CHAPTER 2 Chemistry of Life Living things consist

























- Slides: 72

CHAPTER 2 Chemistry of Life

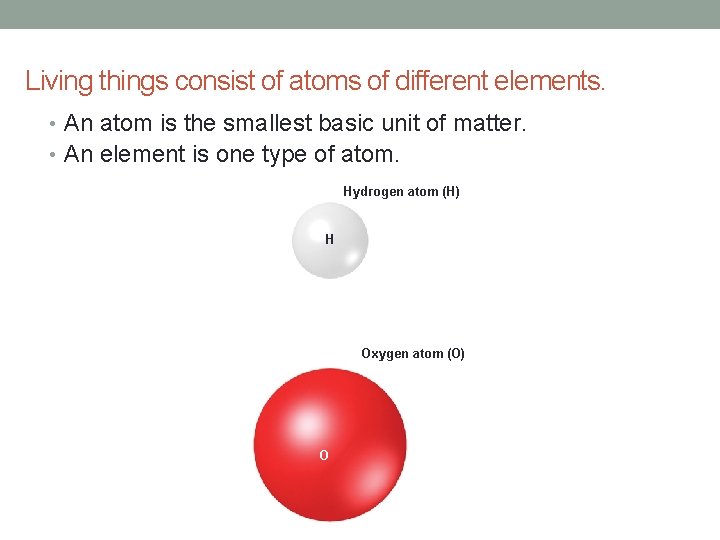
Living things consist of atoms of different elements. • An atom is the smallest basic unit of matter. • An element is one type of atom. Hydrogen atom (H) H Oxygen atom (O) O

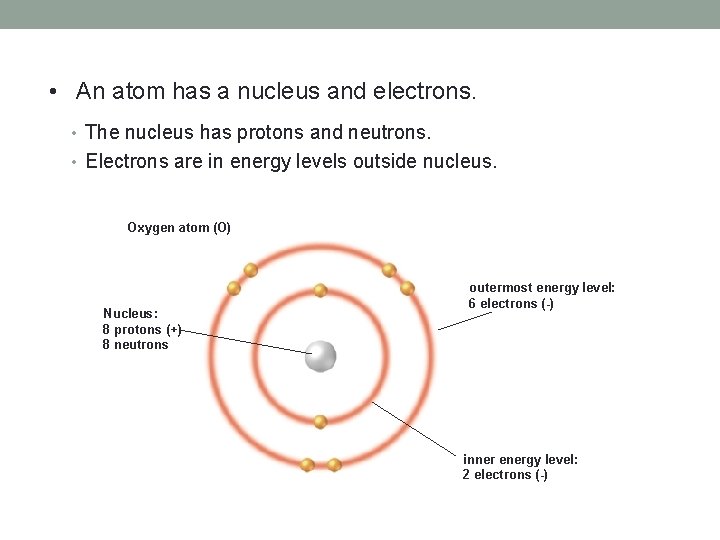
• An atom has a nucleus and electrons. • The nucleus has protons and neutrons. • Electrons are in energy levels outside nucleus. Oxygen atom (O) Nucleus: 8 protons (+) 8 neutrons outermost energy level: 6 electrons (-) inner energy level: 2 electrons (-)

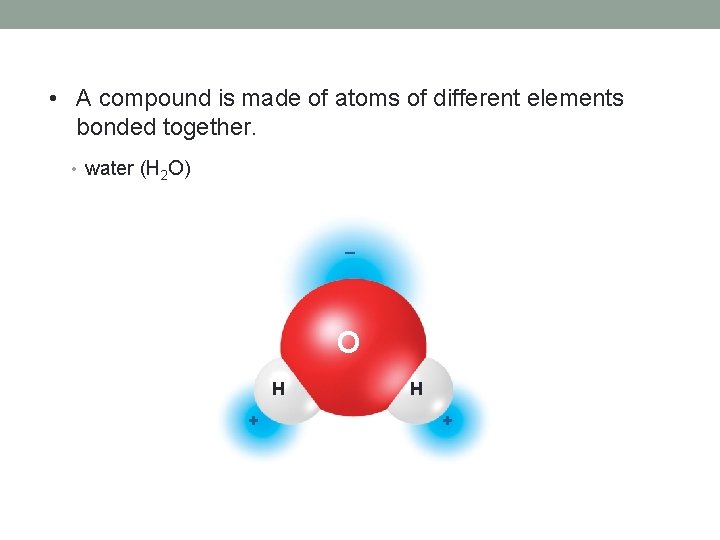
• A compound is made of atoms of different elements bonded together. • water (H 2 O) _ O H +

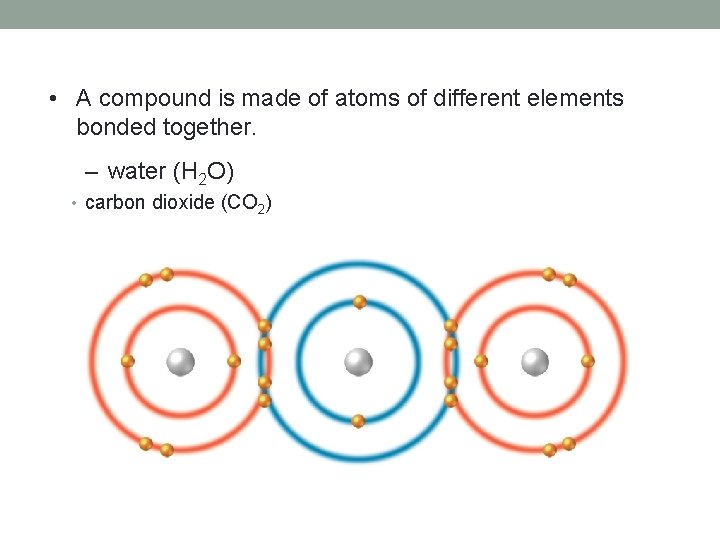
• A compound is made of atoms of different elements bonded together. – water (H 2 O) • carbon dioxide (CO 2)

• A compound is made of atoms of different elements bonded together. – water (H 2 O) – carbon dioxide (CO 2) • many other carbon-based compounds in living things

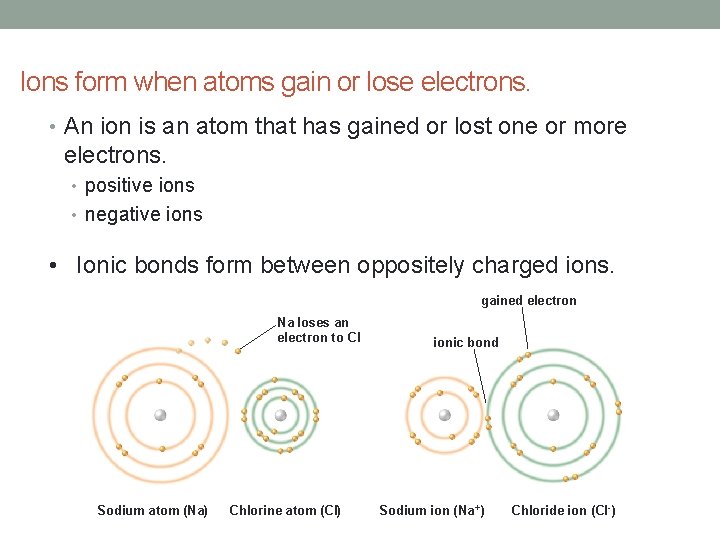
Ions form when atoms gain or lose electrons. • An ion is an atom that has gained or lost one or more electrons. • positive ions • negative ions • Ionic bonds form between oppositely charged ions. gained electron Na loses an electron to CI Sodium atom (Na) Chlorine atom (CI) ionic bond Sodium ion (Na +) Chloride ion (CI -)

