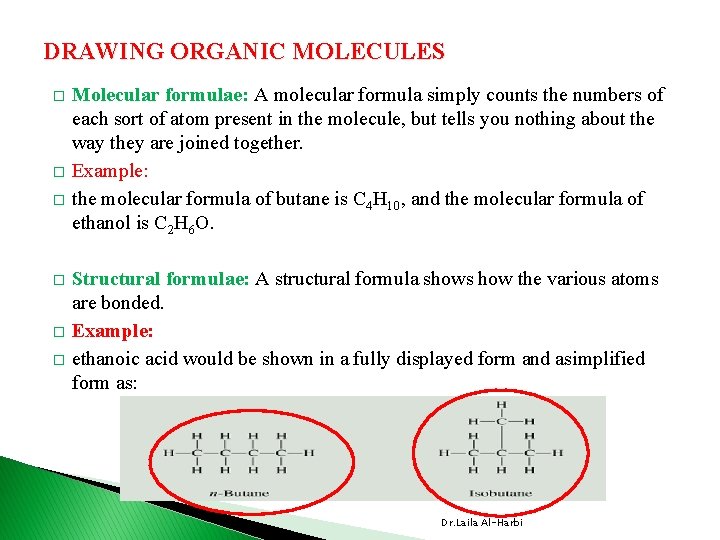
DRAWING ORGANIC MOLECULES � � � Molecular formulae: A molecular formula simply counts the numbers of each sort of atom present in the molecule, but tells you nothing about the way they are joined together. Example: the molecular formula of butane is C 4 H 10, and the molecular formula of ethanol is C 2 H 6 O. Structural formulae: A structural formula shows how the various atoms are bonded. Example: ethanoic acid would be shown in a fully displayed form and asimplified form as: Dr. Laila Al-Harbi

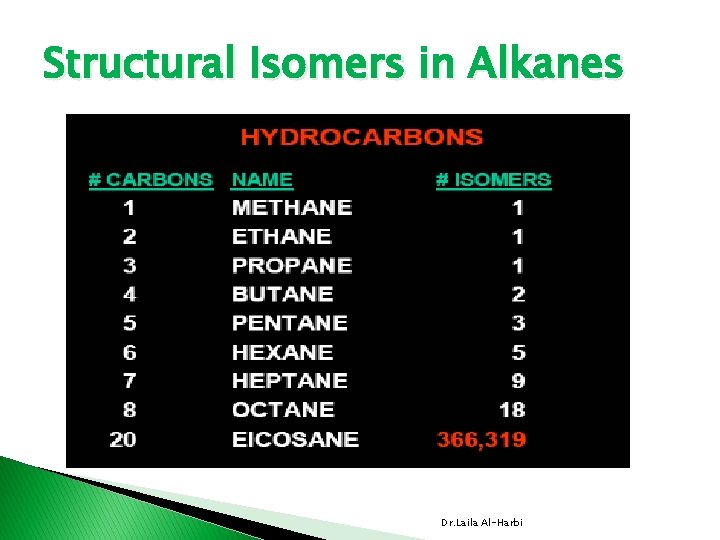
Structural Isomers in Alkanes Dr. Laila Al-Harbi

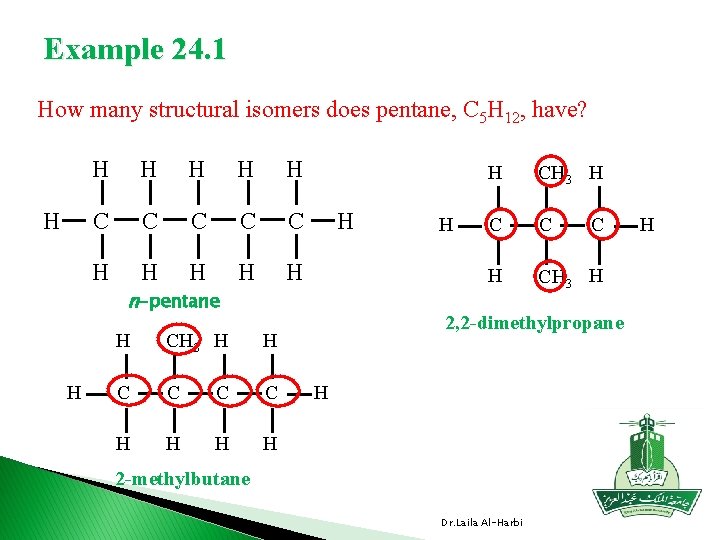
Example 24. 1 How many structural isomers does pentane, C 5 H 12, have? H H H C C C H H H n-pentane H H CH 3 H H C C H H H CH 3 H C C H CH 3 H C 2, 2 -dimethylpropane H 2 -methylbutane Dr. Laila Al-Harbi H

