Chapter 2 Chemistry of Life Living Things Consist







- Slides: 31

Chapter 2: Chemistry of Life

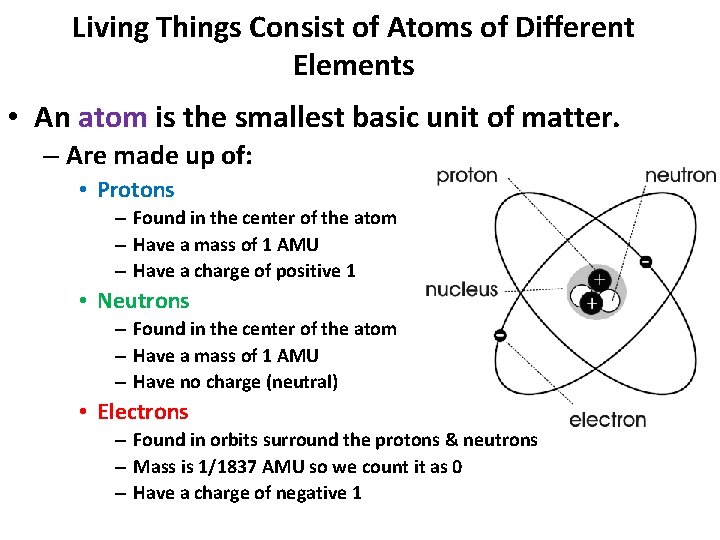
Living Things Consist of Atoms of Different Elements • An atom is the smallest basic unit of matter. – Are made up of: • Protons – Found in the center of the atom – Have a mass of 1 AMU – Have a charge of positive 1 • Neutrons – Found in the center of the atom – Have a mass of 1 AMU – Have no charge (neutral) • Electrons – Found in orbits surround the protons & neutrons – Mass is 1/1837 AMU so we count it as 0 – Have a charge of negative 1

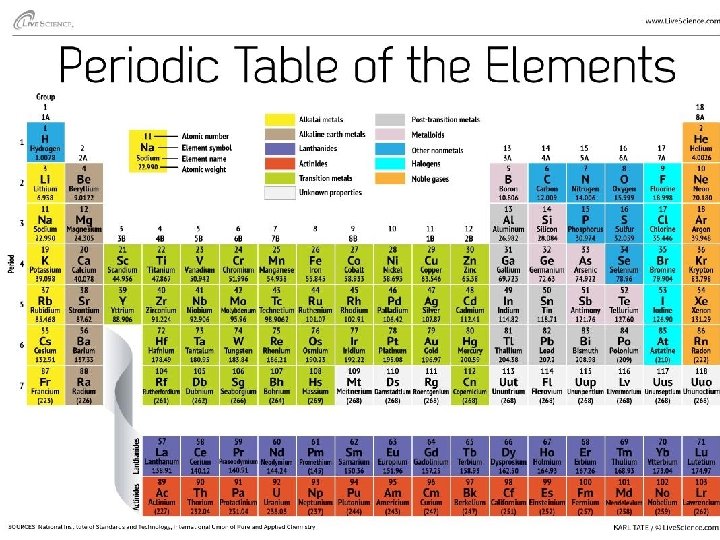
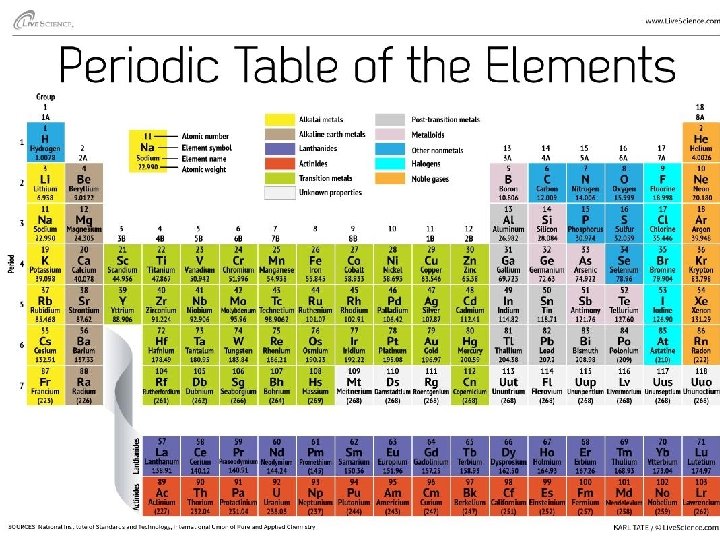
• An element is one particular type of atom, and it cannot be broken down into a simpler substance by ordinary chemical means – Carbon (C), Sodium (Na), Sulfur (S), … – Oxygen (O 2), Hydrogen (H 2), . . . – Differ in the number of protons they have. This is their Atomic Number and no two elements have the same Atomic number – The number of protons (which equals the number of electrons), gives the element its physical and chemical properties