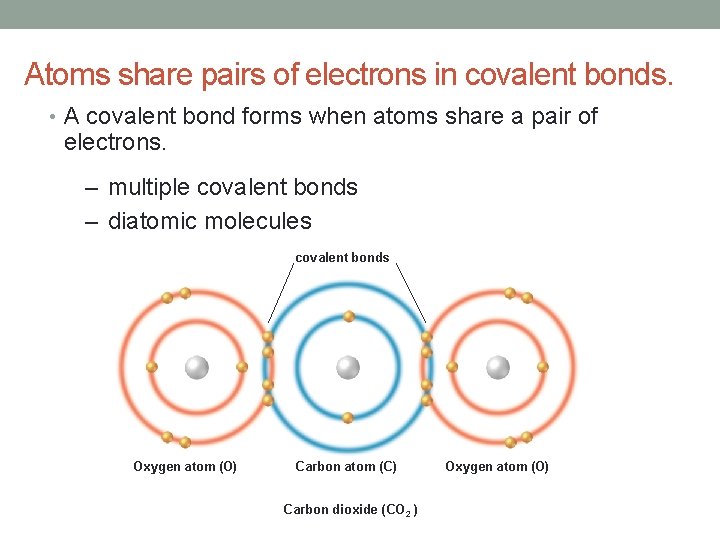
Atoms share pairs of electrons in covalent bonds. • A covalent bond forms when atoms share a pair of electrons. – multiple covalent bonds – diatomic molecules covalent bonds Oxygen atom (O) Carbon atom (C) Carbon dioxide (CO 2 ) Oxygen atom (O)

2. 2 Properties of Water

Water • Needed by all know forms of life • ~75% of Earth’s surface is covered with water • Water refers to liquid form. (Solid and gas forms also seen) • Total Water on Earth

Structure and Properties of Water • What are some you can think of? • Tasteless & odorless • Transparent…Why is that important? • Some marine organisms need sunlight to make food http: //www. european-coatings. com/var/ezflow_site/storage/images/european-coatings/home/raw-materialstechnologies/functional-coatings/relationship-between-surface-wettability-and-transparency-in-four-water-conditions/4481225 -1 eng-GB/Relationship-between-surface-wettability-and-transparency-in-four-water-conditions. jpg

Chemical Structure of Water • One atom of O, two Atoms of H • O attracts negatively charged e- more than the H • Therefore O has a slightly – charge and the H has a slightly + charge • This makes it a polar molecule because it has a different electrical charge between different parts of the same molecule

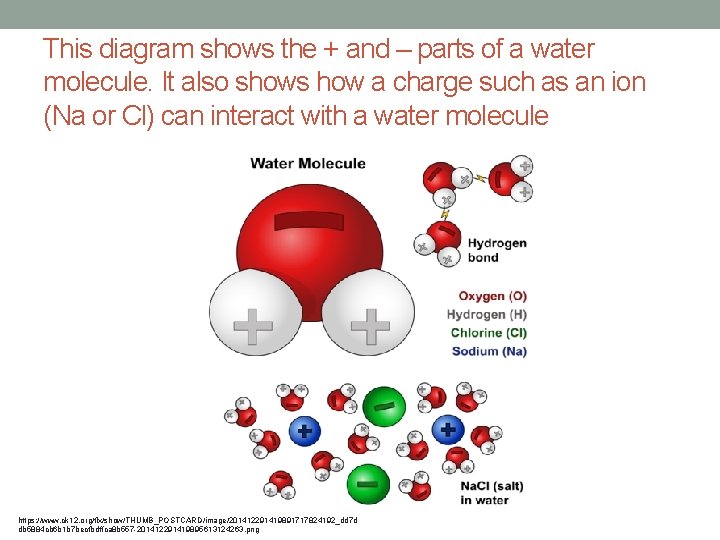
This diagram shows the + and – parts of a water molecule. It also shows how a charge such as an ion (Na or Cl) can interact with a water molecule https: //www. ck 12. org/flx/show/THUMB_POSTCARD/image/201412291419891717824192_dd 7 d db 5884 cb 6 b 1 b 7 becfbdffca 8 b 557 -201412291419895613124263. png

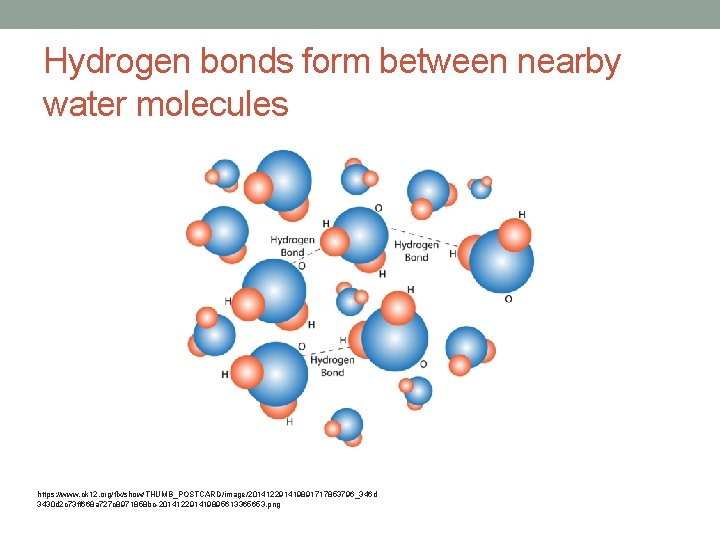
• Opposites attract • H+ end of one water molecule is attracted to the O- end of a nearby molecule • This forms a Hydrogen bond (weak) • Bonds between molecules are not as strong as inside molecules • They are strong enough to hold together nearby molecules • Cohesion - between two water molecules • Adhesion – attraction between two different molecules

Hydrogen bonds form between nearby water molecules https: //www. ck 12. org/flx/show/THUMB_POSTCARD/image/201412291419891717853796_346 d 3430 d 2 c 73 ff 668 a 727 c 8971858 bc-201412291419895613365653. png

Properties of Water • Water sticks together (cohesion) examples? https: //www. ck 12. org/flx/show/THUMB_POSTCARD/image/201412291419891717895411_4 e 0 e 50 a 24 b 1 bf 2 b 454 c 2 e 5736 ea 4 f 95 d-201412291419895613631251. jpg

• H bonds cause a higher boiling point (100 C, 212 F) • Higher boiling point causes most water on Earth to be in liquid state, not gas state • All living things on Earth need water • H bonds cause water to expand when frozen • Causes ice to have lower density than liquid water (ice floats) http: //militaryhuntingandfishing. com/wp-content/uploads/2015/01/Ice-fishing-Canada. jpg

Water and Life • Human= ~70% water not counting fat (varies) • Why so much Water? • Substances the body needs are dissolved in water • Biochemical reactions occur in water • 2 Important biochemical reactions: • 1. photosynthesis: 6 CO 2 + 6 H 2 O + Energy → C 6 H 12 O 6 + 6 O 2 • 2. cellular respiration: C 6 H 12 O 6 + 6 O 2 → 6 CO 2 + 6 H 2 O + Energy. • Just about all life processes involve water

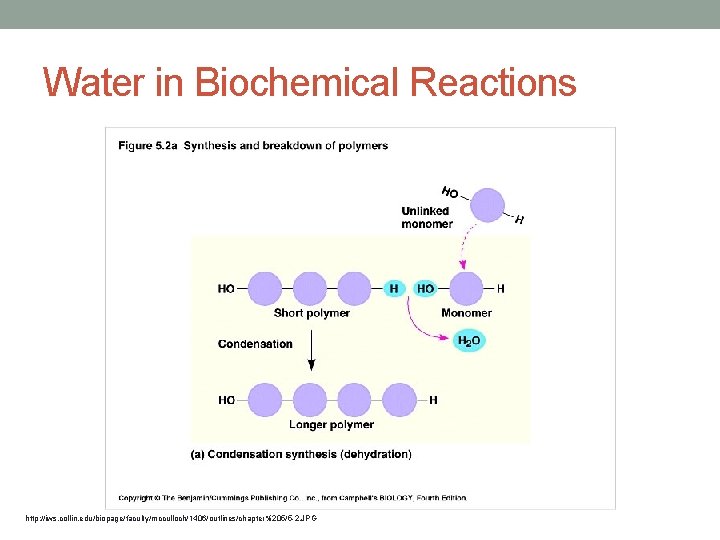
Water in Biochemical Reactions http: //iws. collin. edu/biopage/faculty/mcculloch/1406/outlines/chapter%205/5 -2. JPG

