Chapter 14 Heat and Temperature Temperature Energy Transfer

![Section 1 - Temperature • Key Questions: • 1] What does temperature have to Section 1 - Temperature • Key Questions: • 1] What does temperature have to](https://slidetodoc.com/presentation_image_h2/cd68af5d7dfb874bd2b8bf88a1da81e5/image-5.jpg)
![Section 2 – Heat Transfer • Key Questions: • 1] How does energy transfer Section 2 – Heat Transfer • Key Questions: • 1] How does energy transfer](https://slidetodoc.com/presentation_image_h2/cd68af5d7dfb874bd2b8bf88a1da81e5/image-12.jpg)


![Heat Flow Conceptual Practice • Scenarios – Explain what is happening: • 1] You Heat Flow Conceptual Practice • Scenarios – Explain what is happening: • 1] You](https://slidetodoc.com/presentation_image_h2/cd68af5d7dfb874bd2b8bf88a1da81e5/image-24.jpg)




![Section 3 – Using Heat • Key Questions: • 1] What happens to heat Section 3 – Using Heat • Key Questions: • 1] What happens to heat](https://slidetodoc.com/presentation_image_h2/cd68af5d7dfb874bd2b8bf88a1da81e5/image-31.jpg)




- Slides: 40

Chapter 14 Heat and Temperature: Temperature Energy Transfer Using Heat

TN Standards • CLE 3202. 2. 3 – Examine the applications and effects of heat energy • CLE. 3202. 2. 6 – Investigate the Law of Conservation of Energy • CLE. 3202. TE. 3 – Explain the relationship between the properties of a material and the use of the material in the application of a technology

TN Standards • SPI. 3231. 2. 1 – Relate temperature changes with the changes of kinetic energy and the flow of heat energy

Bellwork • What is temperature? • Average kinetic energy of particles in a material
![Section 1 Temperature Key Questions 1 What does temperature have to Section 1 - Temperature • Key Questions: • 1] What does temperature have to](https://slidetodoc.com/presentation_image_h2/cd68af5d7dfb874bd2b8bf88a1da81e5/image-5.jpg)
Section 1 - Temperature • Key Questions: • 1] What does temperature have to do with energy? • 2] What three temperature scales are commonly used? • 3] What makes things feel hot or cold?

Temperature and Energy • Kinetic theory of matter – Matter is made of small particles always moving – Higher temperature, more motion – Large particles move slower • The temperature of a substance is proportional to the average kinetic energy of the substance’s particles • All particles have kinetic energy ( atomic )

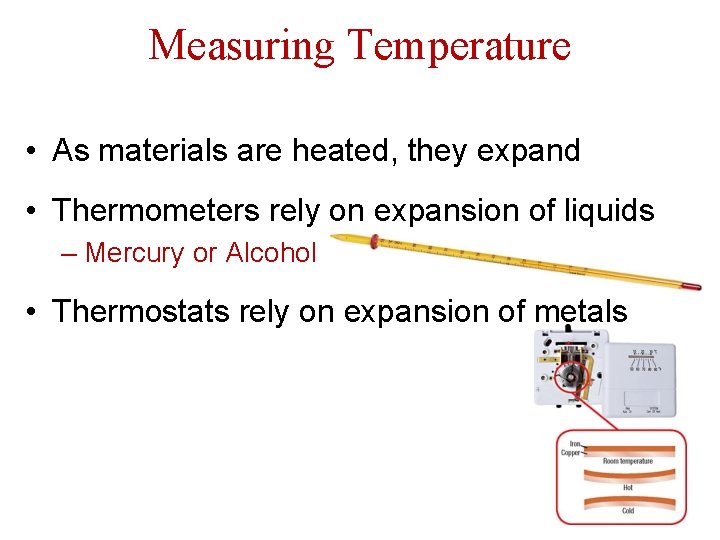
Measuring Temperature • As materials are heated, they expand • Thermometers rely on expansion of liquids – Mercury or Alcohol • Thermostats rely on expansion of metals