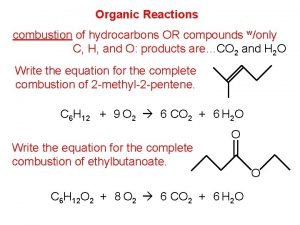
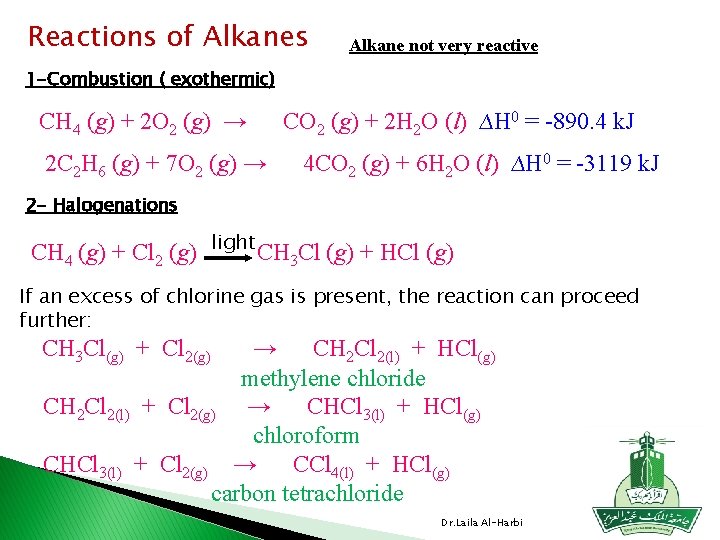
Reactions of Alkanes Alkane not very reactive 1 -Combustion ( exothermic) CH 4 (g) + 2 O 2 (g) → 2 C 2 H 6 (g) + 7 O 2 (g) → CO 2 (g) + 2 H 2 O (l) ∆H 0 = -890. 4 k. J 4 CO 2 (g) + 6 H 2 O (l) ∆H 0 = -3119 k. J 2 - Halogenations CH 4 (g) + Cl 2 (g) light CH 3 Cl (g) + HCl (g) If an excess of chlorine gas is present, the reaction can proceed further: CH 3 Cl(g) + Cl 2(g) → CH 2 Cl 2(l) + HCl(g) methylene chloride CH 2 Cl 2(l) + Cl 2(g) → CHCl 3(l) + HCl(g) chloroform CHCl 3(l) + Cl 2(g) → CCl 4(l) + HCl(g) carbon tetrachloride Dr. Laila Al-Harbi

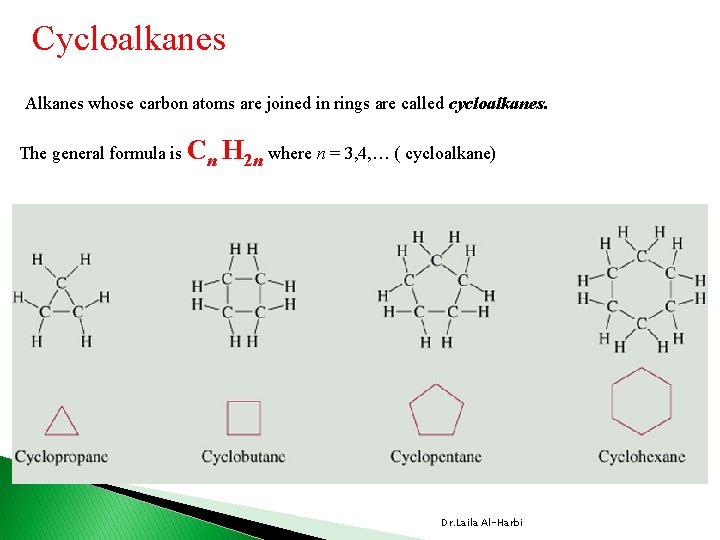
Cycloalkanes Alkanes whose carbon atoms are joined in rings are called cycloalkanes. The general formula is Cn H 2 n where n = 3, 4, … ( cycloalkane) Dr. Laila Al-Harbi