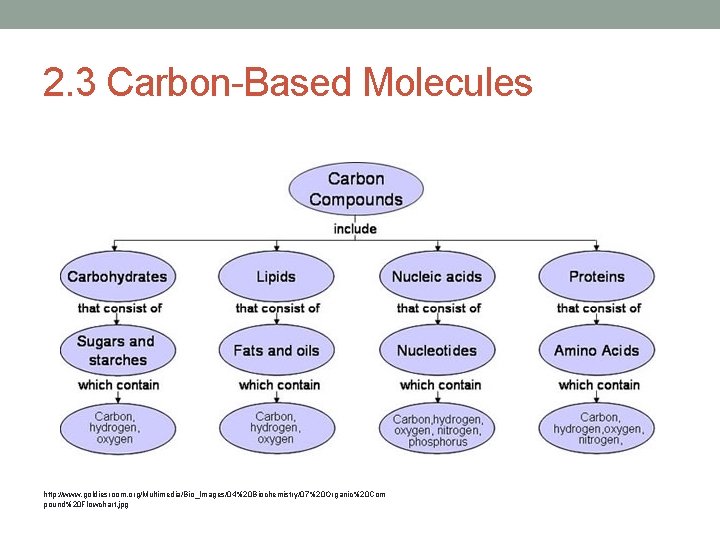
2. 3 Carbon-Based Molecules http: //www. goldiesroom. org/Multimedia/Bio_Images/04%20 Biochemistry/07%20 Organic%20 Com pound%20 Flowchart. jpg

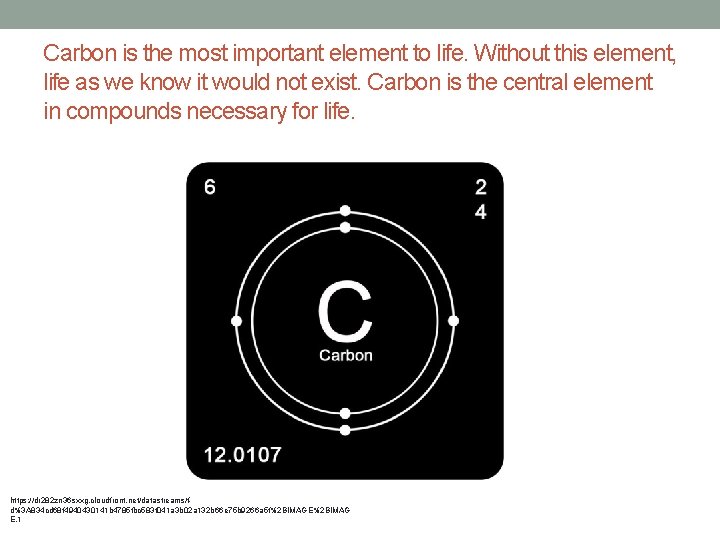
Carbon is the most important element to life. Without this element, life as we know it would not exist. Carbon is the central element in compounds necessary for life. https: //dr 282 zn 36 sxxg. cloudfront. net/datastreams/fd%3 A 834 cd 68 f 4940430141 b 4785 fbc 583 f 041 a 3 b 02 a 132 b 66 e 75 b 9266 a 5 f%2 BIMAGE%2 BIMAG E. 1

The significance of Carbon • A compound in living things is an Organic Compound • Organic compounds make up cells/other structures and carry out life processes • Carbon is the main element in organic compounds (important) http: //ww 2. coastal. edu/kingw/psyc 460/molecules/organic_molecules. jpg

Compounds • Compound: substance that consists of two or more elements (always the same) • The smallest part of a compound is a molecule • A molecule of water always contains 2 H atoms and 1 O atom • Water is not an organic compound https: //dr 282 zn 36 sxxg. cloudfront. net/datastreams/fd%3 Abf 34 ad 1 d 559 d 476 bbe 62 fe 8278 fc 9 e 8 f 088 e 04 df 8 ba 2405 a 6 a 72 a 87 f%2 BIMAGE_THUMB_L ARGE%2 BIMAGE_THUMB_LARGE. 1

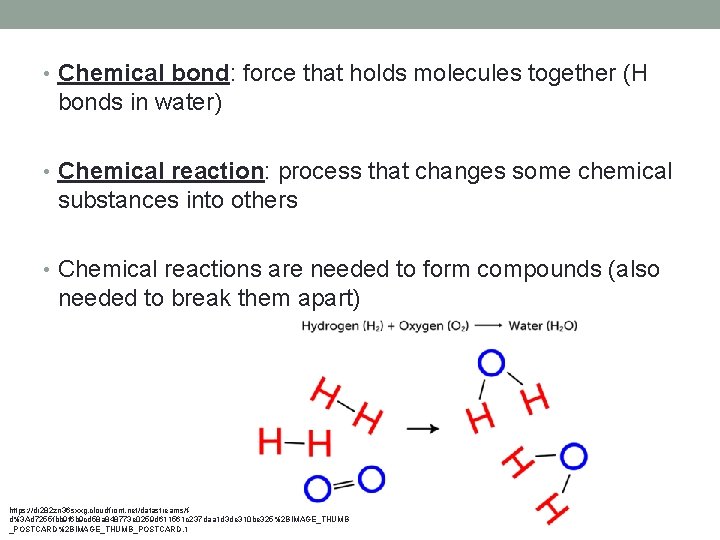
• Chemical bond: force that holds molecules together (H bonds in water) • Chemical reaction: process that changes some chemical substances into others • Chemical reactions are needed to form compounds (also needed to break them apart) https: //dr 282 zn 36 sxxg. cloudfront. net/datastreams/fd%3 Ad 7255 fbb 9 f 6 b 9 cd 58 a 848773 e 0259 d 611561 c 237 daa 1 d 3 de 310 be 325%2 BIMAGE_THUMB _POSTCARD%2 BIMAGE_THUMB_POSTCARD. 1

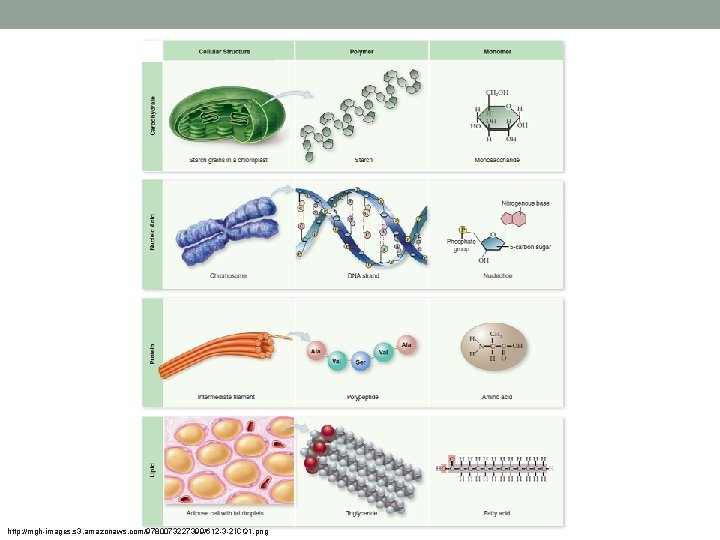
Carbon • Why is carbon needed for life? • It forms stable bonds with many elements including C • C forms a variety of complex and simple molecules (10 million C based compounds) • The millions can be grouped into 4 main types (macromolecules) • 1. carbohydrates • 2. lipids • 3. proteins • 4. nucleic acids

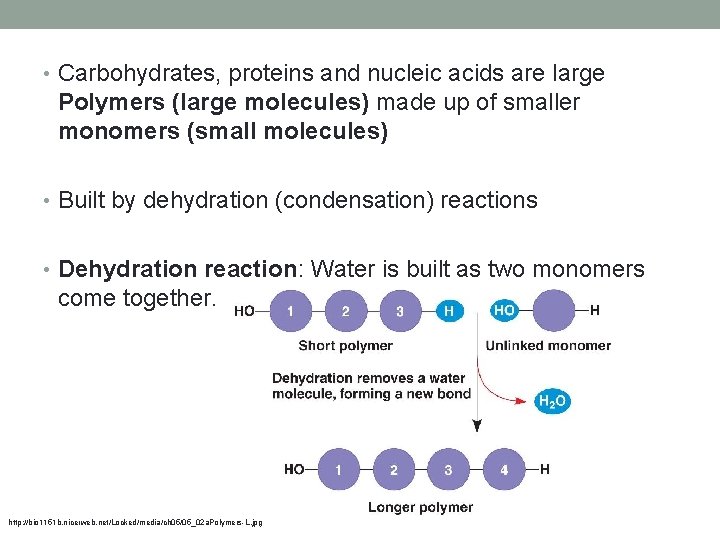
• Carbohydrates, proteins and nucleic acids are large Polymers (large molecules) made up of smaller monomers (small molecules) • Built by dehydration (condensation) reactions • Dehydration reaction: Water is built as two monomers come together. http: //bio 1151 b. nicerweb. net/Locked/media/ch 05/05_02 a. Polymers-L. jpg

http: //mgh-images. s 3. amazonaws. com/9780073227399/612 -3 -2 ICQ 1. png

http: //4. bp. blogspot. com/_207 DNIa. Lgc/TBS 99 j. Vr. Lu. I/AAAAAFw/ADLT 6 H 9 h. PYU/s 1600/Carbon+compounds. gif