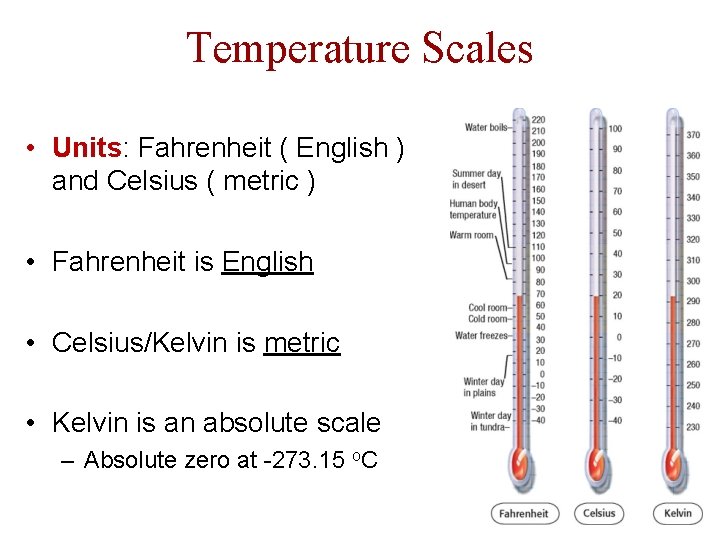
Temperature Scales • Units: Fahrenheit ( English ) and Celsius ( metric ) • Fahrenheit is English • Celsius/Kelvin is metric • Kelvin is an absolute scale – Absolute zero at -273. 15 o. C

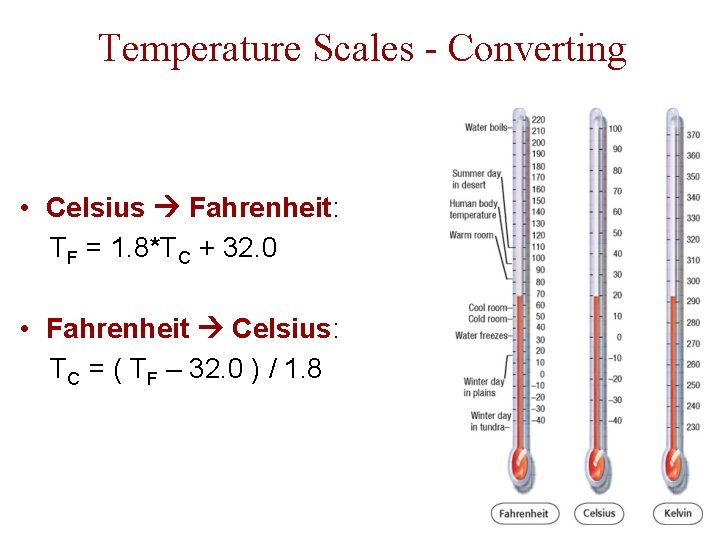
Temperature Scales - Converting • Celsius Fahrenheit: TF = 1. 8*TC + 32. 0 • Fahrenheit Celsius: TC = ( TF – 32. 0 ) / 1. 8

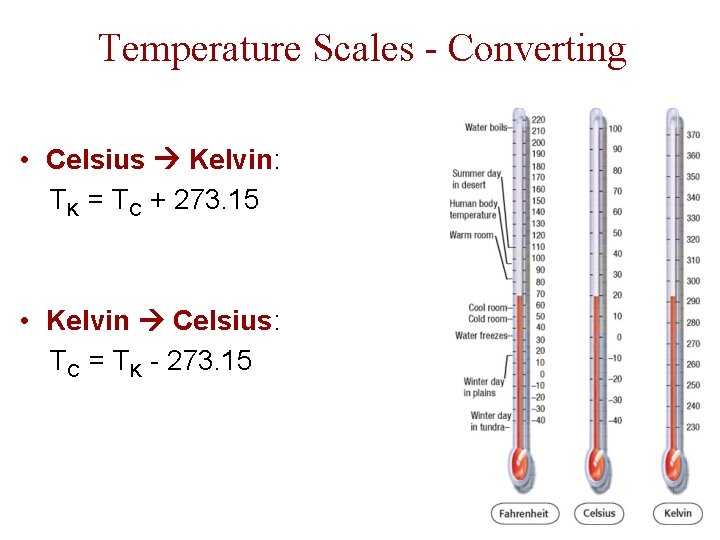
Temperature Scales - Converting • Celsius Kelvin: TK = TC + 273. 15 • Kelvin Celsius: TC = TK - 273. 15