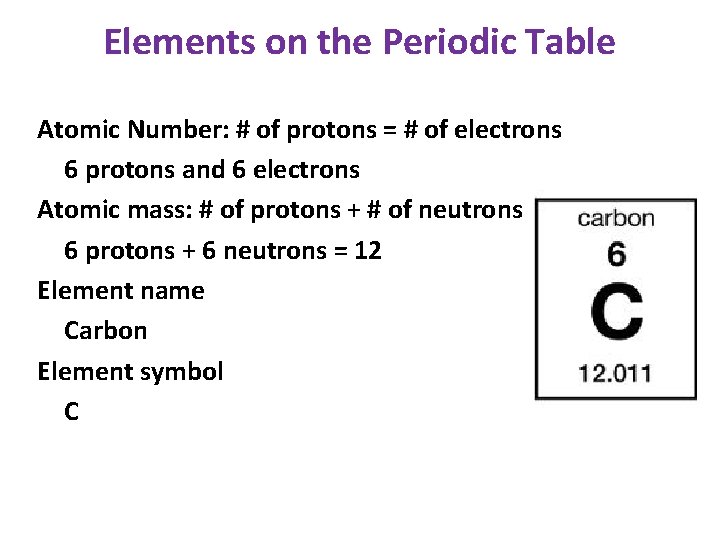
Elements on the Periodic Table Atomic Number: # of protons = # of electrons 6 protons and 6 electrons Atomic mass: # of protons + # of neutrons 6 protons + 6 neutrons = 12 Element name Carbon Element symbol C

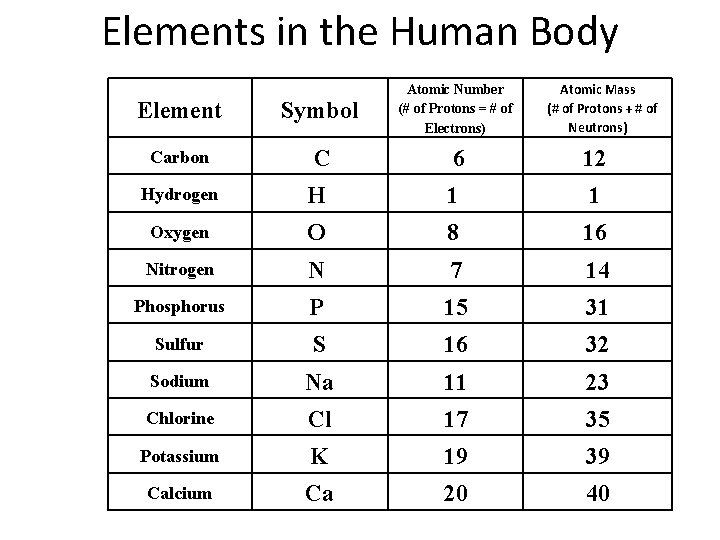
Elements in the Human Body Atomic Mass (# of Protons + # of Neutrons) Element Symbol Atomic Number (# of Protons = # of Electrons) Carbon C 6 12 Hydrogen H 1 1 Oxygen O 8 16 Nitrogen N 7 14 Phosphorus P 15 31 Sulfur S 16 32 Sodium Na 11 23 Chlorine Cl 17 35 Potassium K 19 39 Calcium Ca 20 40

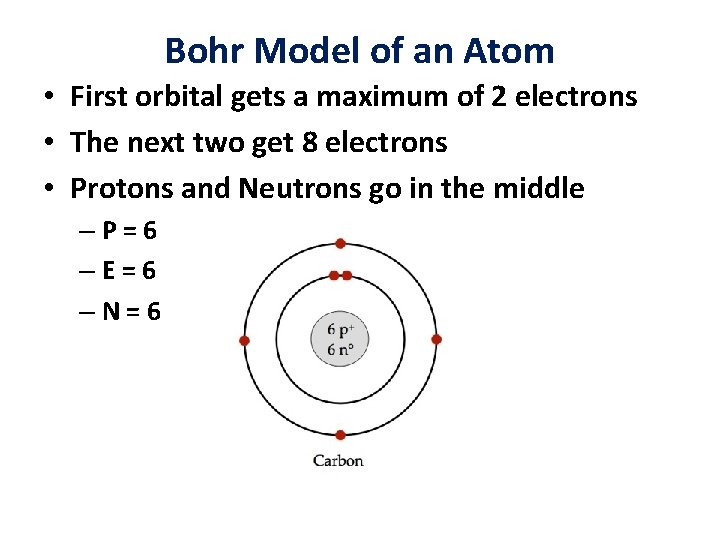
Bohr Model of an Atom • First orbital gets a maximum of 2 electrons • The next two get 8 electrons • Protons and Neutrons go in the middle –P=6 –E=6 –N=6

