Cell Injury and Cell Death Nirush Lertprasertsuke M









































- Slides: 48

Cell Injury and Cell Death Nirush Lertprasertsuke, M. D. Department of Pathology Faculty of Medicine , Chiang Mai University

Cell Injury • • Normal cell: homeostasis Sublethal injury: reversible injury Irreversible injury Cell death

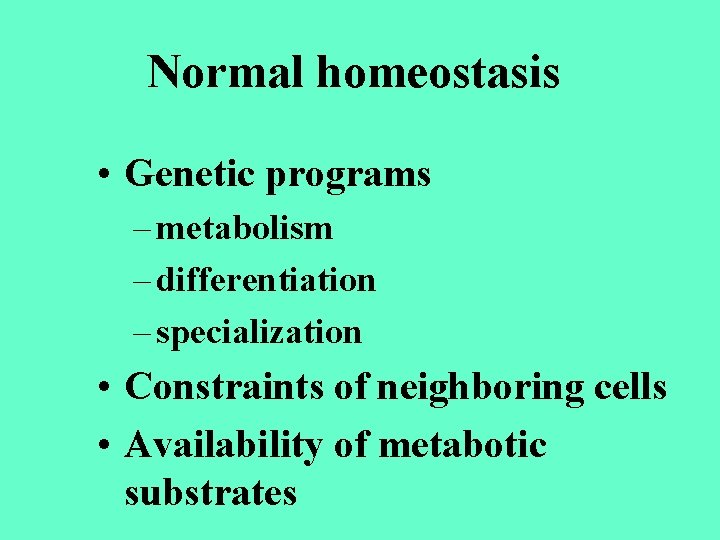
Normal homeostasis • Genetic programs – metabolism – differentiation – specialization • Constraints of neighboring cells • Availability of metabotic substrates

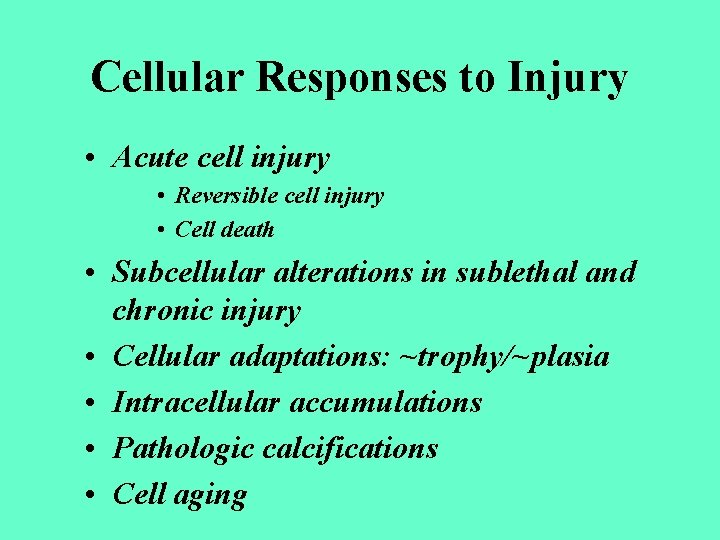
Cellular Responses to Injury • Acute cell injury • Reversible cell injury • Cell death • Subcellular alterations in sublethal and chronic injury • Cellular adaptations: ~trophy/~plasia • Intracellular accumulations • Pathologic calcifications • Cell aging

Causes of cell injury • • Oxygen Deprivation: hypoxia/ischemia Physical agents Chemical agents and drugs Infectious agents Immunologic reactions Genetic derangements Nutritional imbalances: self-imposed

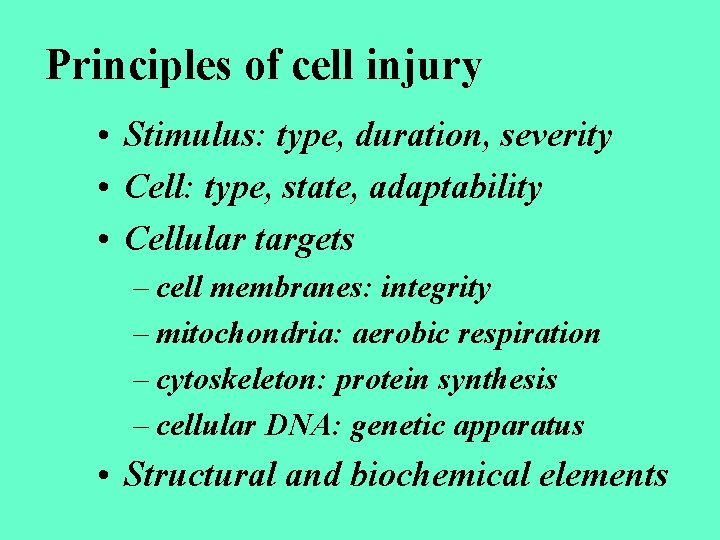
Principles of cell injury • Stimulus: type, duration, severity • Cell: type, state, adaptability • Cellular targets – cell membranes: integrity – mitochondria: aerobic respiration – cytoskeleton: protein synthesis – cellular DNA: genetic apparatus • Structural and biochemical elements

