CELL INJURY lecture 1 Sufia Husain Assistant Prof




















- Slides: 41

CELL INJURY (lecture 1) Sufia Husain Assistant Prof & Consultant KKUH, Riyadh.

CELL INJURY When the cell is exposed to an injurious agent or stress, a sequence of events follows that is loosely termed cell injury. Cell injury is reversible up to a certain point, but if the stimulus persists or is severe enough from the beginning, the cell reaches a point of no return and suffers irreversible cell injury and ultimately cell death. Cell death, is the ultimate result of cell injury

CELL DEATH There are two principal patterns of cell death, necrosis and apoptosis. Necrosis is the type of cell death that occurs after ischemia and chemical injury, and it is always pathologic. Apoptosis occurs when a cell dies through activation of an internally controlled suicide program. It is designed to eliminate unwanted cells during embryogenesis and in various physiologic processes and certain pathologic conditions.

Causes of Cell Injury 1) Oxygen Deprivation (Hypoxic cell injury). It common cause of cell injury and cell death. Hypoxia can be due to a) Ischemia (obstruction of arterial blood flow), the most common cause. b) inadequate oxygenation of the blood due to cardiorespiratory failure, hypotension, shock etc. c) loss of the oxygen-carrying capacity of the blood, as in anemia d) carbon monoxide poisoning. Depending on the severity of the hypoxic state, cells may adapt, undergo injury, or die. Also some cell types are more vulnerable to hypoxic injury then others e. g. neurons are most susceptible followed by cardiac muscle, hepatocytes and then skeletal muscles.

Causes of Cell Injury cont. 2)Physical Agents e. g. mechanical trauma, burns and deep cold, sudden changes in atmospheric pressure, radiation, and electric shock 3)Chemical Agents and Drugs e. g. oxygen, in high concentrations, poisons, such as arsenic, cyanide, or mercuric salts, environmental and air pollutants, insecticides, herbicides, industrial and occupational hazards, alcohol and narcotic drugs and therapeutic drugs. 4)Infectious Agents e. g. bacteria, fungi, viruses and parasites. 5) Immunologic Reactions. 6) Genetic Derangements. 7) Nutritional Imbalances

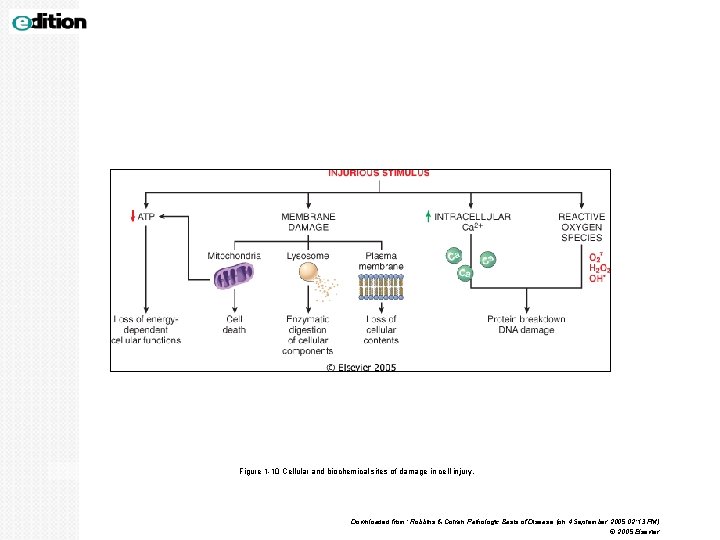
MECHANISM OF CELL INJURY 1. Depletion of ATP 2. Mitochondrial damage. 3. Ribosomal damage 4. Nuclear damage 5. Defects in membrane permeability/ cell membrane damage 6. Influx of intracellular calcium and loss of calcium homeostasis. 7. Accumulation of oxygen-derived free radicals (oxidative stress)