Temperature & Energy Transfer • When you feel “hot” or “cold” you are detecting a temperature difference • You are also feeling the affects of energy transfer • Temperature changes indicate an energy transfer – temperature difference between two objects is felt as heat • Heat is the energy transferred between objects of different temperature
![Section 2 Heat Transfer Key Questions 1 How does energy transfer Section 2 – Heat Transfer • Key Questions: • 1] How does energy transfer](https://slidetodoc.com/presentation_image_h2/cd68af5d7dfb874bd2b8bf88a1da81e5/image-12.jpg)
Section 2 – Heat Transfer • Key Questions: • 1] How does energy transfer happen? • 2] What do conductors and insulators do? • 3] What makes something a good conductor of heat?

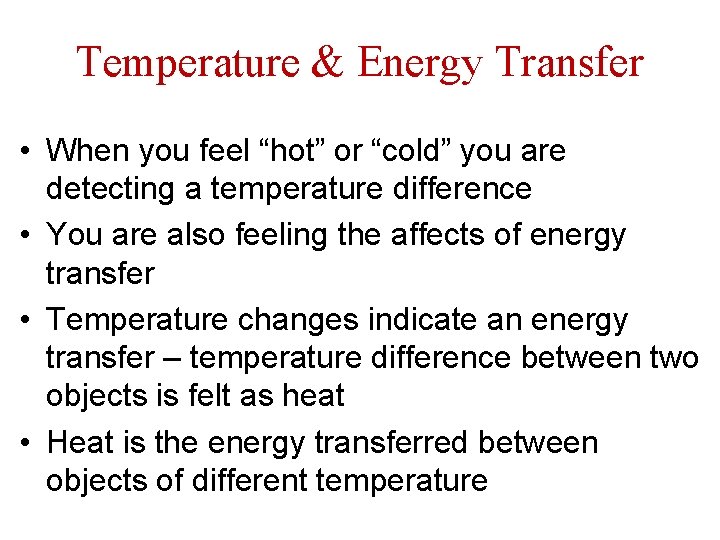
Energy Transfer ( Heat Flow ) • What is happening in each picture • Explain how heat is flowing ( ID how heat goes from one object to another ) • What might be happening on the atomic level?