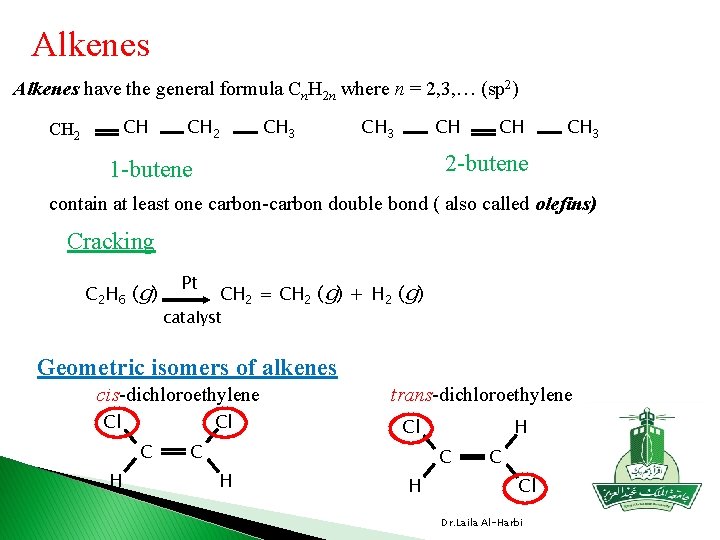
Alkenes have the general formula Cn. H 2 n where n = 2, 3, … (sp 2) CH CH 2 CH 3 CH CH 3 2 -butene 1 -butene contain at least one carbon-carbon double bond ( also called olefins) Cracking C 2 H 6 (g) Pt CH 2 = CH 2 (g) + H 2 (g) catalyst Geometric isomers of alkenes cis-dichloroethylene Cl Cl C H trans-dichloroethylene Cl H C Cl Dr. Laila Al-Harbi

Which of the following compounds has geometrical isomer ? H Cl C H H C C H Cl NO , Geometrical isomer NO 2 Cl Cl NO 2 Cl H C Cl C C H yes , it can have geometrical isomer NO , Geometrical isomer Cl Cl C H yes , it can have geometrical isomer Dr. Laila Al-Harbi

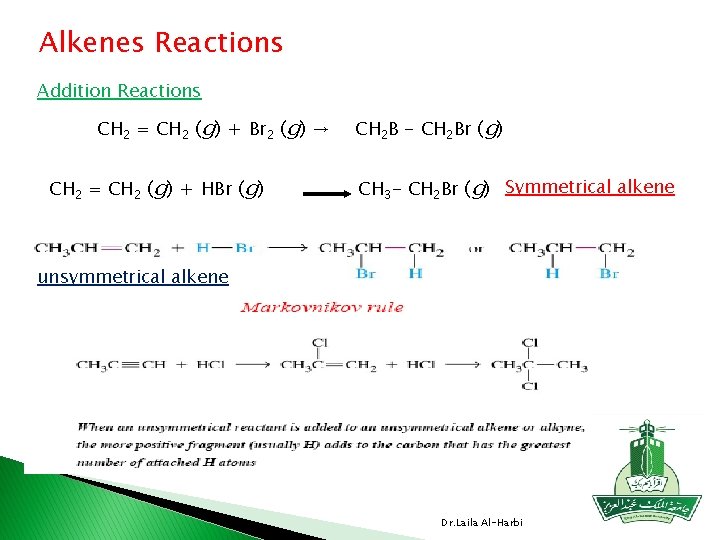
Alkenes Reactions Addition Reactions CH 2 = CH 2 (g) + Br 2 (g) → CH 2 = CH 2 (g) + HBr (g) CH 2 B - CH 2 Br (g) CH 3 - CH 2 Br (g) Symmetrical alkene unsymmetrical alkene Dr. Laila Al-Harbi