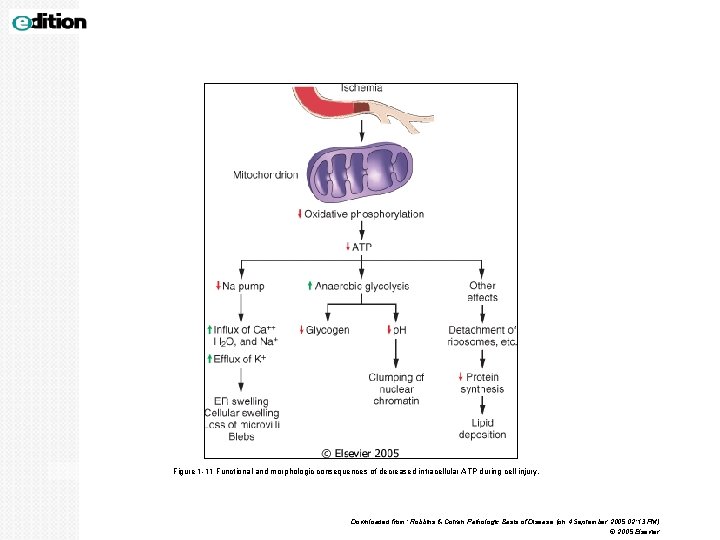
MECHANISM OF CELL INJURY 1. DEPLETION OF ATP: ATP depletion and decreased ATP synthesis are associated with both hypoxic and chemical (toxic) injury. ATP is required for normal function within the cell. ATP is produced in two ways. 1. The major pathway is oxidative phosphorylation of adenosine diphosphate. 2. The second is the glycolytic pathway, which generate ATP in absence of oxygen using glucose derived from body fluids or from glycogen •

MECHANISM OF CELL INJURY cont. 2. MITOCHONDRIAL DAMAGE Mitochondria is necessary for aerobic respiration of the cell. Seen in hypoxia and cyanide poisoning. Mitochondria are important targets for all types of injury, specially hypoxic injury and injury by toxic substaces like cyanide. Mitochondria can also be damaged by increases of cytosolic Ca 2+ and by breakdown of phospholipids 3. RIBOSOMAL DAMAGE Ribosomes are necessary for protein synthesis and any ribosomal damage leads to altered protein synthesi e. g. alcohol associated damage of liver cells and bacterial infection.

MECHANISM OF CELL INJURY cont. 4. NUCLEAR DAMAGE It can be caused by virus, radiation or free radicals. 5. DEFECTS IN MEMBRANE PERMEABILITY Membrane damage may be the result of hypoxia and ATP depletion and calcium-modulated activation of phospholipases. It can also be damaged directly by certain bacterial toxins, viral proteins, complement mediated lysis with the help of membrane attack complex and by free radicals (reactive oxygen species).

MECHANISM OF CELL INJURY cont. 6. INFLUX OF INTRACELLULAR CALCIUM AND LOSS OF CALCIUM HOMEOSTASIS. Ischemia causes an increase in cytosolic calcium concentration. Increased Ca 2+ in turn activates a number of enzymes which cause damage, e. g. Ø ATPases (used in ATP depletion) Ø phospholipases (which causes membrane damage) Ø proteases (breaks down both membrane and cytoskeletal proteins) Ø endonucleases (responsible for DNA and chromatin fragmentation).

MECHANISM OF CELL INJURY cont. 7. ACCUMULATION OF OXYGEN-DERIVED FREE RADICALS (OXIDATIVE STRESS) § Small amounts of partially reduced reactive oxygen forms are produced as a byproduct of mitochondrial respiration. They are referred to as reactive oxygen species/free radicals. § Free radicals are chemical species that have single unpaired electron in an outer orbit. § The common free radicals are superoxide anion radical (O 2 -), hydrogen peroxide (H 2 O 2), and hydroxyl ions (OH). Nitric oxide (NO), an important chemical mediator generated by various cells, can act as a free radical. § Some free radicals damage lipids, proteins, and nucleic acids. The main effects of these reactive species/ free radicals are: 1. Lipid peroxidation of membranes: leads to membrane, organellar, and cellular damage. 2. Oxidative modification of proteins: leads to protein fragmentation. 3. Lesions in DNA: This DNA damagecan lead to cell aging and malignant transformation of cells

MECHANISM OF CELL INJURY cont. The production of free radicals are intiated within cells in several ways. They are called the free radical generating systems and include the following: a) Absorption of radiant energy (e. g. , ultraviolet light, xrays or any other type of radiation). b) Enzymatic metabolism of exogenous chemicals or drugs. c) The reduction-oxidation reactions that occur during normal metabolic processes. During normal respiration, small amounts of free radicals are produced. d) Transition metals such as iron and copper can trigger production.