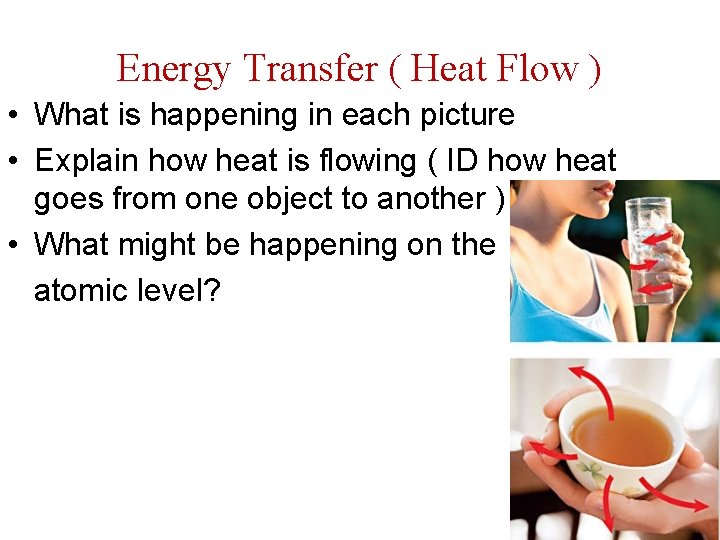
Different Methods of Transfer

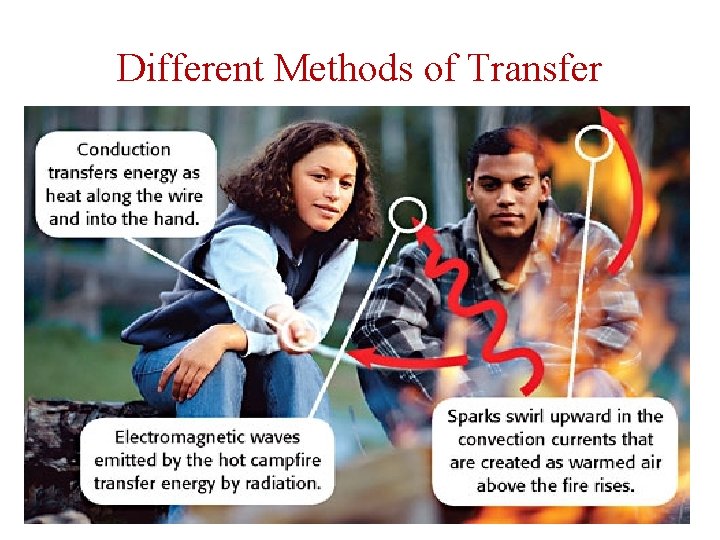
Different Methods of Transfer • Conduction occurs between objects in direct contact • Thermal Conduction–heat source is one object

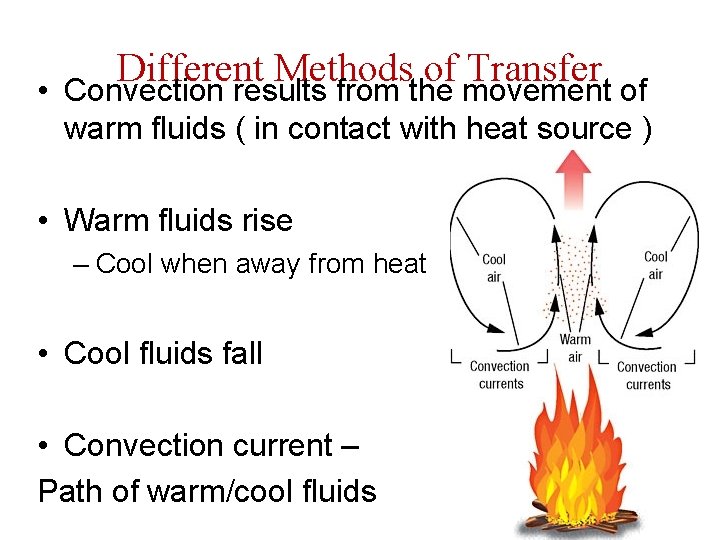
Different Methods of Transfer • Convection results from the movement of warm fluids ( in contact with heat source ) • Warm fluids rise – Cool when away from heat • Cool fluids fall • Convection current – Path of warm/cool fluids

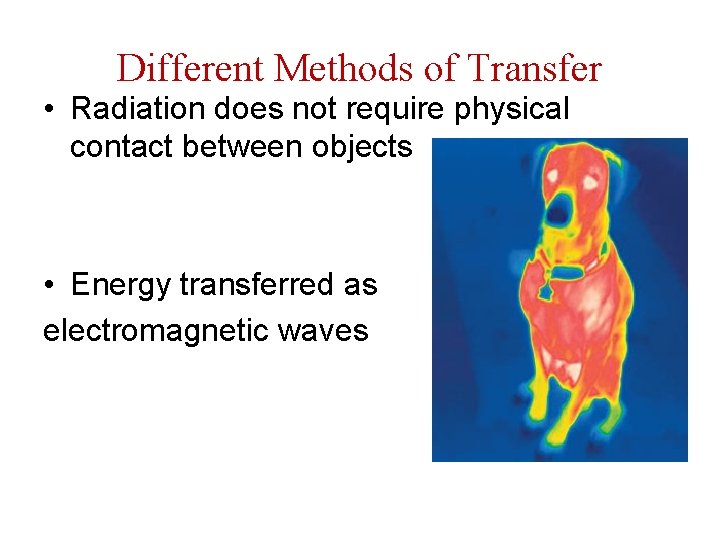
Different Methods of Transfer • Radiation does not require physical contact between objects • Energy transferred as electromagnetic waves

Conductors & Insulators • A conductor is a material through which energy can be easily transferred as heat • An insulator is a material that transfers energy poorly • Heat energy is transferred through particle collisions

Conductors & Insulators • Heat energy is transferred through particle collisions • Gases – poor conductors – Why? • Denser materials usually are better conductors than less dense • Metals – very good conductors • Plastics – poor conductors

Bellwork – 11/21/14 • When you melt ice, is heat going to be added to the water, or removed from it? • Add heat