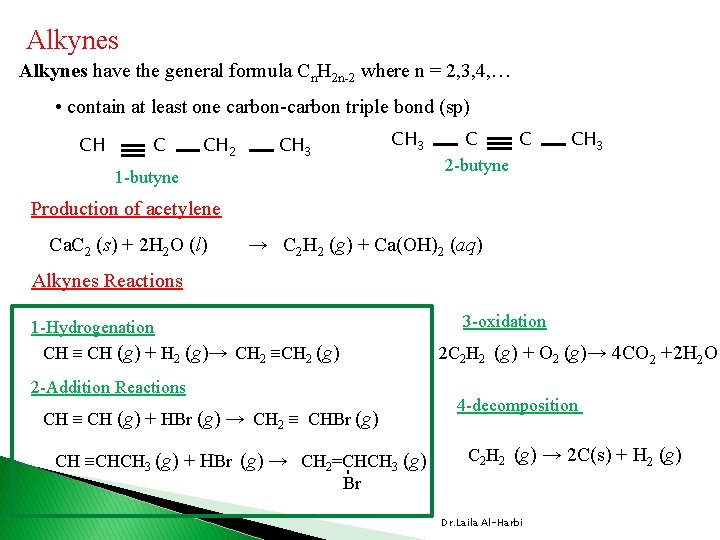
Alkynes have the general formula Cn. H 2 n-2 where n = 2, 3, 4, … • contain at least one carbon-carbon triple bond (sp) CH C CH 2 CH 3 1 -butyne C C 2 -butyne CH 3 Production of acetylene Ca. C 2 (s) + 2 H 2 O (l) → C 2 H 2 (g) + Ca(OH)2 (aq) Alkynes Reactions 3 -oxidation 1 -Hydrogenation CH ≡ CH (g) + H 2 (g)→ CH 2 ≡CH 2 (g) 2 C 2 H 2 (g) + O 2 (g)→ 4 CO 2 +2 H 2 O 2 -Addition Reactions CH ≡ CH (g) + HBr (g) → CH 2 ≡ CHBr (g) C 2 H 2 (g) → 2 C(s) + H 2 (g) - CH ≡CHCH 3 (g) + HBr (g) → CH 2=CHCH 3 (g) Br 4 -decomposition Dr. Laila Al-Harbi

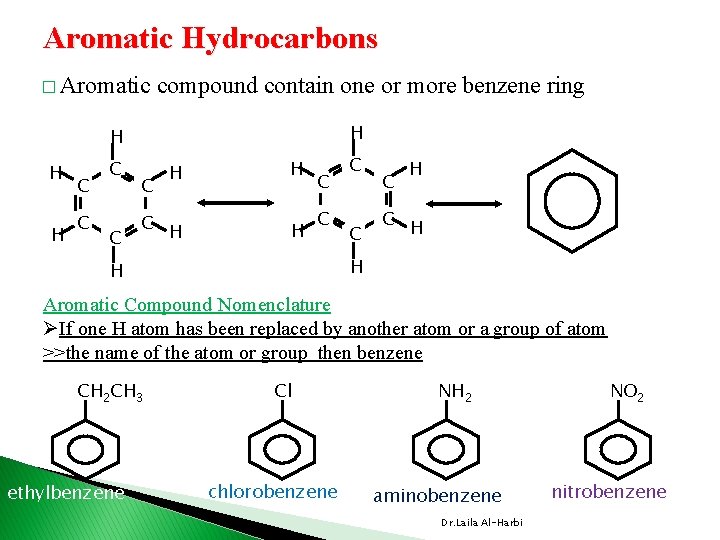
Aromatic Hydrocarbons � Aromatic compound contain one or more benzene ring H H H H C C C H H Aromatic Compound Nomenclature ØIf one H atom has been replaced by another atom or a group of atom >>the name of the atom or group then benzene CH 2 CH 3 ethylbenzene Cl chlorobenzene NH 2 aminobenzene Dr. Laila Al-Harbi NO 2 nitrobenzene