MECHANISM OF CELL INJURY cont. Cells have developed multiple mechanisms to remove free radicals and § therefore minimize injury caused by these products. There are several § substances that contribute to inactivation of free radical reactions. They § are called as the free radical scavenging system. These include the § following: Ø Antioxidants: Examples vitamins E, A and C (ascorbic acid). Ø Enzymes which break down hydrogen peroxide and superoxide anion e. g. Catalase, Superoxide dismutases, and Glutathione peroxidase. § Any imbalance between free radical-generating and radicalscavenging systems results in oxidative stress causing cell injury. § Free radical-mediated damage are seen in chemical and radiation injury, ischemia-reperfusion injury, cellular aging, and microbial killing by phagocytes. §

Figure 1 -10 Cellular and biochemical sites of damage in cell injury. Downloaded from: Robbins & Cotran Pathologic Basis of Disease (on 4 September 2005 02: 13 PM) © 2005 Elsevier

Figure 1 -11 Functional and morphologic consequences of decreased intracellular ATP during cell injury. Downloaded from: Robbins & Cotran Pathologic Basis of Disease (on 4 September 2005 02: 13 PM) © 2005 Elsevier

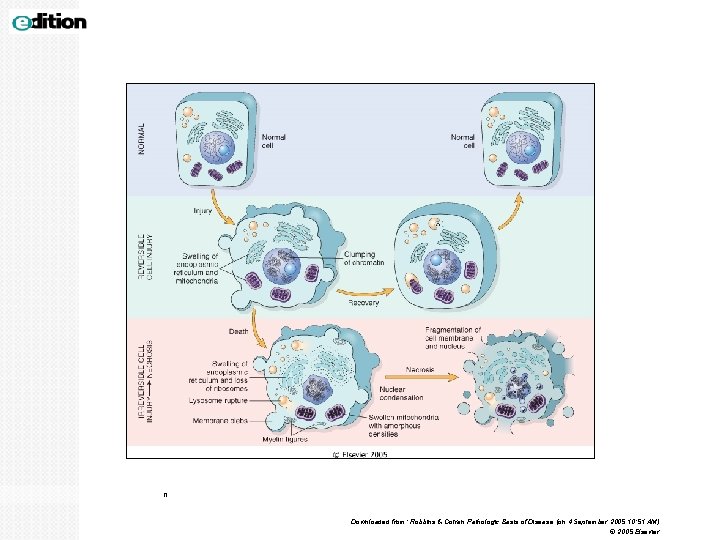
Reversible Cell Injury The type of injury the time duration of injury and the severity of injury will determine the extent of cell damage i. e. whether the injury is reversible or irreversible. Earliest changes associated with cell injury are ◦ Cytoplasmic eosinophilia ◦ decreased generation of ATP and defects in protein synthesis ◦ loss of cell membrane integrity, ◦ Mitochondria and endoplasmic reticulum swelling, ◦ Cytoskeletal damage, cytoplasmic swelling and vacuolation, ◦ DNA damage with clumping of nuclear chromatin. Within limits, the cell can compensate for these derangements and, if the injurious stimulus is removed the damage can be reversed.

Irreversible Cell Injury Persistent or excessive injury, however, causes cells to pass the threshold into irreversible injury. Irreversible injury is marked by Ø severe mitochondrial vacuolization and the appearance large, amorphous densities in mitochondria. Ø extensive damage and disruption of plasma membranes, Ø swelling and rupture of lysosomes Ø Nuclear: 1. pyknosis (shrinkage), 2. karyolysis (dissolution) and 3. karyorrhexis (break down) Two phenomena consistently characterize irreversibility. They are: 1)the inability to reverse mitochondrial dysfunction (lack of oxidative phosphorylation and ATP generation) even after removal of the original injury. 2)profound loss in membrane function.

n Downloaded from: Robbins & Cotran Pathologic Basis of Disease (on 4 September 2005 10: 51 AM) © 2005 Elsevier

