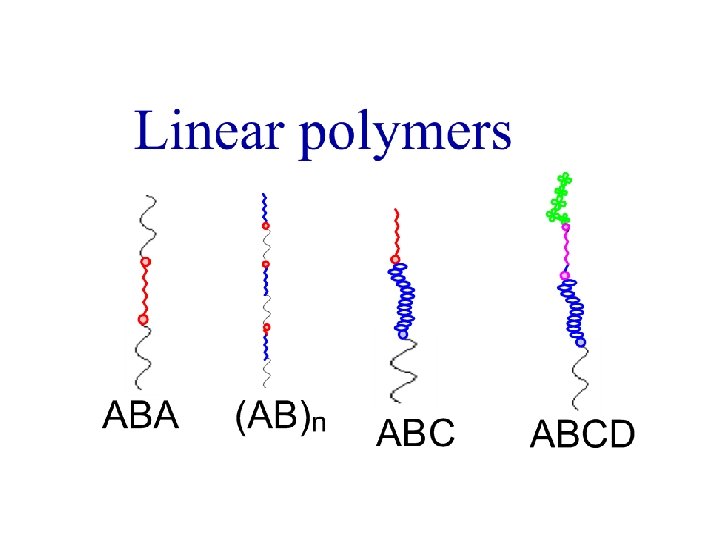
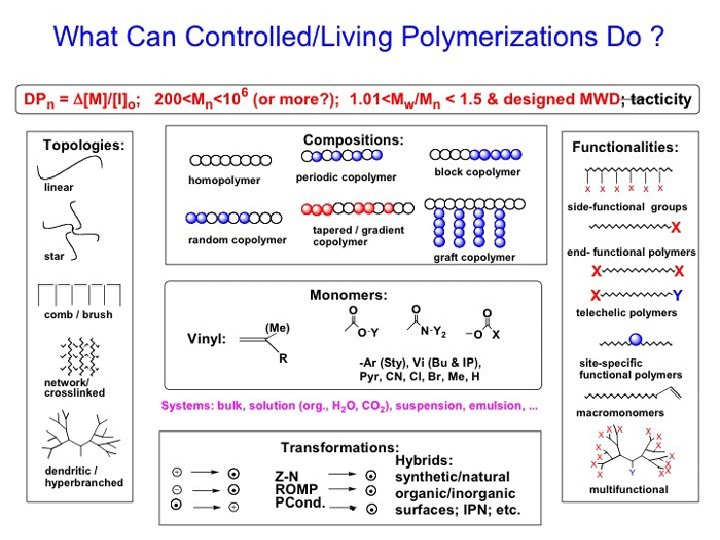
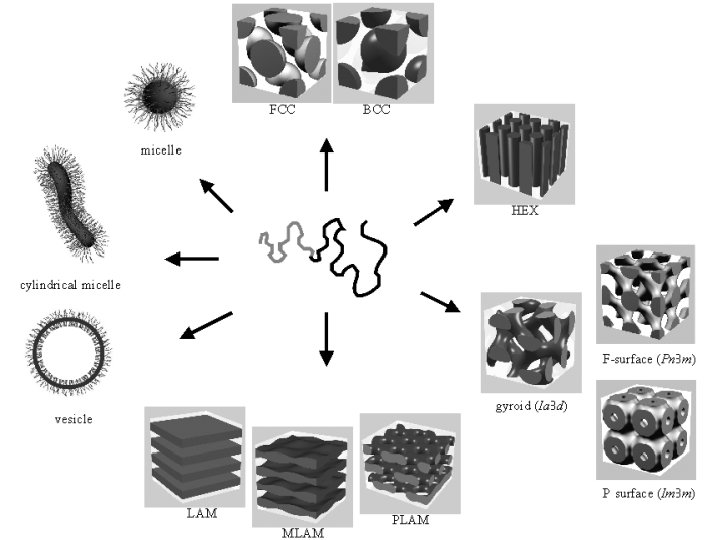
Architectures of polymers Linear polymers PE PP PS










- Slides: 66


聚合物的结构 Architectures of polymers 线形聚合物 Linear polymers 均聚物 PE, PP, PS, PVC etc. 嵌段共聚物 无规共聚物 交替共聚物


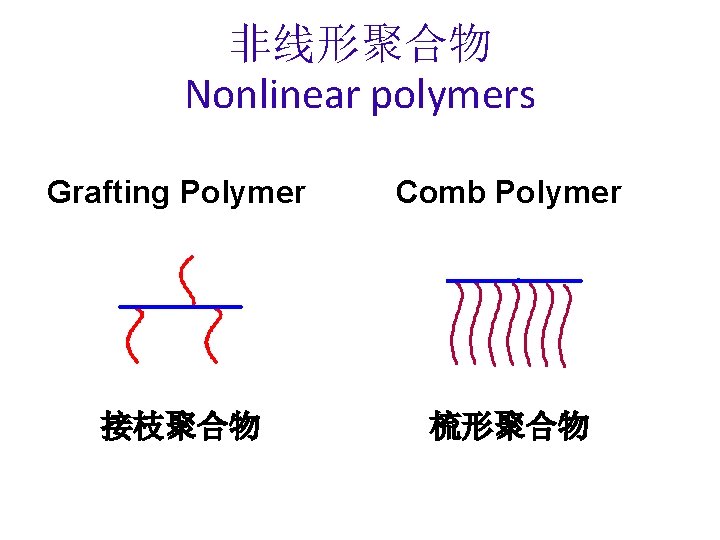
非线形聚合物 Nonlinear polymers Grafting Polymer Comb Polymer 接枝聚合物 梳形聚合物