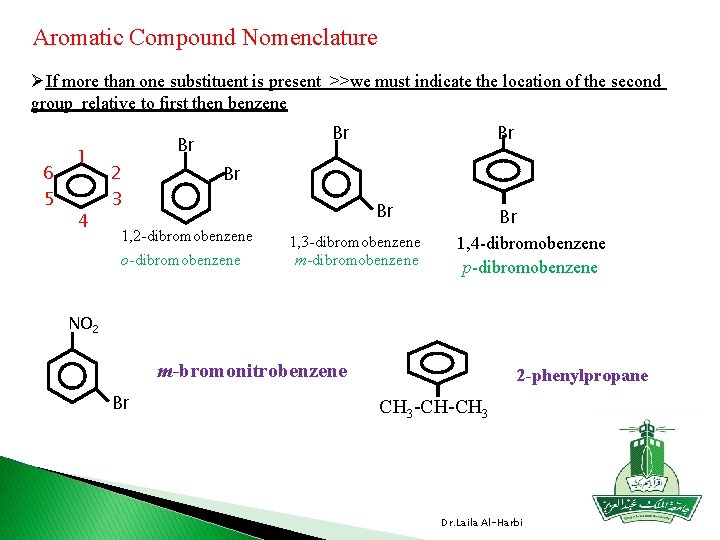
Aromatic Compound Nomenclature ØIf more than one substituent is present >>we must indicate the location of the second group relative to first then benzene 6 5 1 4 Br Br 2 3 Br Br Br 1, 2 -dibromobenzene o-dibromobenzene 1, 3 -dibromobenzene m-dibromobenzene Br 1, 4 -dibromobenzene p-dibromobenzene NO 2 m-bromonitrobenzene Br 2 -phenylpropane CH 3 -CH-CH 3 Dr. Laila Al-Harbi

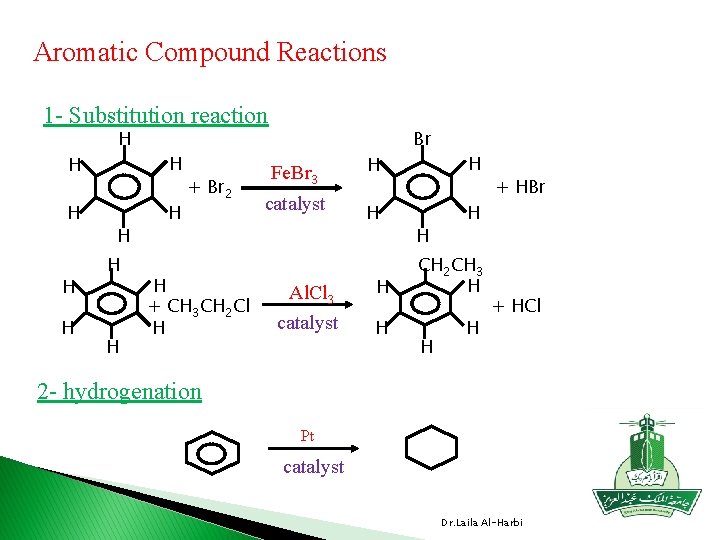
Aromatic Compound Reactions 1 - Substitution reaction H H H H H + Br 2 H + CH 3 CH 2 Cl H Fe. Br 3 catalyst H H Br H H H Al. Cl 3 H CH 2 CH 3 H catalyst H H H + HBr + HCl 2 - hydrogenation Pt catalyst Dr. Laila Al-Harbi