NECROSIS. Necrosis refers to a spectrum of morphologic changes that follow cell death in living tissue, due to degradative action of enzymes on the injured cell. It occurs in irreversible injury. This may elicit inflammation in the surrounding tissue. There is denaturation of intracellular proteins and enzymatic digestion of the cell. The enzymes used in this degradation are derived either from the lysosomes of the dead cells themselves, in which case the enzymatic digestion is referred to as autolysis, or from the lysosomes of immigrant leukocytes, during inflammatory reactions referred to as heterolysis. (Autolysis is disintegration of cells or tissues by autologous enzymes, it can be seen in cells after death of the organism and in some pathologic conditions)

Morphology of necrosis. Necrotic cells show increased eosinophilia with a glassy homogeneous appearance. The cytoplasm becomes vacuolated and appears moth-eaten. Sometimes calcification of the dead cells may occur. By electron microscopy, necrotic cells are characterized by overt discontinuities in plasma membrane, marked dilation of mitochondria with the appearance of large amorphous densities, intracytoplasmic myelin figures, amorphous osmiophilic debris, and aggregates of fluffy material probably representing denatured protein Nuclear changes show one of three patterns, all due to nonspecific breakdown of DNA karyolysis : lysis of nucleus Pyknosis, (also seen in apoptotic cell death) is characterized by nuclear shrinkage. Here the DNA apparently condenses into a solid, shrunken basophilic mass. Karyorrhexis: fragmentation of the nucleus. With the passage of time (a day or two), the nucleus in the necrotic cell totally disappears.

Types of necrosis There are different types of necrosis eg: Ø coagulative necrosis Ø liquefactive necrosis Ø caseous necrosis Ø fat necrosis Ø fibrinoid necrosis

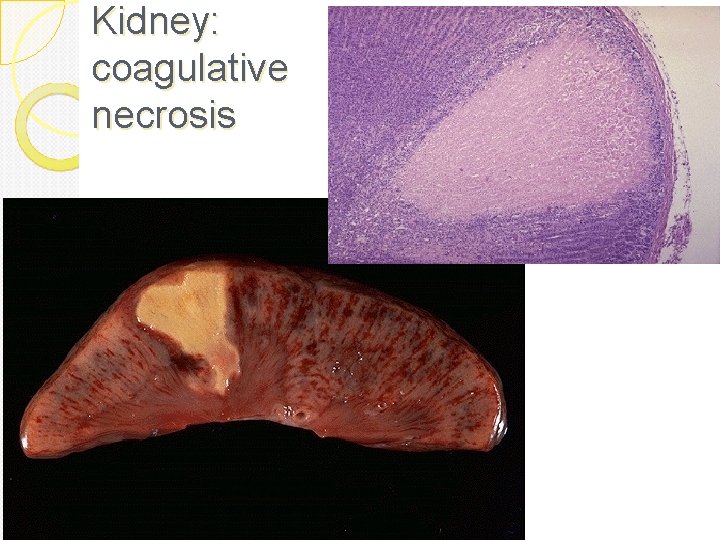
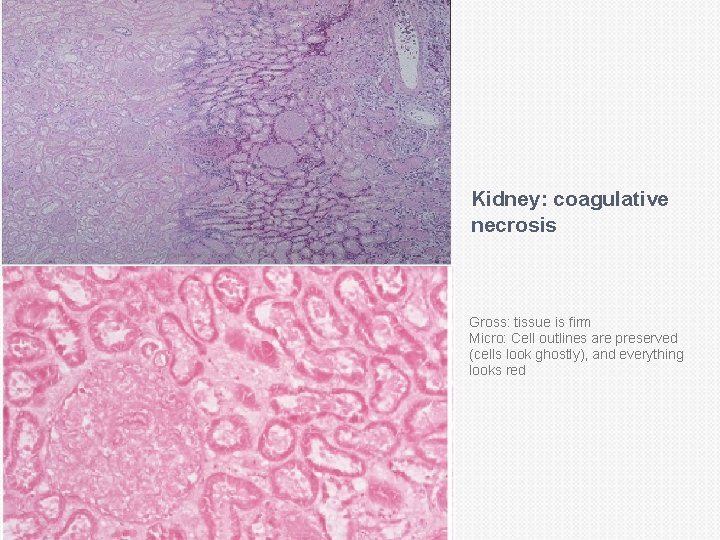
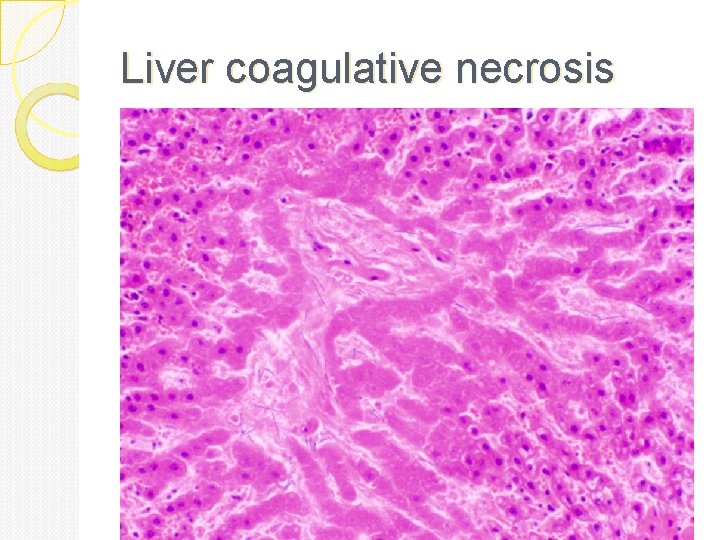
Coagulative necrosis: In it there is preservation of the general tissue architecture and the basic outline of the coagulated cell remains preserved for a span of some days. Ultimately, the necrotic cells are removed by fragmentation and then phagocytosis of the cellular debris by scavenger leukocytes and their proteolytic lysosomal enzymes. Coagulative necrosis is characteristically seen in ischemic/hypoxic death of cells in all tissues (myocardial, kidney, spleen etc) except the brain.