Carbohydrates • Carbohydrate: organic compound such as sugar or starch and is used to store E • Built of small monomers called monosaccharides • Contain only Carbon, Hydrogen and Oxygen • https: //www. ck 12. org/flx/show/image/201412291419891630024152_1 d 1 b 9 dd 1016 afe 4 a 756791 605 e 44 e 54 b-201412291419895625805275. jpg

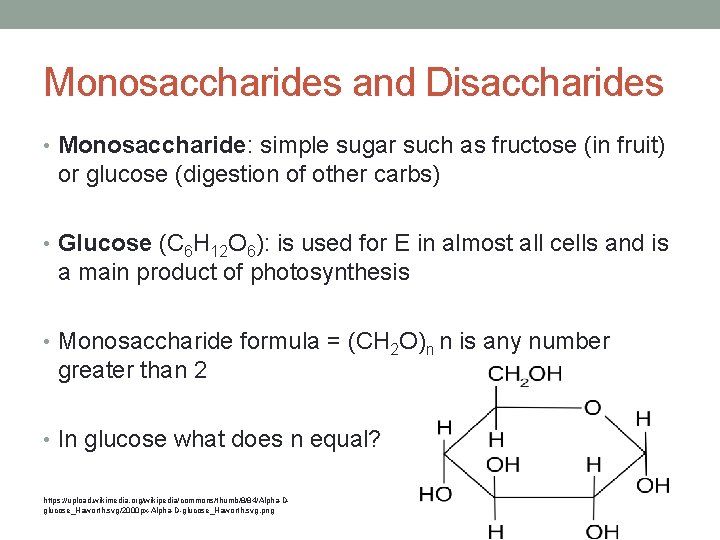
Monosaccharides and Disaccharides • Monosaccharide: simple sugar such as fructose (in fruit) or glucose (digestion of other carbs) • Glucose (C 6 H 12 O 6): is used for E in almost all cells and is a main product of photosynthesis • Monosaccharide formula = (CH 2 O)n n is any number greater than 2 • In glucose what does n equal? https: //upload. wikimedia. org/wikipedia/commons/thumb/8/84/Alpha-Dglucose_Haworth. svg/2000 px-Alpha-D-glucose_Haworth. svg. png

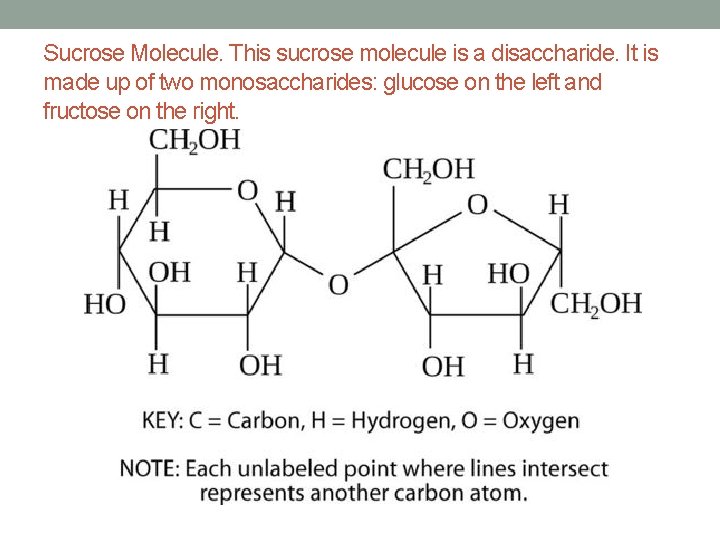
• If two monosaccharides come together they form a disaccharide • Sucrose is an example, contain glucose and fructose • Monosaccharides and disaccharides are called simple sugars • Major source of E in cells

Sucrose Molecule. This sucrose molecule is a disaccharide. It is made up of two monosaccharides: glucose on the left and fructose on the right.

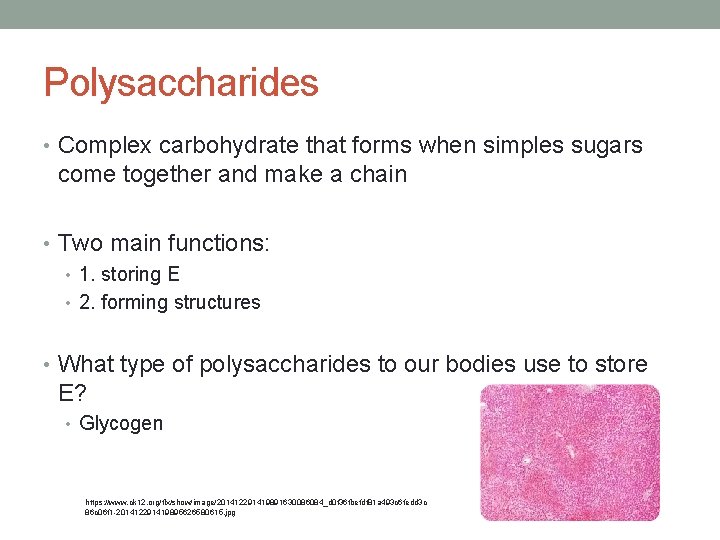
Polysaccharides • Complex carbohydrate that forms when simples sugars come together and make a chain • Two main functions: • 1. storing E • 2. forming structures • What type of polysaccharides to our bodies use to store E? • Glycogen https: //www. ck 12. org/flx/show/image/201412291419891630086084_d 0 f 36 fbefdf 81 a 493 c 6 fedd 3 c 86 c 06 f 1 -201412291419895626580615. jpg

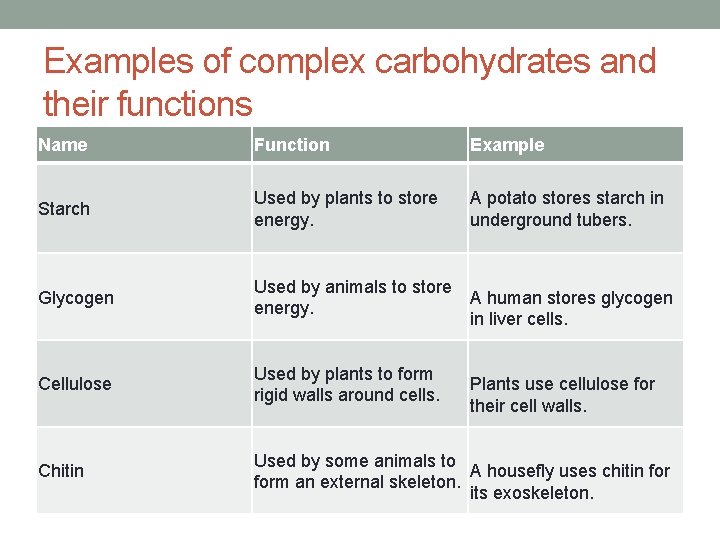
Examples of complex carbohydrates and their functions Name Function Example Starch Used by plants to store energy. A potato stores starch in underground tubers. Glycogen Used by animals to store A human stores glycogen energy. in liver cells. Cellulose Used by plants to form rigid walls around cells. Chitin Used by some animals to A housefly uses chitin form an external skeleton. its exoskeleton. Plants use cellulose for their cell walls.

Lipids • An organic compound like fat or oil • Used by organisms to store E • Lipids are made of repeating units called fatty acids • Two types of fatty acids: • 1. saturated • 2. unsaturated https: //www. ck 12. org/flx/show/image/201412291419891638863740_b 1851 c 58 c 1 beb 264 ad 82 d 6 bfad 88 f 967 -201412291419895623692225. jpg

Saturated Fatty Acids • Carbon atoms are bonded to as many hydrogen atoms as possible in saturated fatty acids • Molecules have straight chains • The straight chains can be packed together very tightly, allowing them to store energy in a compact form • Solid at room temperature. • Animals use them to store energy.