Do Oxygen (16 O 8), Hydrogen (1 H 1), and then Nitrogen (14 N 7),

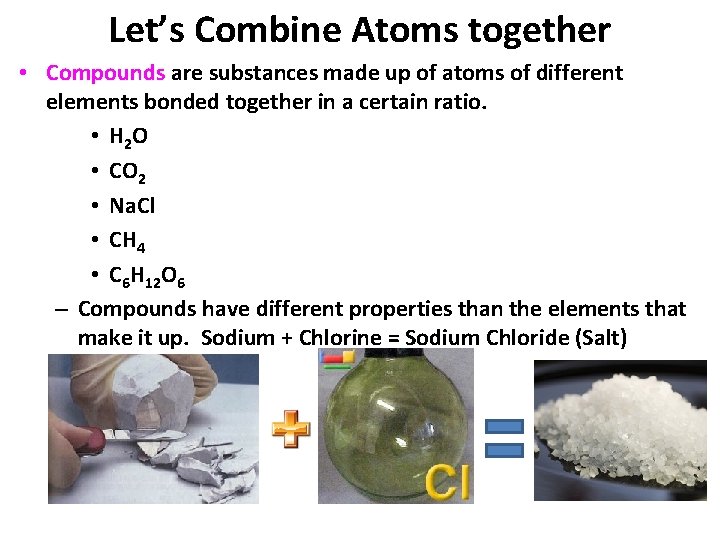
Let’s Combine Atoms together • Compounds are substances made up of atoms of different elements bonded together in a certain ratio. • H 2 O • CO 2 • Na. Cl • CH 4 • C 6 H 12 O 6 – Compounds have different properties than the elements that make it up. Sodium + Chlorine = Sodium Chloride (Salt)

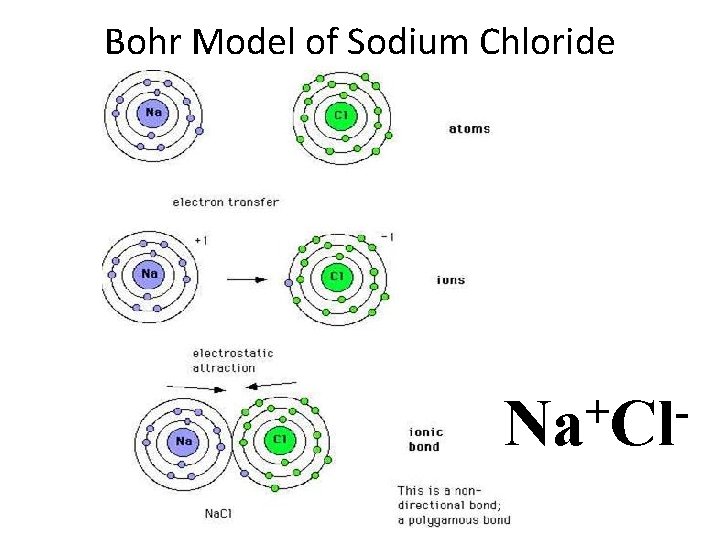
Bohr Model of Sodium Chloride + Na Cl

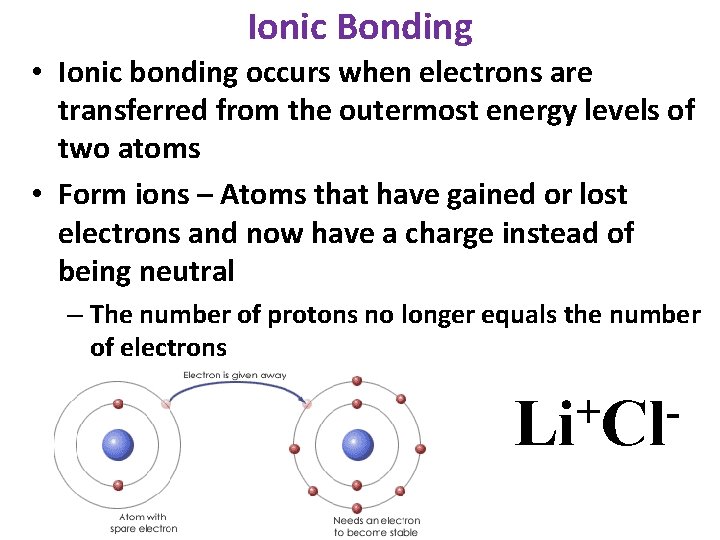
Ionic Bonding • Ionic bonding occurs when electrons are transferred from the outermost energy levels of two atoms • Form ions – Atoms that have gained or lost electrons and now have a charge instead of being neutral – The number of protons no longer equals the number of electrons + Li Cl