Coagulative necrosis: See this in infarcts in any tissue (except brain) Due to loss of blood Gross: tissue is firm Micro: Cell outlines are preserved (cells look ghostly), and everything looks red

Kidney: coagulative necrosis

Kidney: coagulative necrosis Gross: tissue is firm Micro: Cell outlines are preserved (cells look ghostly), and everything looks red

Liver coagulative necrosis

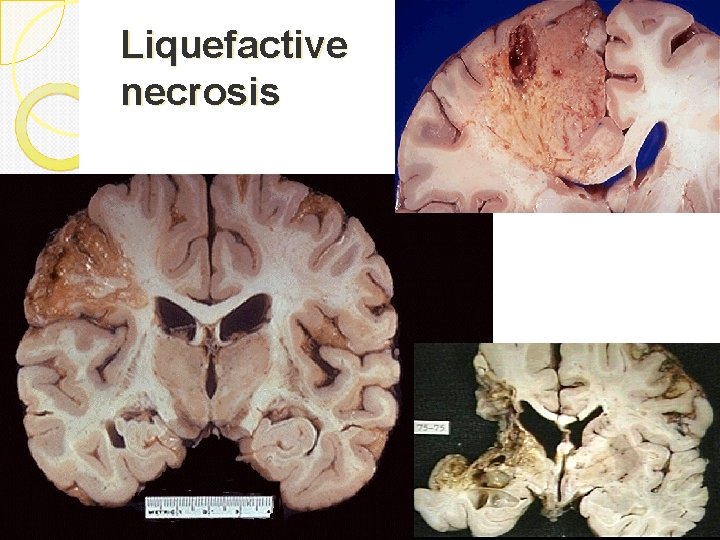
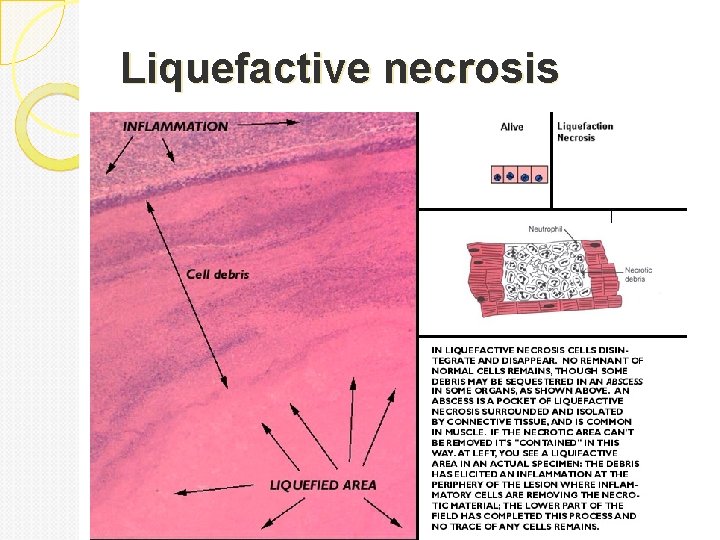
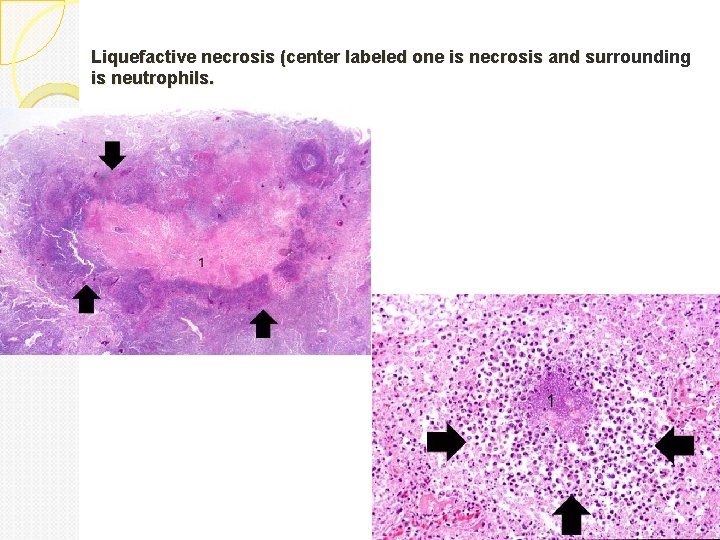
Liquefactive necrosis Is characteristic of infections especially bacterial. It is also seen in hypoxic death of cells within the central nervous system. Due to lots of neutrophils around releasing their toxic contents, “liquefying” the tissue. Liquefaction completely digests the dead cells. The end result is transformation of the tissue into a liquid viscous mass. Gross: tissue is liquidy and creamy yellow because of the presence of dead white cells and is called pus. Micro: lots of neutrophils and cell debris