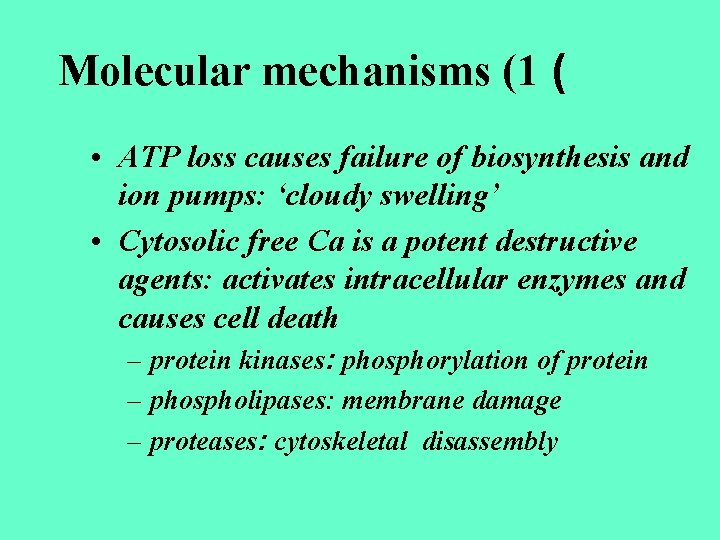
Molecular mechanisms (1 ( • ATP loss causes failure of biosynthesis and ion pumps: ‘cloudy swelling’ • Cytosolic free Ca is a potent destructive agents: activates intracellular enzymes and causes cell death – protein kinases: phosphorylation of protein – phospholipases: membrane damage – proteases: cytoskeletal disassembly




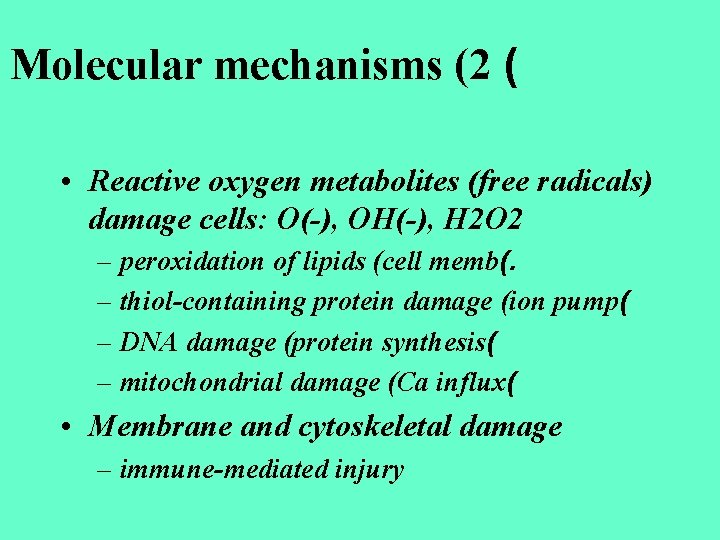
Molecular mechanisms (2 ( • Reactive oxygen metabolites (free radicals) damage cells: O(-), OH(-), H 2 O 2 – peroxidation of lipids (cell memb(. – thiol-containing protein damage (ion pump( – DNA damage (protein synthesis( – mitochondrial damage (Ca influx( • Membrane and cytoskeletal damage – immune-mediated injury


Morphology of Reversible cell injury • Ultrastructural damage to mitochondria – Low-amplitude swelling – )High-amplitude swelling: irreversible( • Swelling of cellular organelles: hydropic degeneration/cloudy swelling • Fatty change: sublethal impairment of metabolism: liver