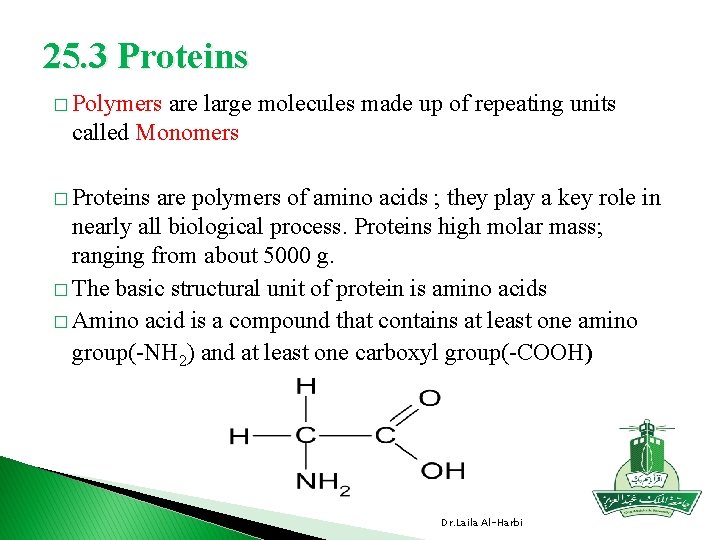
25. 3 Proteins � Polymers are large molecules made up of repeating units called Monomers � Proteins are polymers of amino acids ; they play a key role in nearly all biological process. Proteins high molar mass; ranging from about 5000 g. � The basic structural unit of protein is amino acids � Amino acid is a compound that contains at least one amino group(-NH 2) and at least one carboxyl group(-COOH) Dr. Laila Al-Harbi

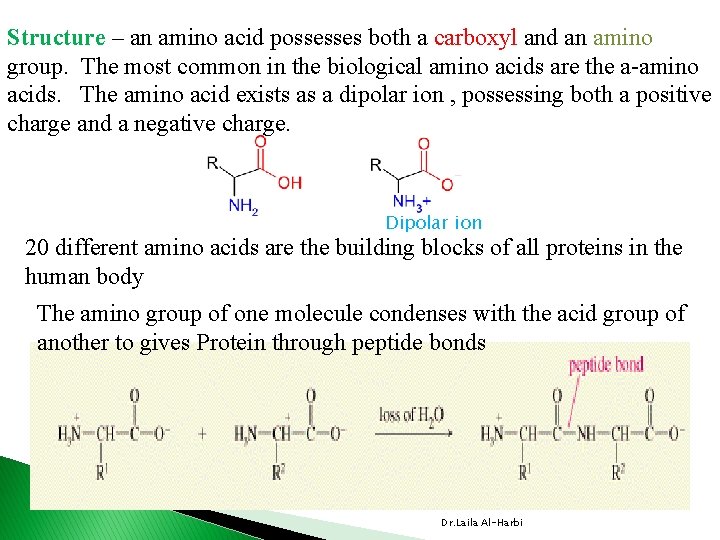
Structure – an amino acid possesses both a carboxyl and an amino group. The most common in the biological amino acids are the a-amino acids. The amino acid exists as a dipolar ion , possessing both a positive charge and a negative charge. Dipolar ion 20 different amino acids are the building blocks of all proteins in the human body The amino group of one molecule condenses with the acid group of another to gives Protein through peptide bonds Dr. Laila Al-Harbi

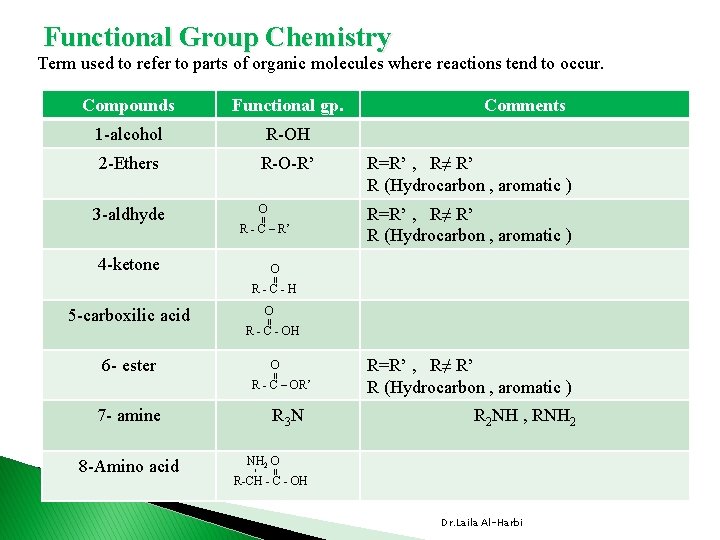
Functional Group Chemistry Term used to refer to parts of organic molecules where reactions tend to occur. Functional gp. 1 -alcohol R-OH 2 -Ethers R-O-R’ R=R’ , R≠ R’ R (Hydrocarbon , aromatic ) O R=R’ , R≠ R’ R (Hydrocarbon , aromatic ) 3 -aldhyde 4 -ketone = Compounds R - C – R’ Comments O = R-C-H R - C - OH O = 6 - ester O = 5 -carboxilic acid R - C – OR’ 7 - amine R 2 NH , RNH 2 O = - 8 -Amino acid R 3 N R=R’ , R≠ R’ R (Hydrocarbon , aromatic ) R-CH - C - OH Dr. Laila Al-Harbi