Still with Ionic Bonding • Elements in Column 1 A will easily bond ionically with elements in Column 7 A. – The Column 1 A elements will donate an electron and therefore have an extra positive charge and become a positive 1 ion – The Column 7 A elements will gain an electron and therefore have an extra negatively charged electron and become a negative 1 ion – What about Column 2 A. Which column will it easily bond ionically with? • Column 6 A and form either a +2 ion or a -2 ion.

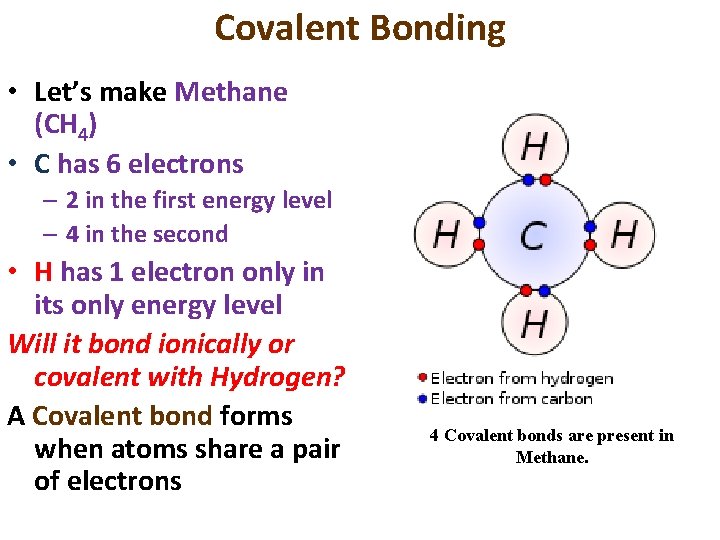
Covalent Bonding • Let’s make Methane (CH 4) • C has 6 electrons – 2 in the first energy level – 4 in the second • H has 1 electron only in its only energy level Will it bond ionically or covalent with Hydrogen? A Covalent bond forms when atoms share a pair of electrons 4 Covalent bonds are present in Methane.
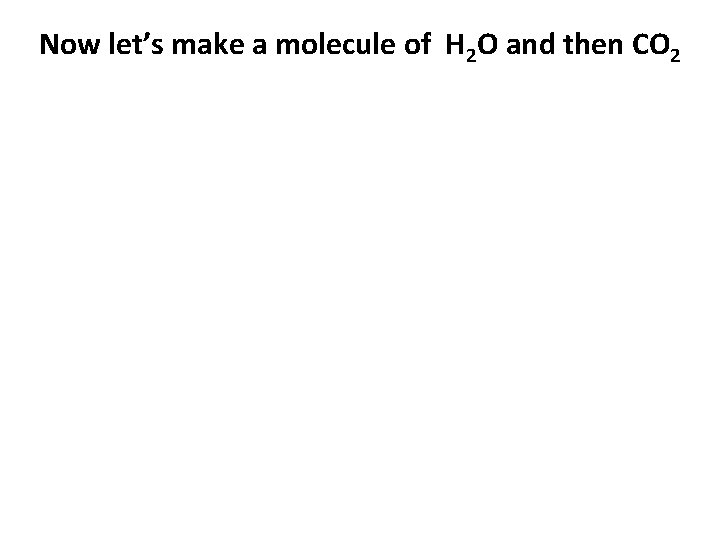
Now let’s make a molecule of H 2 O and then CO 2