Morphology of Cell death • Lysis: Disintegration of cellular structure followed by dissolution • Necrosis: spectrum ofmorphologic changes that follow cell death in living tissue • Apoptosis: “programmed cell death”elimination of unwanted host cells

Necrosis • Concurrent processes : – Enzymic digestion: lysis • autolysis: lysosomes of the dead cells • heterolysis: immigrant leukocytes – Denaturation of proteins • Intense eosinophilia • Nonspecific DNA breakdown – Pyknosis – Karyorhexis – Karyolysis




Patterns of Necrosis • • • Coagulative necrosis Liquefactive necrosis Caseous necrosis Fat necrosis Gangrenous necrosis Fibrinoid necrosis

Coagulative necrosis • • • Dead tissue: firm and pale Intact c. outlines and t. architecture Intracellular acidosis denatures enzymes Occlusion of arterial supply Enzymes used in Dx of tissue damage – Myocardium: CK (MB isoform), AST, LDH – Hepatocytes: ALT – Striated muscle: CK (MM isoform ( – Exocrine pancreas: amylase


Liquefactive necrosis • Semi-liquid viscous tissue • Potent hydrolytic enzymes • Examples – Hypoxic dead in the CNS: lysosomal enzymes of the neurons and the relative lack of extracellular structural protein – Bacterial infection: pus • neutrophil hydrolases: acute inflammation


Caseous necrosis • Soft and white: like cream cheese • Amorphous eosinophilic mass, loss of tissue architecture • Associated with granulomatous inflammation(reaction) in Tuberculosis


Fat necrosis • Hard yellow-gray material: fat tissue • Examples: – Retroperitoneal fat necrosis associated with acute of the pancreas – Traumatic fat necosis: breast, buttock

Gangrenous necosis • Mummified darkened and shrinkage • Coagulative necrosis only or modified by liquefactive necrosis • Dry gangrene: limb (lower leg/toe( • Wet gangrene: hollow viscera (GI tract( – hemorrhage within the tissue


Fibrinoid necrosis • Deposits of fibrin to the wall of necrotic vessels • Causes : – Vasculitis: autoimmune disease – Hypertension


Apoptosis Settings • During development • Homeostatic mechanism to maintain cell populations in tissue: involution • Defense mechanism e. g. immune reaction • Injury – viral infection – low doses of injurious stimuli • Aging

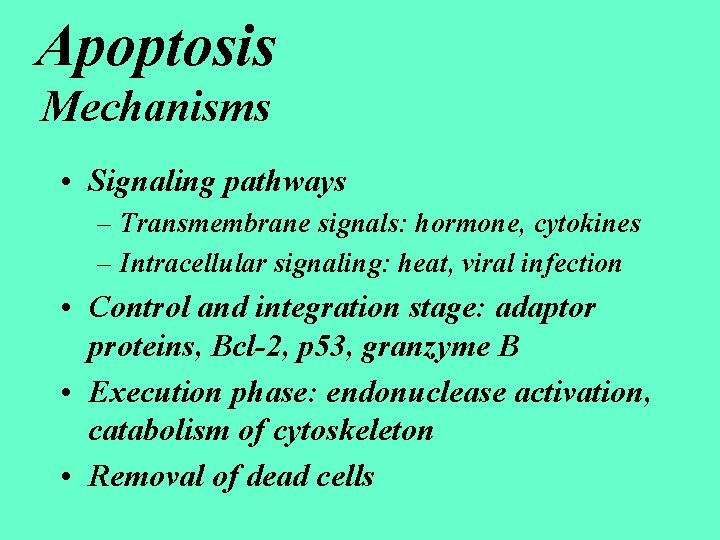
Apoptosis Mechanisms • Signaling pathways – Transmembrane signals: hormone, cytokines – Intracellular signaling: heat, viral infection • Control and integration stage: adaptor proteins, Bcl-2, p 53, granzyme B • Execution phase: endonuclease activation, catabolism of cytoskeleton • Removal of dead cells