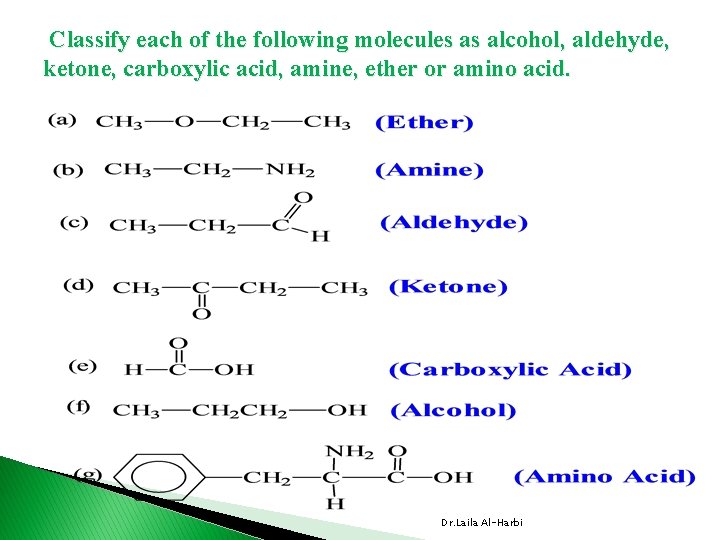
Classify each of the following molecules as alcohol, aldehyde, ketone, carboxylic acid, amine, ether or amino acid. Dr. Laila Al-Harbi
 Chemistry nomenclature
Chemistry nomenclature Ib organic chemistry
Ib organic chemistry Organic vs inorganic chemistry
Organic vs inorganic chemistry Organic chemistry (3rd) edition chapter 1 problem 16s
Organic chemistry (3rd) edition chapter 1 problem 16s Introduction to organic chemistry
Introduction to organic chemistry Chapter 22 review organic chemistry section 1 answers
Chapter 22 review organic chemistry section 1 answers Organic chemistry david klein 3rd edition
Organic chemistry david klein 3rd edition Organic chemistry chapter 9
Organic chemistry chapter 9 What functional group is ch3
What functional group is ch3 Chapter 3 organic chemistry
Chapter 3 organic chemistry Analytical chemistry chapter 1
Analytical chemistry chapter 1 Halohydrin formation
Halohydrin formation Ionic covalent and metallic venn diagram
Ionic covalent and metallic venn diagram Quanto classe e subclasse
Quanto classe e subclasse Pre ap classes vs regular classes
Pre ap classes vs regular classes Water soluble vitamins absorption
Water soluble vitamins absorption Vitamins classification chart
Vitamins classification chart Four types of organic molecules
Four types of organic molecules Decomposition of organic matter equation
Decomposition of organic matter equation Charring test of organic and inorganic compounds
Charring test of organic and inorganic compounds Combustion of organic substances
Combustion of organic substances These are organic compounds made by living things
These are organic compounds made by living things All organic compounds must contain the element
All organic compounds must contain the element Organic compounds must contain
Organic compounds must contain Difference between organic and inorganic
Difference between organic and inorganic Purification and characterization of organic compounds
Purification and characterization of organic compounds Organic compounds such as proteins and starches are too
Organic compounds such as proteins and starches are too Organic vs inorganic compounds
Organic vs inorganic compounds