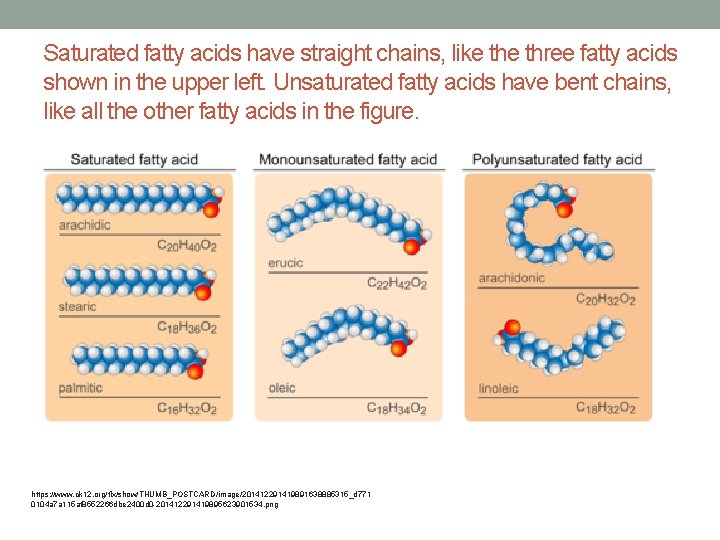
Saturated fatty acids have straight chains, like three fatty acids shown in the upper left. Unsaturated fatty acids have bent chains, like all the other fatty acids in the figure. https: //www. ck 12. org/flx/show/THUMB_POSTCARD/image/201412291419891638885315_d 771 0104 a 7 a 115 af 8552266 dbe 2400 d 0 -201412291419895623901534. png

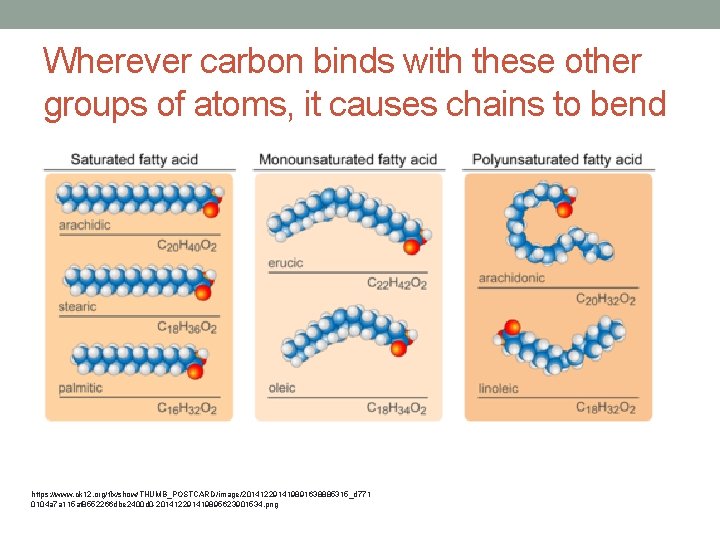
Unsaturated Fatty Acids • Some carbon atoms are not bonded to as many hydrogen atoms • When carbon binds with other groups of atoms, it causes chains to bend • Not packed together as tightly • Liquids at room temperature • Plants use unsaturated fatty acids to store energy

Wherever carbon binds with these other groups of atoms, it causes chains to bend https: //www. ck 12. org/flx/show/THUMB_POSTCARD/image/201412291419891638885315_d 771 0104 a 7 a 115 af 8552266 dbe 2400 d 0 -201412291419895623901534. png

These plant products all contain unsaturated fatty acids. https: //www. ck 12. org/flx/show/THUMB_POSTCARD/image/201412291419891638908347_eb 74 504972 aadccdf 11183 d 76 a 48 c 1 f 7 -201412291419895624137790. jpg

Types of Lipids • Triglycerides: the main form of stored energy in animals. • Phospholipids: the major components of cell membranes. • Steroids: serve as chemical messengers and have other roles.

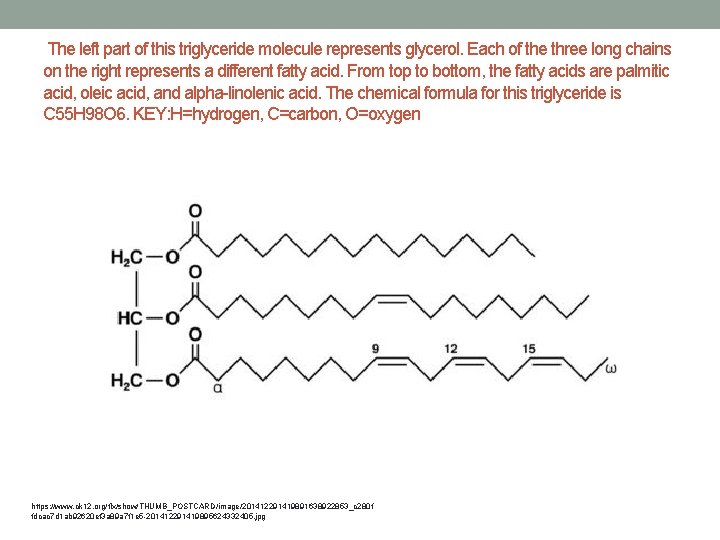
The left part of this triglyceride molecule represents glycerol. Each of the three long chains on the right represents a different fatty acid. From top to bottom, the fatty acids are palmitic acid, oleic acid, and alpha-linolenic acid. The chemical formula for this triglyceride is C 55 H 98 O 6. KEY: H=hydrogen, C=carbon, O=oxygen https: //www. ck 12. org/flx/show/THUMB_POSTCARD/image/201412291419891638922853_c 280 f fdcac 7 d 1 ab 92620 ef 3 a 89 a 7 f 1 e 5 -201412291419895624332405. jpg

Lipids and Diet • Humans need lipids for storing E and forming cell membranes. • Lipids also supply cells with E. • Essential fatty acids must be consumed in food • These include omega-3 and omega-6 fatty acids. • Both of these are needed for important biological processes, not just E.

• Excess dietary lipids can be harmful • Eating too many may cause weight gain. • A high-fat diet may also increase lipid levels in the blood. • Can increase the risk for cardiovascular disease. • Saturated fatty acids, trans fats, and cholesterol are to most common • Cholesterol is mainly responsible for narrowing arteries and causing the disease atherosclerosis

Proteins • Organic molecule made up of monomers called amino acids • 20 different amino acids found in living things • Proteins could contain just a few hundred to thousands of AA https: //www. ck 12. org/flx/show/image/201412291419891647531137_9 a 51 ddcf 1 efd 6 b 4 fd 4252 ba 0 84978 c 25 -201412291419895611792531. jpg

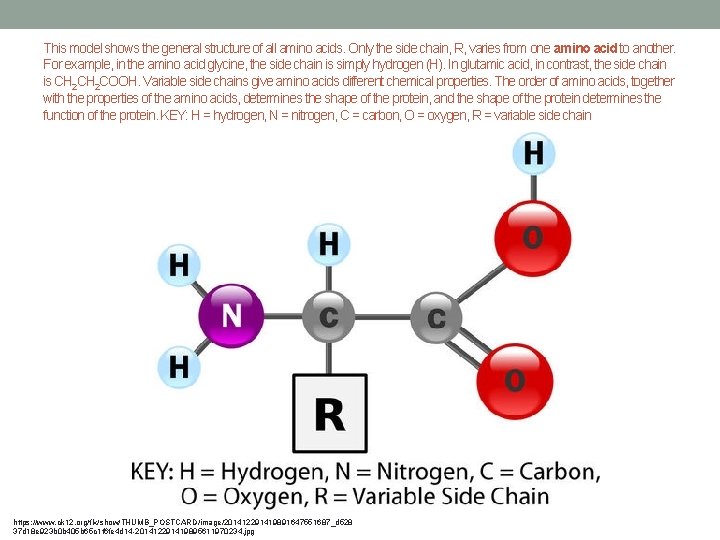
This model shows the general structure of all amino acids. Only the side chain, R, varies from one amino acid to another. For example, in the amino acid glycine, the side chain is simply hydrogen (H). In glutamic acid, in contrast, the side chain is CH 2 COOH. Variable side chains give amino acids different chemical properties. The order of amino acids, together with the properties of the amino acids, determines the shape of the protein, and the shape of the protein determines the function of the protein. KEY: H = hydrogen, N = nitrogen, C = carbon, O = oxygen, R = variable side chain https: //www. ck 12. org/flx/show/THUMB_POSTCARD/image/201412291419891647551687_d 528 37 d 18 e 923 b 0 b 405 b 65 c 1 f 6 fe 4 d 14 -201412291419895611970234. jpg