Liquefactive necrosis

Liquefactive necrosis

Liquefactive necrosis (center labeled one is necrosis and surrounding is neutrophils.

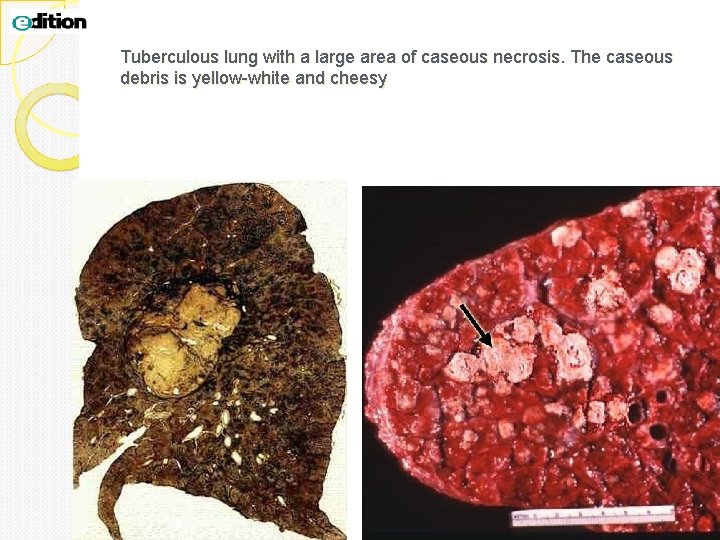
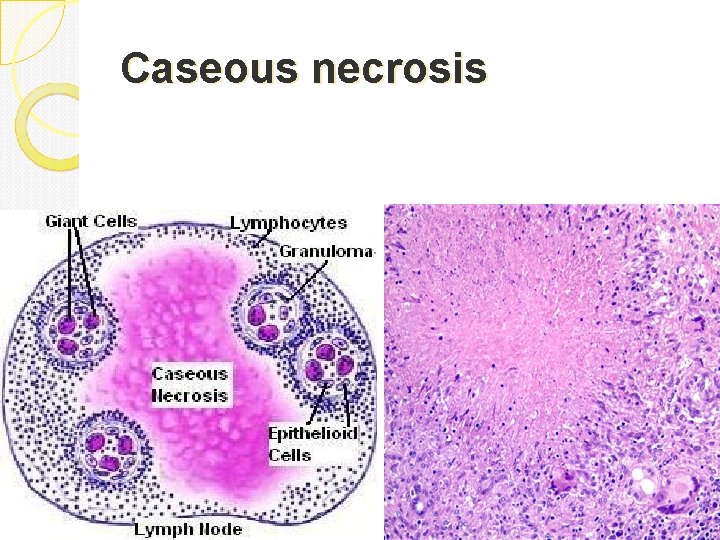
Caseous necrosis is a type of coagulative necrosis classically seen in tuberculous infection. The lung and lymph nodes are commonly involved and patients present with fever, night sweats and respiratory symptoms Gross: White, soft, cheesy-looking (“caseous”) material. The term caseous is derived from the cheesy white gross appearance of the area of necrosis. On microscopic examination, the necrotic area appears as amorphous pink granular debris surrounded by a collar of lymphocytes and macrophages. This in known as granuloma (the body tries to wall off and kill the bug with macrophages). Here the tissue architecture is completely obliterated.