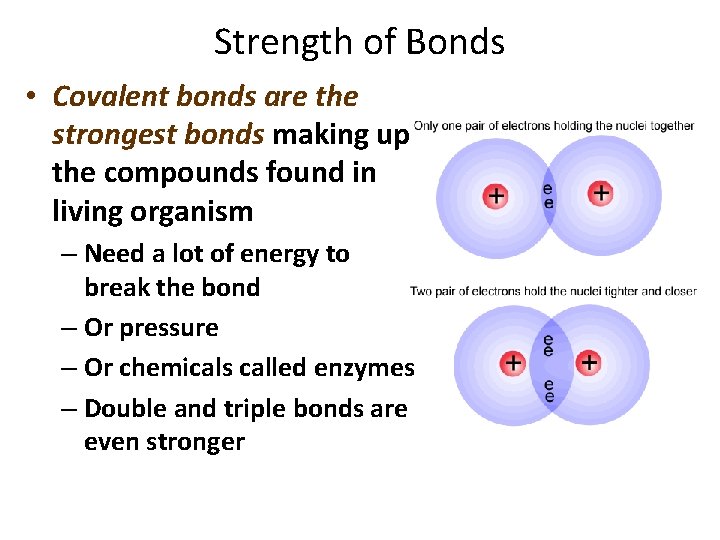
Strength of Bonds • Covalent bonds are the strongest bonds making up the compounds found in living organism – Need a lot of energy to break the bond – Or pressure – Or chemicals called enzymes – Double and triple bonds are even stronger

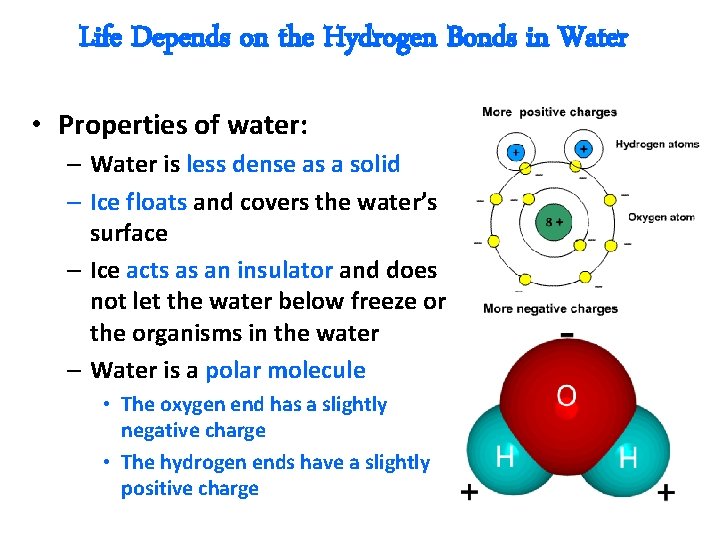
Life Depends on the Hydrogen Bonds in Water • Properties of water: – Water is less dense as a solid – Ice floats and covers the water’s surface – Ice acts as an insulator and does not let the water below freeze or the organisms in the water – Water is a polar molecule • The oxygen end has a slightly negative charge • The hydrogen ends have a slightly positive charge

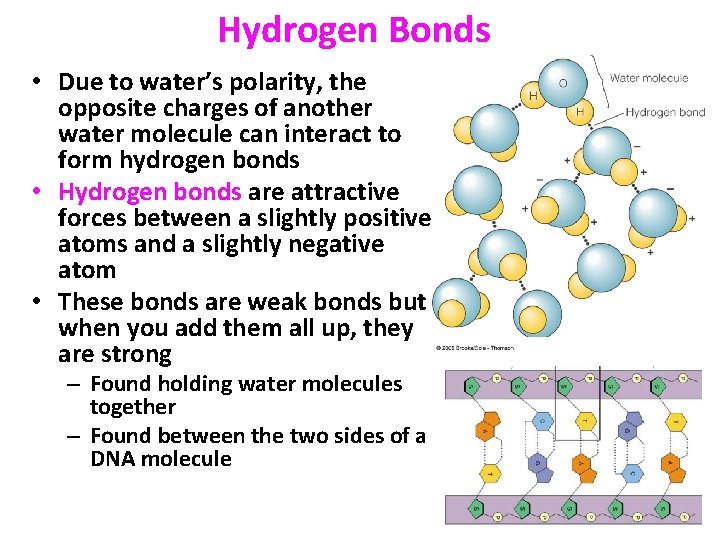
Hydrogen Bonds • Due to water’s polarity, the opposite charges of another water molecule can interact to form hydrogen bonds • Hydrogen bonds are attractive forces between a slightly positive atoms and a slightly negative atom • These bonds are weak bonds but when you add them all up, they are strong – Found holding water molecules together – Found between the two sides of a DNA molecule

Properties of Water due to Hydrogen Bonds 1. High Specific Heat: – The amount of heat needed to raise the temperature of a substance 1`C – Since water has so many hydrogen bonds, it takes a lot of energy to break the bonds to raise its temperature • Once it has absorbed the heat, it takes a lot of energy and time to lower the temperature – Why a watched pot never seems to boil!! – Why it takes a long time in the summer for the ocean or lakes to heat up. – Why water stays warmer in the fall – Why it takes a while for our body temperature to lower