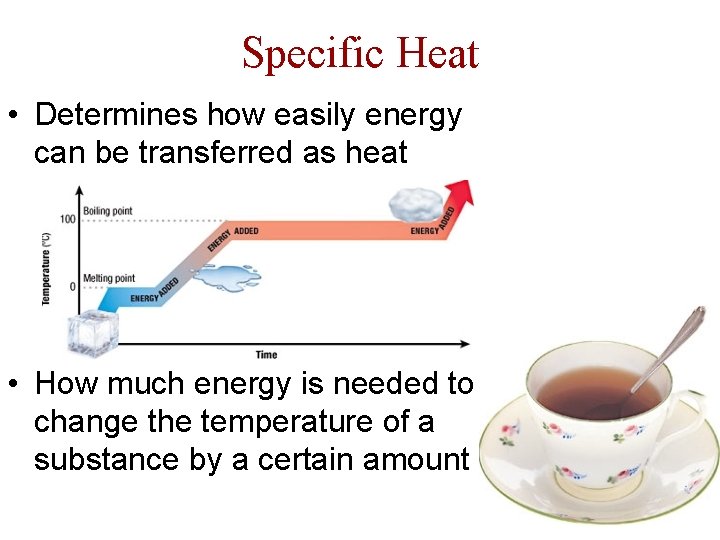
Specific Heat • Determines how easily energy can be transferred as heat • How much energy is needed to change the temperature of a substance by a certain amount

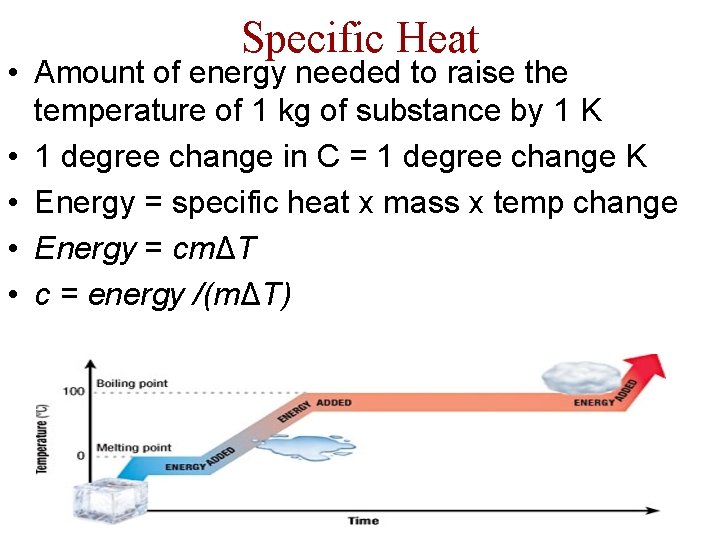
Specific Heat • Amount of energy needed to raise the temperature of 1 kg of substance by 1 K • 1 degree change in C = 1 degree change K • Energy = specific heat x mass x temp change • Energy = cmΔT • c = energy /(mΔT)

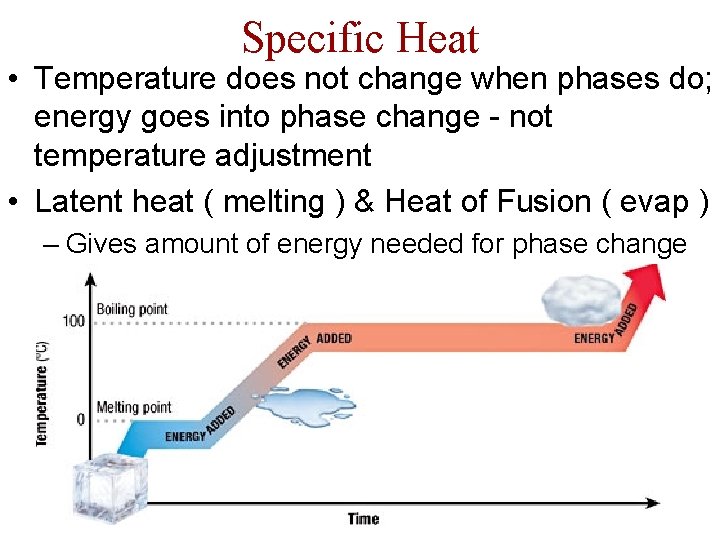
Specific Heat • Temperature does not change when phases do; energy goes into phase change - not temperature adjustment • Latent heat ( melting ) & Heat of Fusion ( evap ) – Gives amount of energy needed for phase change
![Heat Flow Conceptual Practice Scenarios Explain what is happening 1 You Heat Flow Conceptual Practice • Scenarios – Explain what is happening: • 1] You](https://slidetodoc.com/presentation_image_h2/cd68af5d7dfb874bd2b8bf88a1da81e5/image-24.jpg)
Heat Flow Conceptual Practice • Scenarios – Explain what is happening: • 1] You pick up a coffee cup and it is hot • 2] You touch a glass of cold SCHAWEET tea • 3] A breeze makes you shiver