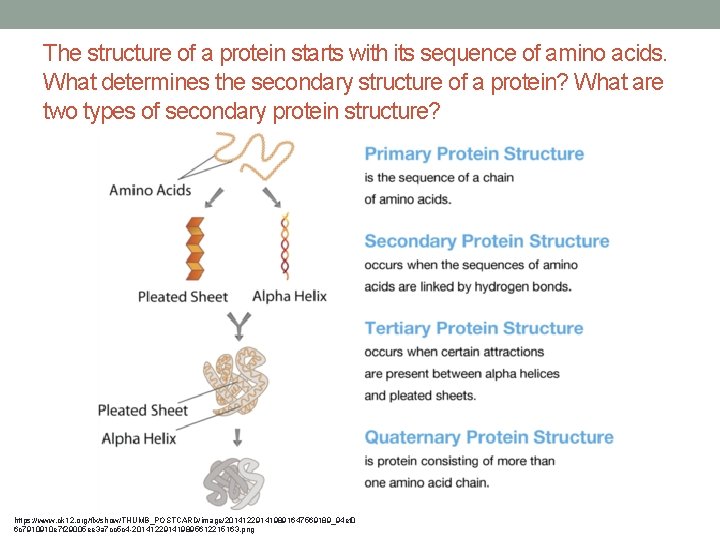
Protein Structure • Amino acids bind together and form a chain called a polypeptide • Proteins consist of one or more polypeptide chains • A protein may have up to four levels of structure. • A protein’s primary structure is its sequence of amino acids. • The complex structures of different proteins give them unique properties, which they need to carry out their various jobs in living organisms. • Protein structure animation

The structure of a protein starts with its sequence of amino acids. What determines the secondary structure of a protein? What are two types of secondary protein structure? https: //www. ck 12. org/flx/show/THUMB_POSTCARD/image/201412291419891647569189_94 ef 0 6 c 7910910 e 7 f 29005 ee 3 a 7 cc 5 c 4 -201412291419895612215163. png

Functions of proteins • Help cells keep their shape (structural proteins) • Make up muscle tissues • Transport items in and out of cells (transport proteins) • Act as signals and receive signals • Enzymes are proteins that speed up chemical reactions in cells • Others are antibodies which bind to foreign substances such as bacteria and target them for destruction. • Carry messages or transport materials (ex. Hemoglobin) • Protein Functions in the body video

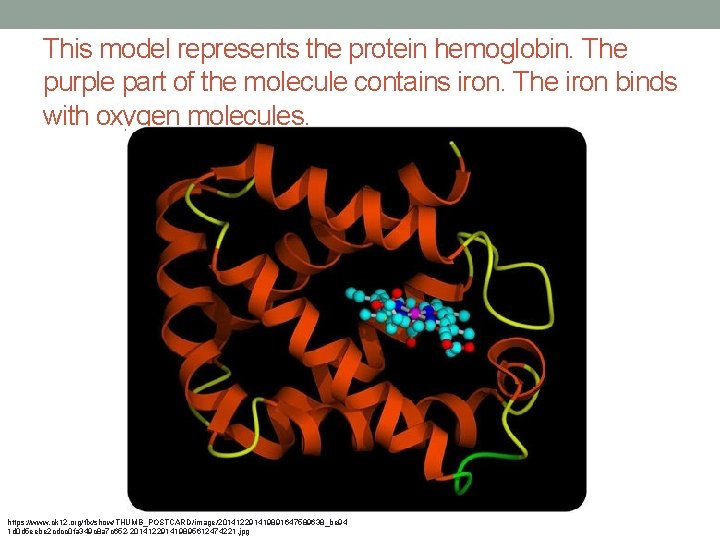
This model represents the protein hemoglobin. The purple part of the molecule contains iron. The iron binds with oxygen molecules. https: //www. ck 12. org/flx/show/THUMB_POSTCARD/image/201412291419891647589638_be 94 1 d 0 d 5 eebe 2 cdcc 0 fa 349 c 8 a 7 c 652 -201412291419895612474221. jpg

Proteins and Diet • Proteins are broken down into amino acids when food is digested • Cells use the AA to build new proteins • Essential amino acids must be consumed in foods • Dietary proteins can be broken down to provide cells with energy.

Nucleic Acids • Organic compound such as DNA or RNA that is built of monomers called nucleotides • The nucleic acid DNA (deoxyribonucleic acid) consists of two chains. • The nucleic acid RNA (ribonucleic acid) consists of just one chain. https: //www. ck 12. org/flx/show/image/201412291419891657593749_a 7 dca 734 fd 0631 b 79 caf 57 a 9 bd 55 e 12 a-201412291419895618456881. jpg

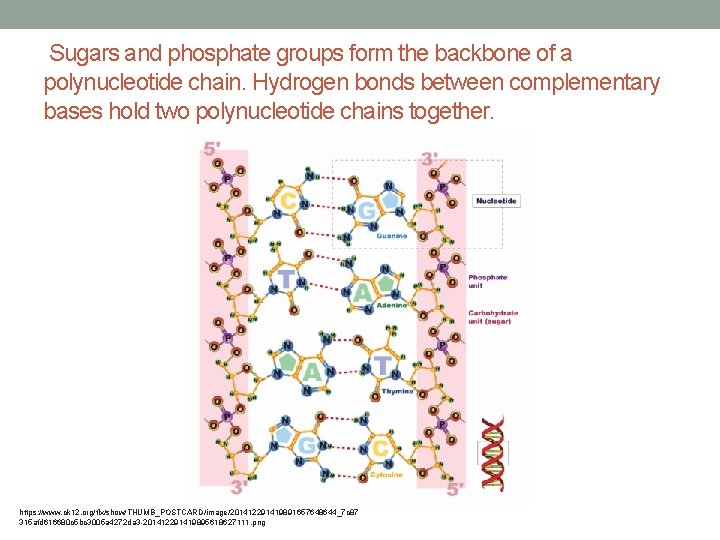
Structure of Nucleic Acids • Each nucleotide consists of three molecules: • 1. sugar • 2. phosphate group • 3. nitrogen base • The sugar of one nucleotide binds to the phosphate group of the next nucleotide. • This is known as the sugar-phosphate backbone.

Sugars and phosphate groups form the backbone of a polynucleotide chain. Hydrogen bonds between complementary bases hold two polynucleotide chains together. https: //www. ck 12. org/flx/show/THUMB_POSTCARD/image/201412291419891657648644_7 c 87 315 afd 616680 c 5 bc 3005 a 4272 da 3 -201412291419895618627111. png

Roles of Nucleic Acids • DNA contains the genetic instructions for the correct sequence of amino acids in proteins • RNA uses the information in DNA to assemble the correct amino acids and help make the protein. • DNA is passed from parents to offspring when organisms reproduce. • This is how inherited characteristics are passed from one generation to the next.

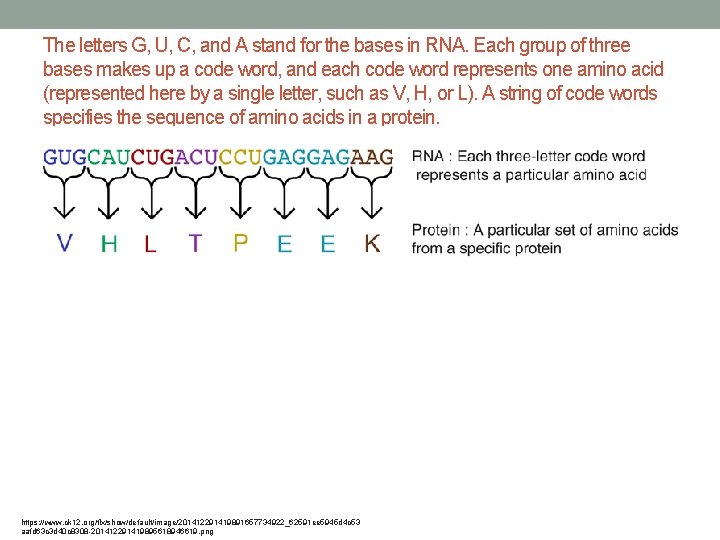
The letters G, U, C, and A stand for the bases in RNA. Each group of three bases makes up a code word, and each code word represents one amino acid (represented here by a single letter, such as V, H, or L). A string of code words specifies the sequence of amino acids in a protein. https: //www. ck 12. org/flx/show/default/image/201412291419891657734922_62591 ee 5945 d 4 c 53 aafd 63 c 3 d 40 c 8308 -201412291419895618946619. png