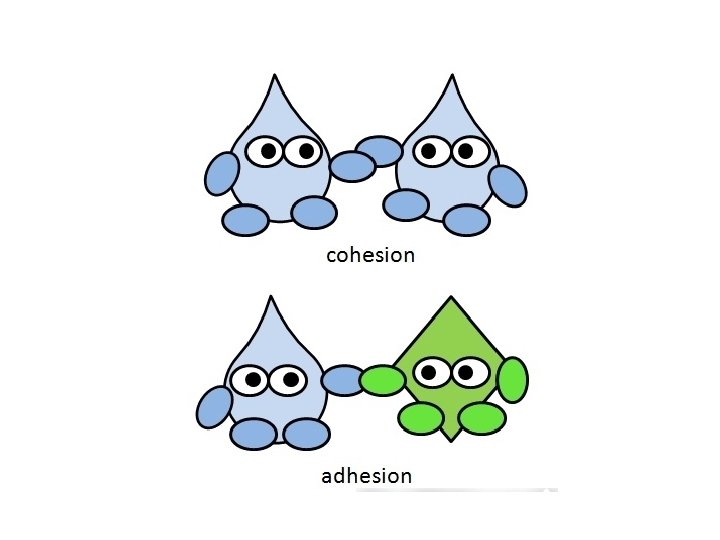
2. Cohesion – Like molecules being attracted to each other. – There is an attractive force holding adjacent water molecules together called hydrogen bonds • • Water beading up on leaves or your window Surface tension allowing the water strider to “walk on water”

3. Adhesion – The attractive force between unlike molecules – Water molecules stick to other molecules IF they are also polar – Water molecules sticking to glass or a web – Water molecules sticking to roots – The meniscus in a graduated cylinder – How water molecules (or our blood since its basically water) can easily be transported through our capillaries (really thin blood vessels)


Water as the Universal Solvent Water makes up about 70% of the mass of living things. For us, its closer to 60%. In order for chemical processes to occur, molecules and ions need to be able to dissolve or go into solution with water. Sugars, oxygen, Vit B & C, minerals such as salt, … Polar compounds or ionic compounds can go into solution with water Non-polar molecules like lipids (fats and oils), fat soluble vitamins like A, D, E, & K can’t. Oil and water do not mix!! They can only dissolve in other nonpolar solvents

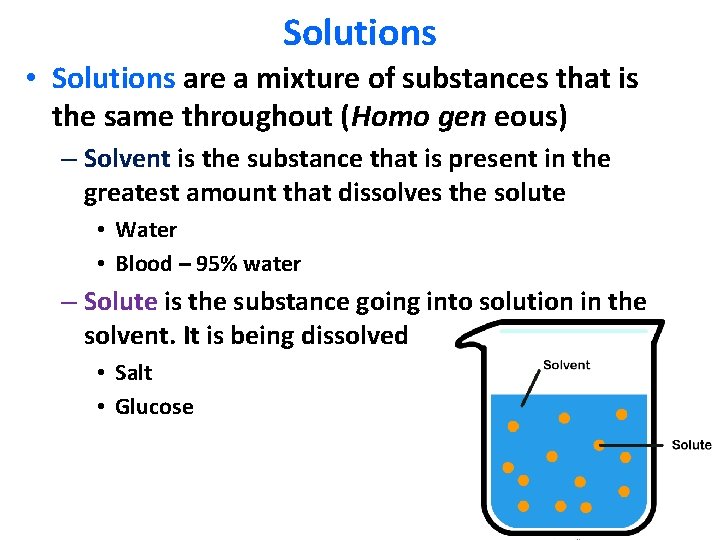
Solutions • Solutions are a mixture of substances that is the same throughout (Homo gen eous) – Solvent is the substance that is present in the greatest amount that dissolves the solute • Water • Blood – 95% water – Solute is the substance going into solution in the solvent. It is being dissolved • Salt • Glucose