Tuberculous lung with a large area of caseous necrosis. The caseous debris is yellow-white and cheesy

Caseous necrosis

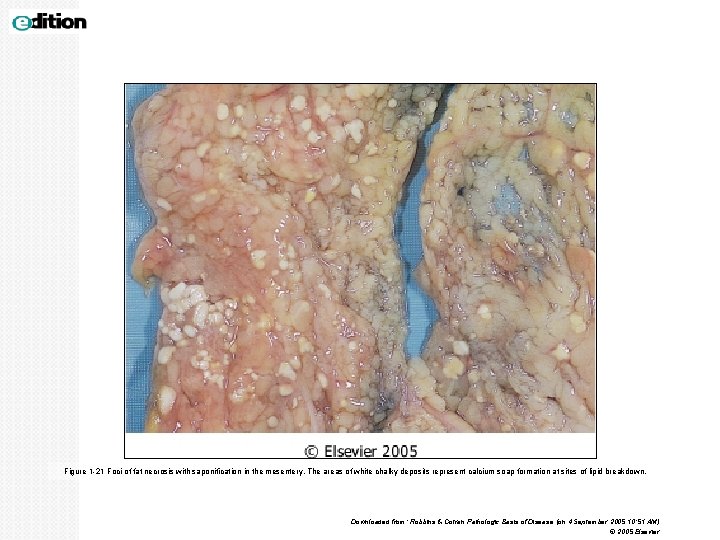
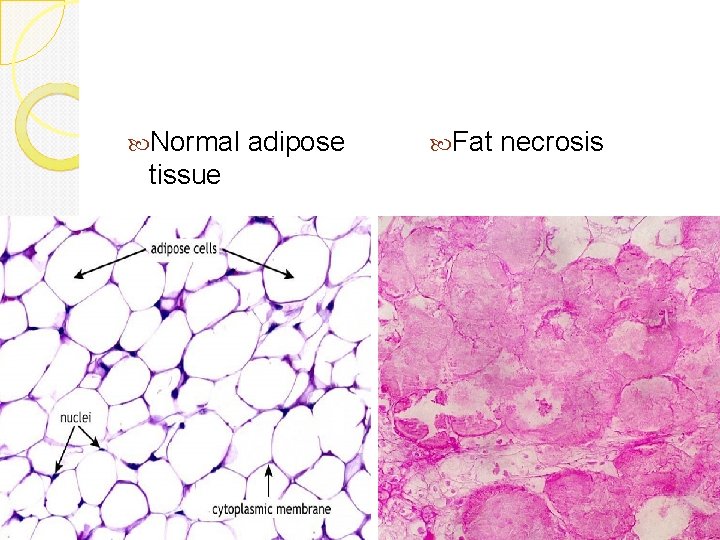
Fat necrosis Is focal areas of fat destruction, due to release of activated pancreatic lipases into the substance of the pancreas and the peritoneal cavity. Seen in acute pancreatitis. Damaged cells release lipases, which split the triglyceride esters within fat cells into glycerol and free fatty acids. The released fatty acids combine with calcium to produce calcium soaps (fat saponification) which grossly look like chalky white areas. Gross: chalky, white areas from the combination of the newlyformed free fatty acids with calcium (saponification) Micro: shadowy outlines of necrotic/dead fat cells; sometimes there is a bluish cast from the calcium deposits, which are basophilic. Some inflammatory cells may be present. Fat necrosis can also be seen in breast fat.