2. 4 Chemical Reactions

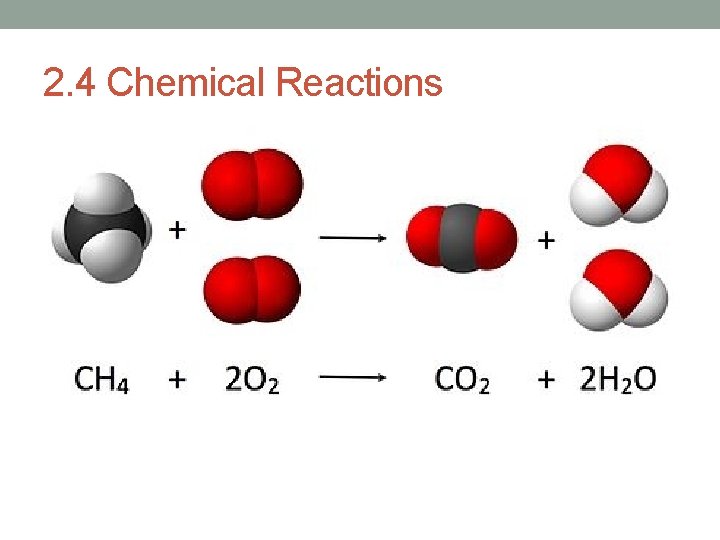
Bonds break and form during chemical reactions. • Chemical reactions change substances into different ones by breaking and forming chemical bonds. • Reactants are changed during a chemical reaction. – Products are made by a chemical reaction.

• Bond energy is the amount of energy that breaks a bond. – Energy is added to break bonds. – Energy is released when bonds form. • A reaction is at equilibrium when reactants and products form at the same rate. CO 2 + H 2 O H 2 CO 3

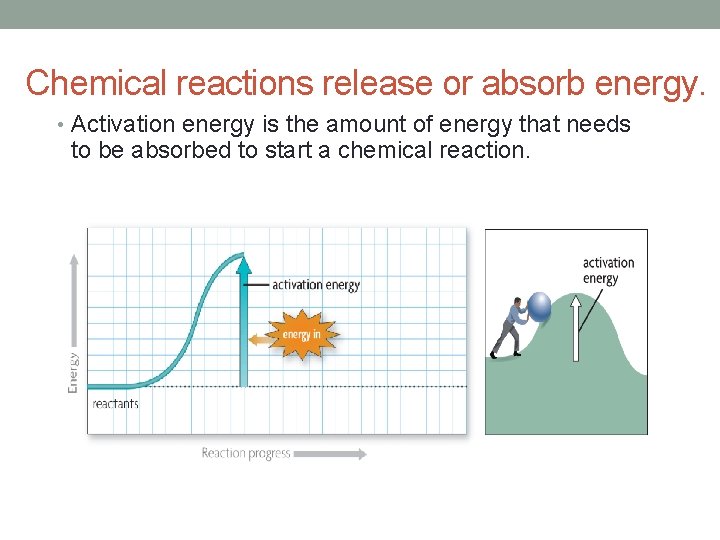
Chemical reactions release or absorb energy. • Activation energy is the amount of energy that needs to be absorbed to start a chemical reaction.

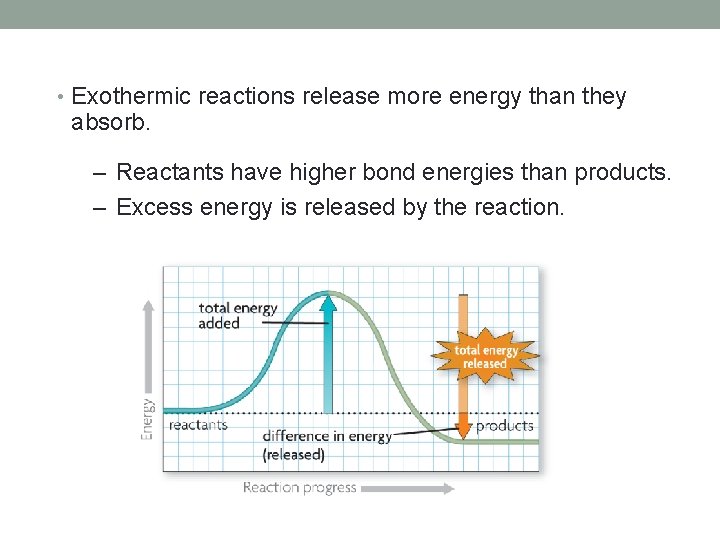
• Exothermic reactions release more energy than they absorb. – Reactants have higher bond energies than products. – Excess energy is released by the reaction.

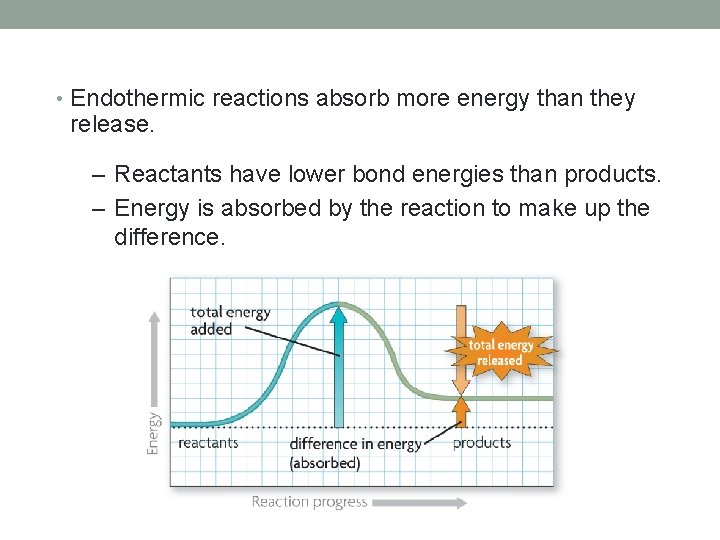
• Endothermic reactions absorb more energy than they release. – Reactants have lower bond energies than products. – Energy is absorbed by the reaction to make up the difference.

Enzymes

Enzymes and Biochemical Reactions • Most chemical reactions within organisms would be impossible under the conditions in cells (ex. Temp to low) • The rate of reactions must be increased by a catalyst • Catalyst: a chemical that speeds up chemical reactions called enzymes (biological catalysts) http: //upload. wikimedia. org/wikipedia/commons/d/d 9/S, S-Jacobsen's-catalyst-from-xtal-3 Dballs. png

• Enzymes are not reactants • They help the reactants but are not used up in the reactions • They may be used many times • Enzymes are highly specific for particular chemical reactions • Catalyze only one or a few types of reactions.

• Efficient in speeding up reactions • Can catalyze up to several million reactions per second • A typical biochemical reaction might take hours or even days to occur under normal cellular conditions without an enzyme but less than a second with an enzyme • Enzymes video

Importance of Enzymes • Enzymes are involved in most of the biochemical reactions • Enzymes allow reactions to occur at the rate necessary for life. • In animals, an important function of enzymes is to help digest food. • Digestive enzymes speed up reactions that break down large molecules of carbohydrates, proteins, and fats into smaller molecules the body can use. • Without digestive enzymes, animals would not be able to break down food molecules quickly enough to provide the energy and nutrients they need to survive.