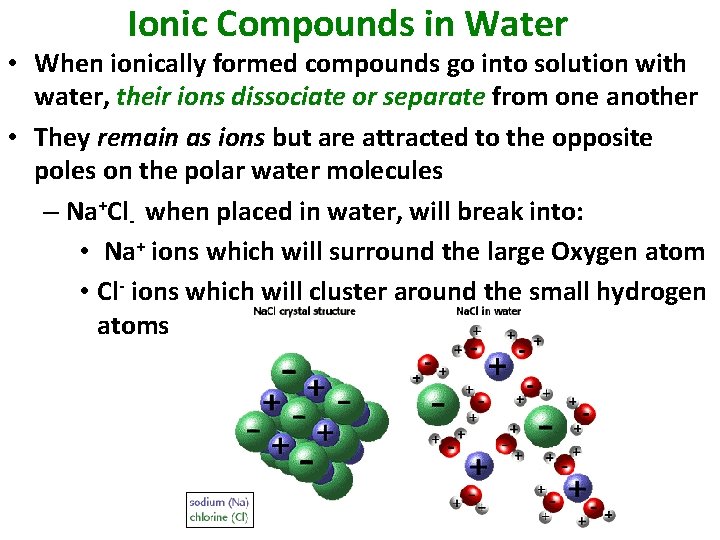
Ionic Compounds in Water • When ionically formed compounds go into solution with water, their ions dissociate or separate from one another • They remain as ions but are attracted to the opposite poles on the polar water molecules – Na+Cl- when placed in water, will break into: • Na+ ions which will surround the large Oxygen atom • Cl- ions which will cluster around the small hydrogen atoms

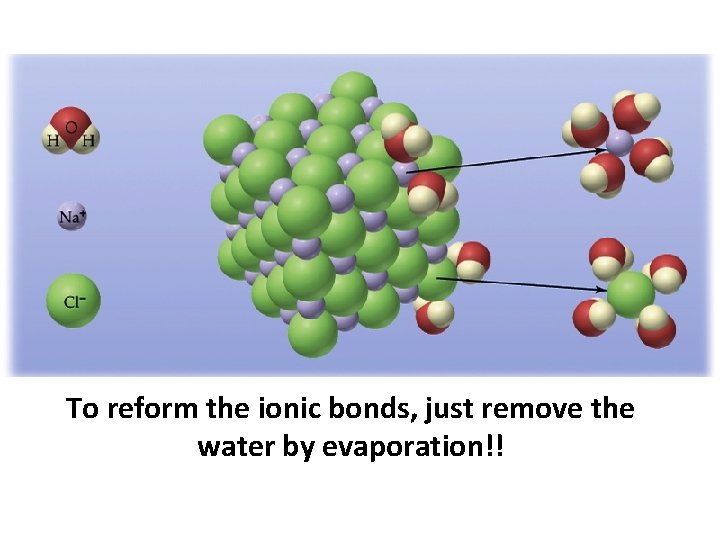
To reform the ionic bonds, just remove the water by evaporation!!

Acids and Bases Acids are compounds that when placed in water, release a proton or Hydrogen ion (H+) into the water. – HCl, H 2 SO 4, Citric Acid – Sour tasting – Corrosive

Bases or alkaline compounds remove H+ ions when in solution and have an excess of OHions (hydroxide ions) – Ammonia, Bleach, Blood, Bile, Soap… – Bitter tasting – Slimy to touch

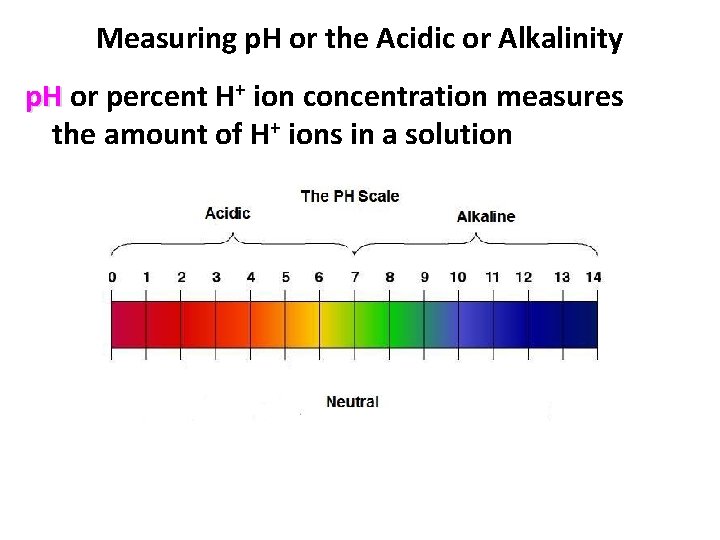
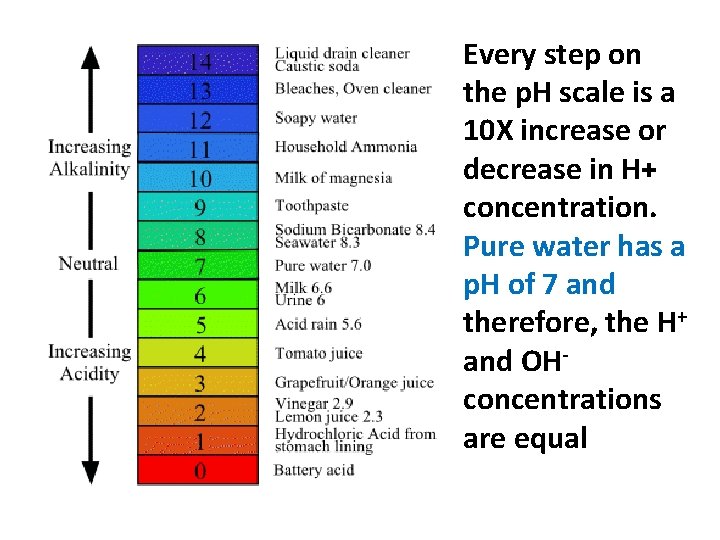
Measuring p. H or the Acidic or Alkalinity p. H or percent H+ ion concentration measures the amount of H+ ions in a solution