Heat Flow Conceptual Practice • Which substance can you heat the quickest? • One with a large or small heat capacity?

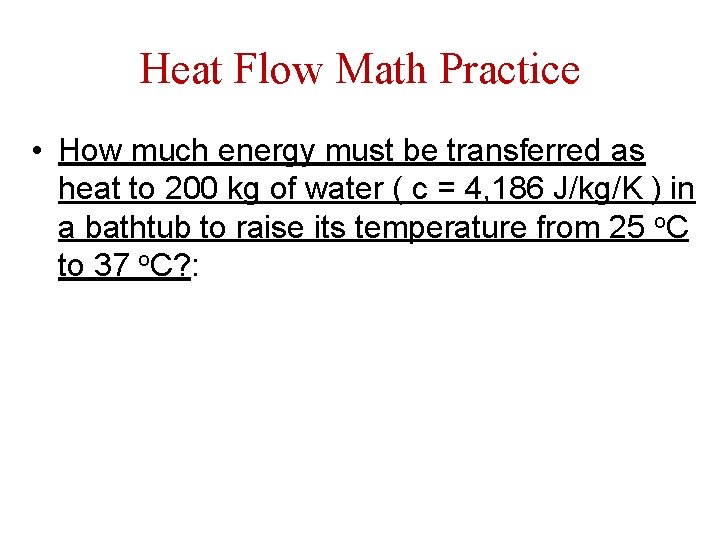
Heat Flow Math Practice • How much energy must be transferred as heat to 200 kg of water ( c = 4, 186 J/kg/K ) in a bathtub to raise its temperature from 25 o. C to 37 o. C? :

Specific Heat Example • The temperature of a substance increases by 7 K when 1850 J is added to a 5 kg quantity of the substance. What is the specific heat ( c )?

Specific Heat Example • The temperature of a substance increases by 7 K when 1850 J is added to a 5 kg quantity of the substance. What is the specific heat ( c )? • 1850 / (5 * 7 ) = 52. 9 J/(kg*K)

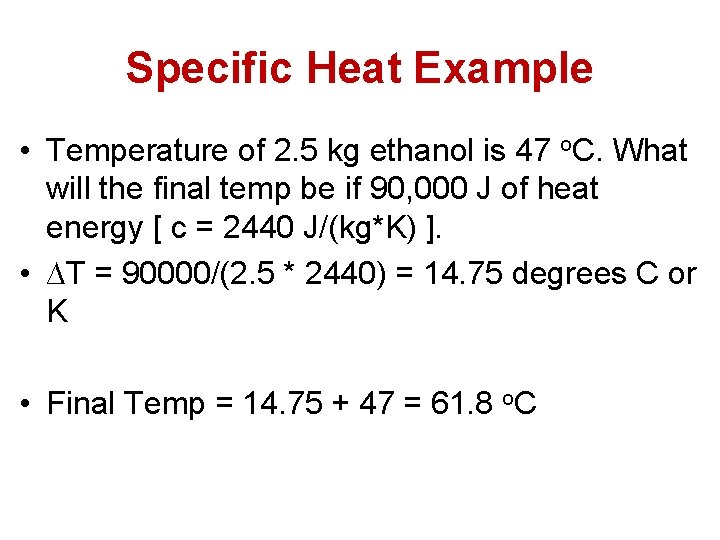
Specific Heat Example • Temperature of 2. 5 kg ethanol is 47 o. C. What will the final temp be if 90, 000 J of heat energy [ c = 2440 J/(kg*K) ].