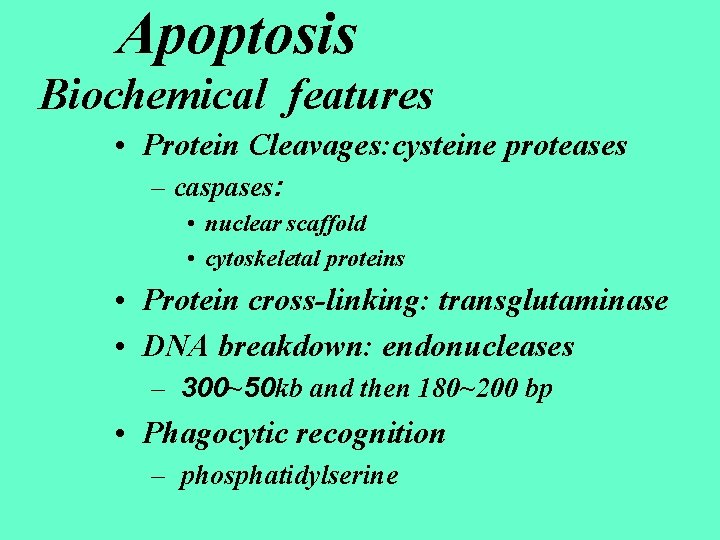
Apoptosis Biochemical features • Protein Cleavages: cysteine proteases – caspases: • nuclear scaffold • cytoskeletal proteins • Protein cross-linking: transglutaminase • DNA breakdown: endonucleases – 300~50 kb and then 180~200 bp • Phagocytic recognition – phosphatidylserine

Apoptosis Morphology • Cell shrinkage • Chromatin condensation • Formation of cytoplasmic blebs and apoptotic bodies • Phagocytosis of apoptotic cells/bodies • Single cell or small clusters with intense eosinophilic cytoplasm and dense chromatin fragments




 Intentional injury examples
Intentional injury examples Cell injury and inflammation
Cell injury and inflammation Dry vs wet gangrene
Dry vs wet gangrene What is death
What is death Russell bodies
Russell bodies Types of necrosis
Types of necrosis Cell injury
Cell injury Myelin figures in reversible cell injury
Myelin figures in reversible cell injury Example of physiological hyperplasia
Example of physiological hyperplasia Mitochondrial swelling
Mitochondrial swelling Injury prevention safety and first aid
Injury prevention safety and first aid Chapter 25 suicide and nonsuicidal self injury
Chapter 25 suicide and nonsuicidal self injury A spill at parsenn bowl knee injury and recovery
A spill at parsenn bowl knee injury and recovery Nrl head injury recognition and referral form
Nrl head injury recognition and referral form Workplace fatality prevention
Workplace fatality prevention Safety practices and sports injury management pictures
Safety practices and sports injury management pictures Unintentional injury examples
Unintentional injury examples Somi brace
Somi brace Serious injury and fatality prevention
Serious injury and fatality prevention Denuding tower
Denuding tower Prokaryotic and eukaryotic cells
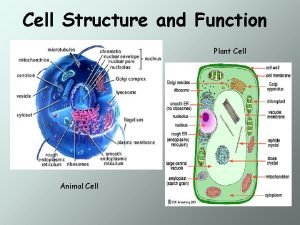
Prokaryotic and eukaryotic cells Plant vs animal cells organelles
Plant vs animal cells organelles Plant cell structure
Plant cell structure Nucleolus function in animal cell
Nucleolus function in animal cell Primary cell and secondary cell
Primary cell and secondary cell Difference between plant and animal cell
Difference between plant and animal cell Events of the cell cycle
Events of the cell cycle Prokaryotic cell and eukaryotic cell
Prokaryotic cell and eukaryotic cell The scientist mathias schleiden studied _______ in ______.
The scientist mathias schleiden studied _______ in ______. Idealized plant cell
Idealized plant cell Walker cell and hadley cell
Walker cell and hadley cell Cell cycle and cell division
Cell cycle and cell division Similarities between plant and animal cells venn diagram
Similarities between plant and animal cells venn diagram Phases of cell cycle
Phases of cell cycle Difference between galvanic cell and electrolytic cell
Difference between galvanic cell and electrolytic cell Rigid outer covering of plant cells
Rigid outer covering of plant cells Priapisml
Priapisml What is incident report form
What is incident report form Unilateral superior laryngeal nerve injury
Unilateral superior laryngeal nerve injury Vocal cord positions
Vocal cord positions Median cricothyroid ligament
Median cricothyroid ligament Peroneal nerve palsy
Peroneal nerve palsy Parasthesisa
Parasthesisa Most common site of ureteric injury during hysterectomy
Most common site of ureteric injury during hysterectomy Iss score
Iss score Liver injury grading
Liver injury grading Liver injury grading
Liver injury grading Generous adjective
Generous adjective Wound certificate
Wound certificate