Enzyme Function • Enzymes lower the activation energy of chemical reactions. • Activation energy: the energy needed to start a chemical reaction. • Enzyme animation

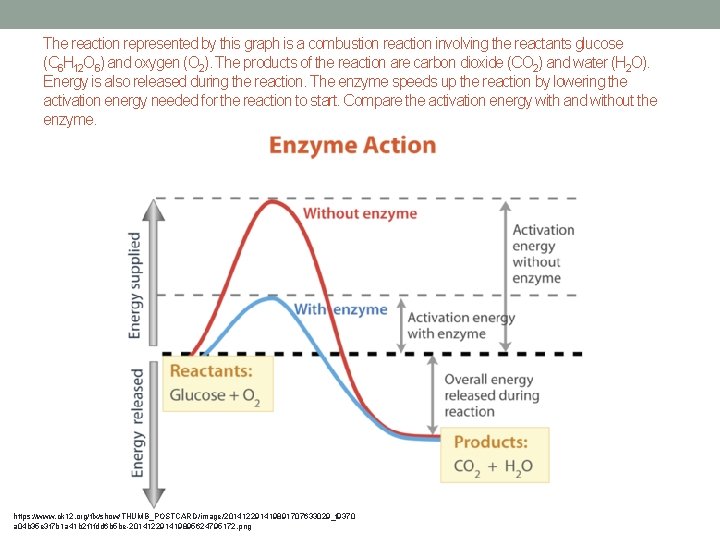
The reaction represented by this graph is a combustion reaction involving the reactants glucose (C 6 H 12 O 6) and oxygen (O 2). The products of the reaction are carbon dioxide (CO 2) and water (H 2 O). Energy is also released during the reaction. The enzyme speeds up the reaction by lowering the activation energy needed for the reaction to start. Compare the activation energy with and without the enzyme. https: //www. ck 12. org/flx/show/THUMB_POSTCARD/image/201412291419891707633029_f 9370 a 04 b 35 e 3 f 7 b 1 a 41 b 2 f 1 fdd 6 b 5 be-201412291419895624795172. png

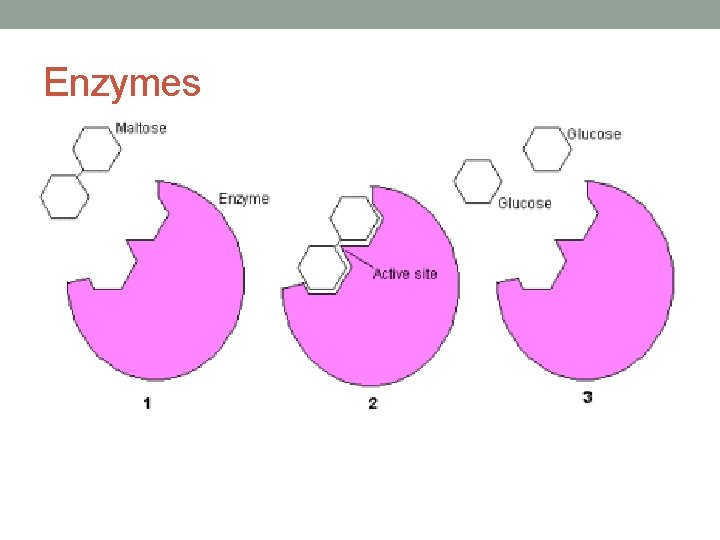
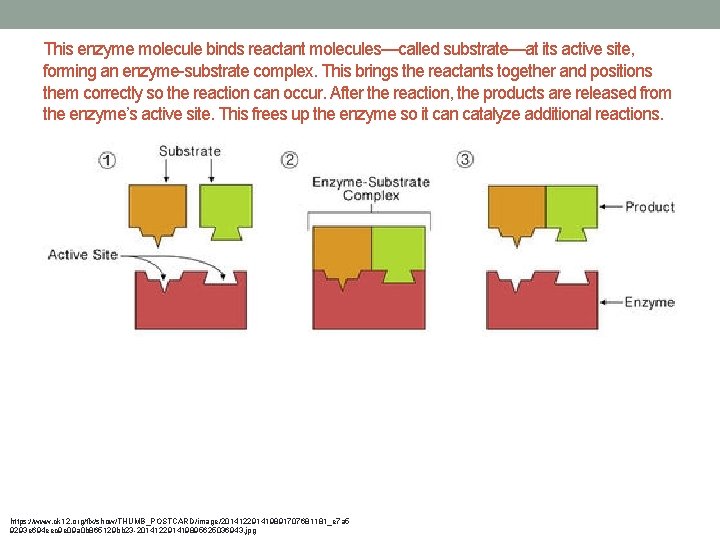
• Enzymes bring reactants together, don’t have to expend energy moving until they collide at random. • Enzymes bind reactant molecules (called the substrate), tightly and specifically, at a site on the enzyme molecule called the active site • Active site is specific for the reactants of the biochemical reaction the enzyme catalyzes. (puzzle pieces) • Enzymes also position reactants correctly which allows the molecules to interact with less energy. • Enzymes also allow reactions to occur by different pathways that have lower activation energy.

This enzyme molecule binds reactant molecules—called substrate—at its active site, forming an enzyme-substrate complex. This brings the reactants together and positions them correctly so the reaction can occur. After the reaction, the products are released from the enzyme’s active site. This frees up the enzyme so it can catalyze additional reactions. https: //www. ck 12. org/flx/show/THUMB_POSTCARD/image/201412291419891707681181_e 7 a 5 9293 e 694 eec 9 e 09 a 0 b 865129 bb 23 -201412291419895625036943. jpg

• The activities of enzymes also depend on the temperature, ionic conditions, and the p. H of the surroundings. • Many enzymes lose function at lower and higher temperatures. • At higher temperatures, an enzyme’s shape deteriorates. • Only when the temperature comes back to normal does the enzyme regain its shape and normal activity.
 Pyramid hesd
Pyramid hesd The smallest living unit of all living things is
The smallest living unit of all living things is Looking at living things
Looking at living things What do plants do at night
What do plants do at night Life cycle of all living things
Life cycle of all living things What does mrs nerg stand for
What does mrs nerg stand for Chapter 7 the evolution of living things answers
Chapter 7 the evolution of living things answers Chapter 7 the evolution of living things answers
Chapter 7 the evolution of living things answers Yeast is living or nonliving
Yeast is living or nonliving Living non living dead
Living non living dead Chapter 1 living a healthy life answers
Chapter 1 living a healthy life answers Living by chemistry unit 2 smells answers
Living by chemistry unit 2 smells answers Living by chemistry
Living by chemistry Living by chemistry solutions
Living by chemistry solutions What is catalystfive
What is catalystfive Lesson 81 drop in molecular views
Lesson 81 drop in molecular views Living by chemistry
Living by chemistry Living by chemistry
Living by chemistry Living by chemistry
Living by chemistry Chapter 2 the chemistry of life section review 2-2
Chapter 2 the chemistry of life section review 2-2 Why is water important to living things
Why is water important to living things Non living things in grassland
Non living things in grassland Kingdoms of living things
Kingdoms of living things Life's structure and classification answers key
Life's structure and classification answers key Living things meaning
Living things meaning Ecosystem living and nonliving things
Ecosystem living and nonliving things Is hagfish slime a lipid
Is hagfish slime a lipid Living things table
Living things table Linnaeus
Linnaeus 6 kingdoms of life characteristics
6 kingdoms of life characteristics Living things 20
Living things 20 Everything that is alive needs energy
Everything that is alive needs energy Food chains producers
Food chains producers Five kingdoms of life
Five kingdoms of life Ecosystem living and nonliving things
Ecosystem living and nonliving things Gets its energy from eating living things
Gets its energy from eating living things Kingdoms of archaea
Kingdoms of archaea Domain of living things
Domain of living things The 8 levels of classification
The 8 levels of classification Key to the world
Key to the world Categories of living things
Categories of living things 5 groups of living things
5 groups of living things Domain of living things
Domain of living things What are the 7 classifications of living things
What are the 7 classifications of living things Thampi’s torrent frog
Thampi’s torrent frog How living things are organized
How living things are organized Structural adaptations examples
Structural adaptations examples Carolus linnaeus
Carolus linnaeus Living things 20
Living things 20 3 domain system of classification
3 domain system of classification Changes in living things
Changes in living things Tissues group together to form
Tissues group together to form Abiotic things in a pond
Abiotic things in a pond Non living things outside
Non living things outside Classification of vertebrates
Classification of vertebrates 7 characteristics of life
7 characteristics of life What are the six kingdoms of life
What are the six kingdoms of life Lesson 6: protists: 2
Lesson 6: protists: 2 Phartenogenesis
Phartenogenesis 6 characteristics of living things
6 characteristics of living things All about plants
All about plants What is characteristic of living things
What is characteristic of living things Movement characteristics of living things
Movement characteristics of living things Classifying living things lesson 1 answer key
Classifying living things lesson 1 answer key Biosphere and geosphere
Biosphere and geosphere Domain kingdom phylum
Domain kingdom phylum What are the six characteristics of living things
What are the six characteristics of living things Why are enzymes important to living things?
Why are enzymes important to living things? How do viruses differ from living things
How do viruses differ from living things Unicellular vs multicellular activity
Unicellular vs multicellular activity Five kingdoms of living things
Five kingdoms of living things What function does atp carry out in living things
What function does atp carry out in living things How living things obtain energy worksheet answers
How living things obtain energy worksheet answers