Specific Heat Example • Temperature of 2. 5 kg ethanol is 47 o. C. What will the final temp be if 90, 000 J of heat energy [ c = 2440 J/(kg*K) ]. • ∆T = 90000/(2. 5 * 2440) = 14. 75 degrees C or K • Final Temp = 14. 75 + 47 = 61. 8 o. C
![Section 3 Using Heat Key Questions 1 What happens to heat Section 3 – Using Heat • Key Questions: • 1] What happens to heat](https://slidetodoc.com/presentation_image_h2/cd68af5d7dfb874bd2b8bf88a1da81e5/image-31.jpg)
Section 3 – Using Heat • Key Questions: • 1] What happens to heat energy when it is transferred? • 2] What do heat engines do?

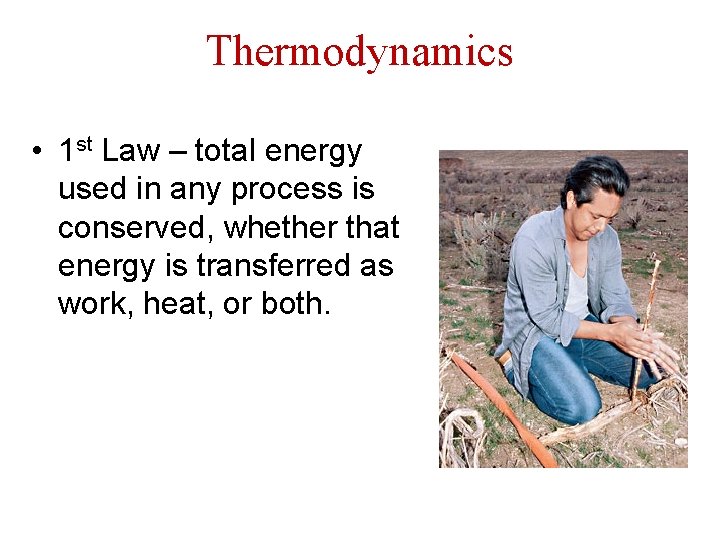
Thermodynamics • 1 st Law – total energy used in any process is conserved, whether that energy is transferred as work, heat, or both.

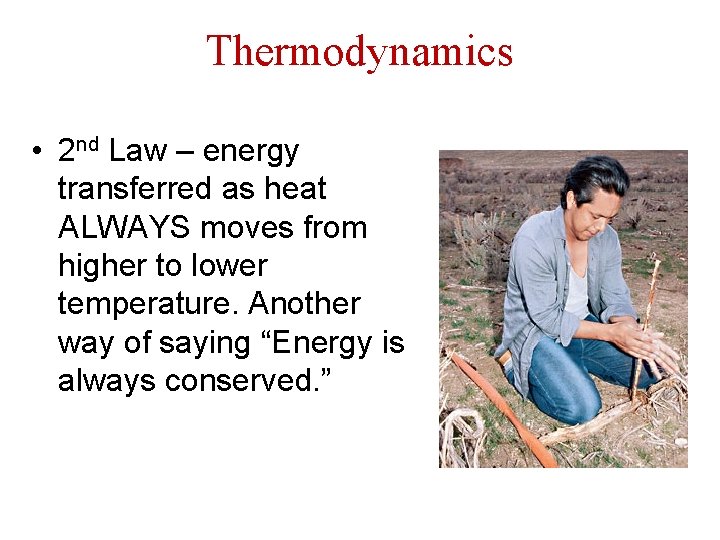
Thermodynamics • 2 nd Law – energy transferred as heat ALWAYS moves from higher to lower temperature. Another way of saying “Energy is always conserved. ”