Figure 1 -21 Foci of fat necrosis with saponification in the mesentery. The areas of white chalky deposits represent calcium soap formation at sites of lipid breakdown. Downloaded from: Robbins & Cotran Pathologic Basis of Disease (on 4 September 2005 10: 51 AM) © 2005 Elsevier

Normal adipose tissue Fat necrosis

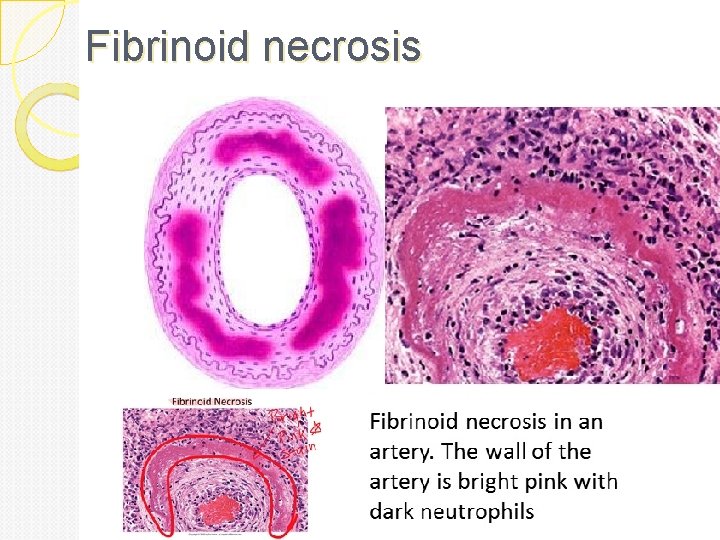
Fibrinoid necrosis Is marked by the deposition of fibrin like proteinaceous material in the arterial walls, which appears smudgy and acidophilic. It is seen in immune mediated vascular damage like autoimmune diseases such as polyarteritis nodosa. It is also seen in malignant hypertension. Gross: changes too small to see grossly Micro: vessel walls are thickened and

Fibrinoid necrosis

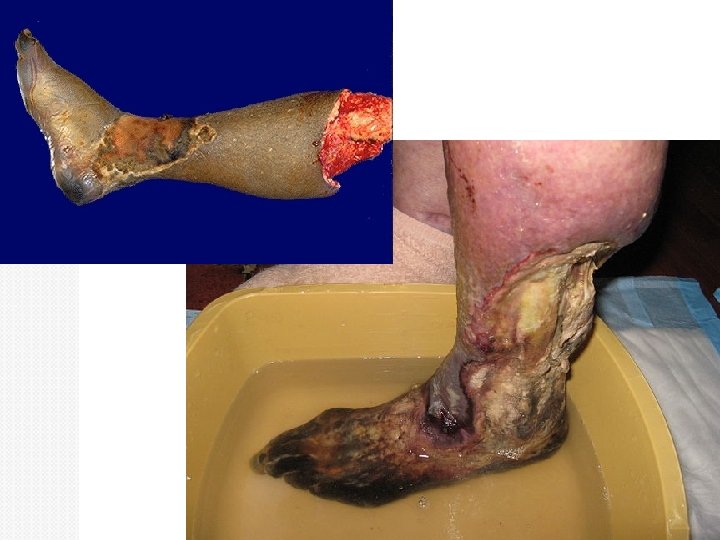
Gangrenous necrosis See this when an entire limb loses blood supply and dies (usually the lower leg). This isn’t really a different kind of necrosis, but people use the term clinically so it’s worth knowing about. Is a term used by surgoens. It is usually applied to a limb, generally the lower leg, that has lost its blood supply and has undergone coagulation necrosis. Gross: skin looks black and dead; underlying tissue is in varying stages of decomposition and is foul smelling. Micro: initially there is coagulative necrosis from the loss of blood supply (this stage is called “dry gangrene”); if there is superimposed bacterial infection, then liquefactive necrosis developes (this stage is called “wet gangrene”). The bacteria is usually gram positive Clostridia species. They are derived from the gut or the soil and can thrive in low oxygen states.