Every step on the p. H scale is a 10 X increase or decrease in H+ concentration. Pure water has a p. H of 7 and therefore, the H+ and OHconcentrations are equal

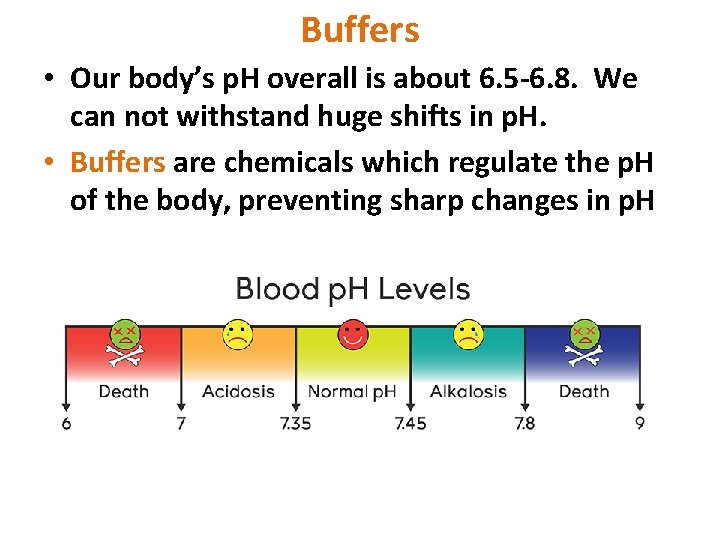
Buffers • Our body’s p. H overall is about 6. 5 -6. 8. We can not withstand huge shifts in p. H. • Buffers are chemicals which regulate the p. H of the body, preventing sharp changes in p. H
 Venn diagram living and non living
Venn diagram living and non living What is the smallest living unit of life
What is the smallest living unit of life Storyline online
Storyline online What is the process?
What is the process? Life cycle of all living things
Life cycle of all living things What does mrs nerg stand for
What does mrs nerg stand for Chapter 7 the evolution of living things answers
Chapter 7 the evolution of living things answers Chapter 7 the evolution of living things answers
Chapter 7 the evolution of living things answers Moss living or nonliving
Moss living or nonliving Living non living dead
Living non living dead A person at the midpoint of the health continuum is
A person at the midpoint of the health continuum is Living by chemistry unit 2 smells answers
Living by chemistry unit 2 smells answers Living by chemistry
Living by chemistry Living by chemistry solutions
Living by chemistry solutions What is catalystfive
What is catalystfive Drop in molecular views answer key
Drop in molecular views answer key Living by chemistry
Living by chemistry Living by chemistry
Living by chemistry Getting connected ionic compounds
Getting connected ionic compounds Chapter 2 the chemistry of life section review 2-2
Chapter 2 the chemistry of life section review 2-2 Why is water important to living things
Why is water important to living things Freshwater non living things
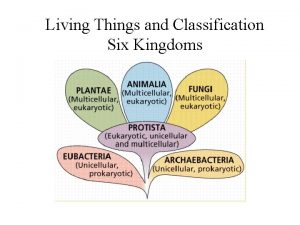
Freshwater non living things 5 kingdoms of living things
5 kingdoms of living things Life's structure and classification answers key
Life's structure and classification answers key Living things meaning
Living things meaning Ecosystems examples
Ecosystems examples Cho cho chon chonp
Cho cho chon chonp Living things grow images
Living things grow images Jackal linnaean system
Jackal linnaean system Taxonomic kingdoms
Taxonomic kingdoms Living things 20
Living things 20 Food chain begins with
Food chain begins with