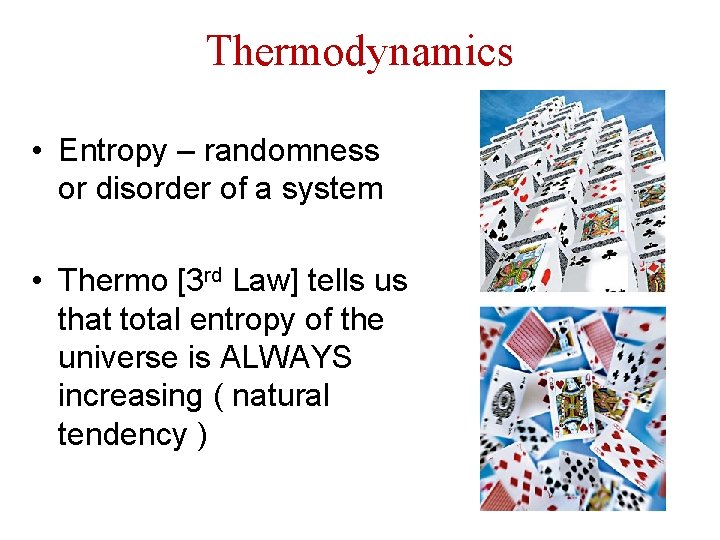
Thermodynamics • Entropy – randomness or disorder of a system • Thermo [3 rd Law] tells us that total entropy of the universe is ALWAYS increasing ( natural tendency )

What is a System? • A “system” is defined as the area, volume and/or group of objects being analyzed • Systems can be open or closed • Closed system – like a can of coke or soup that has not been opened • Open system – once you open that can of coke or soup

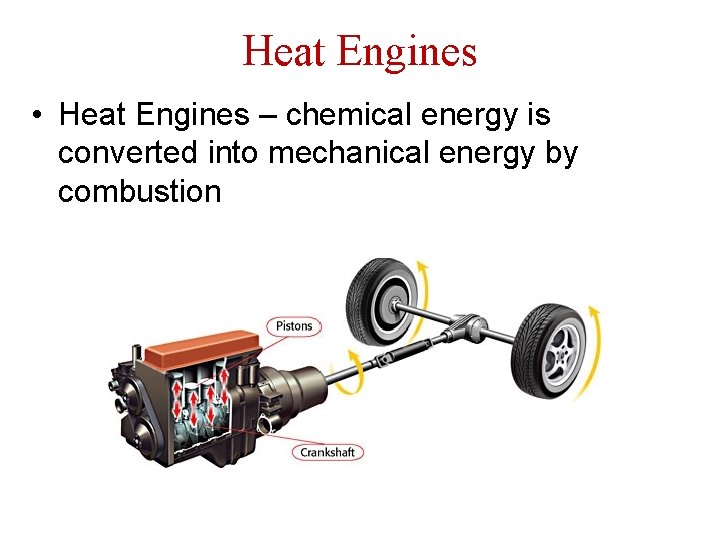
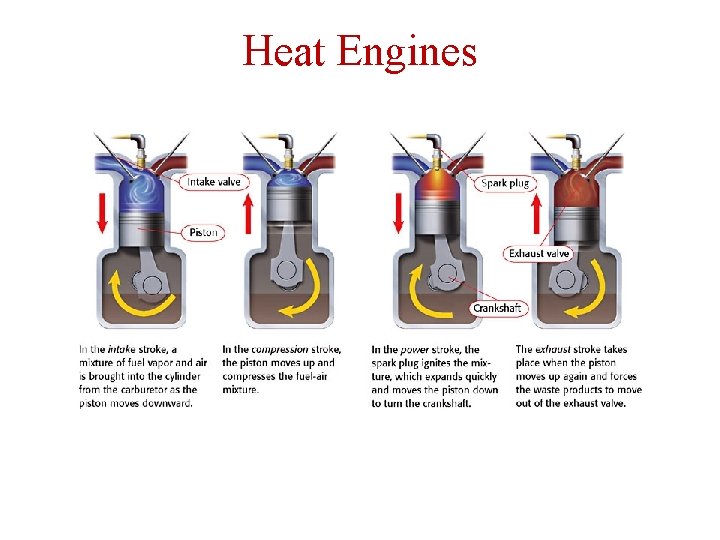
Heat Engines • Heat Engines – chemical energy is converted into mechanical energy by combustion

Heat Engines

Using Heat • Rubbing alcohol applied to the skin – what happens/what do you observe? • Why?

Using Heat • Rubbing alcohol applied to the skin – what happens/what do you observe? • Why?

Using Heat • cooling/heating processes utilize this • SWEATING! • Fluids—liquids & gases—are chosen that easily evaporate and condense • Evaporation – energy is absorbed by the fluid/sweat ( from surrounding air ) • Condensation – energy is released by the fluid/moisture ( absorbed by air )