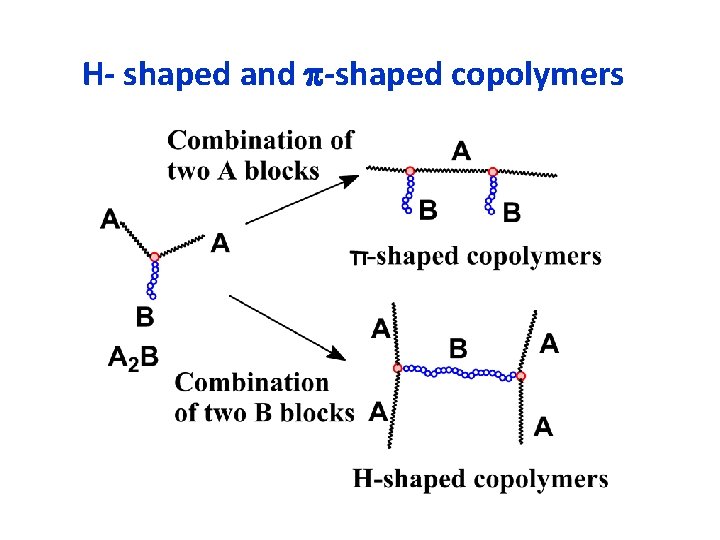
H- shaped and -shaped copolymers

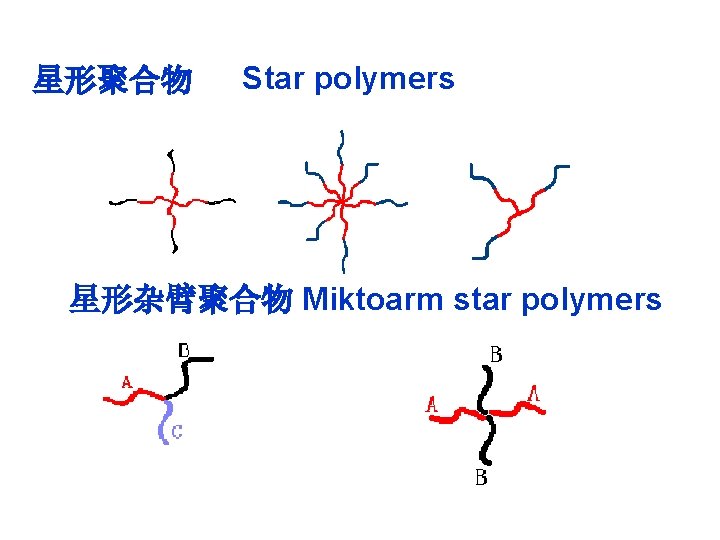
星形聚合物 Star polymers 星形杂臂聚合物 Miktoarm star polymers

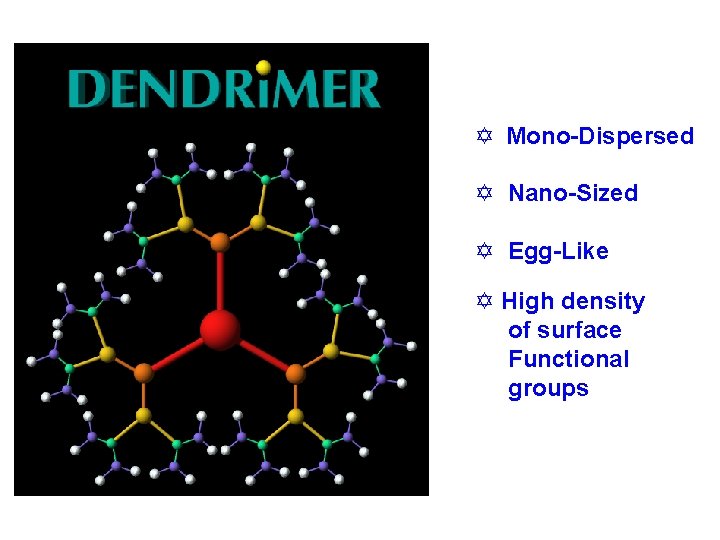
Y Mono-Dispersed Y Nano-Sized Y Egg-Like Y High density of surface Functional groups

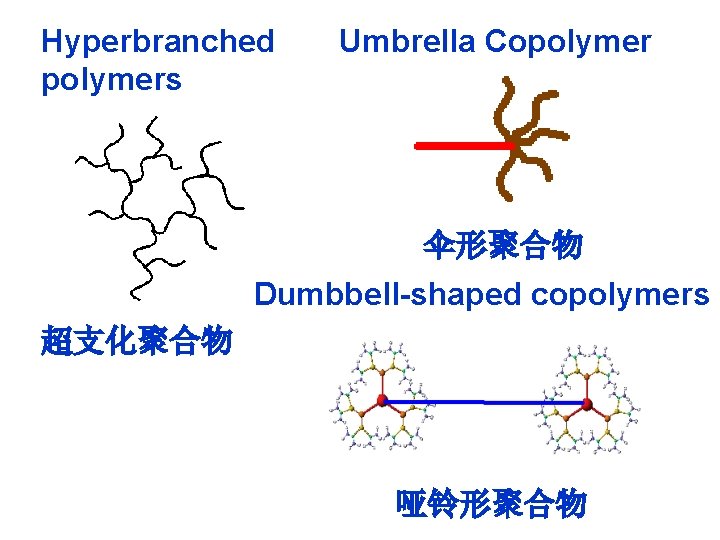
Hyperbranched polymers Umbrella Copolymer 伞形聚合物 Dumbbell-shaped copolymers 超支化聚合物 哑铃形聚合物


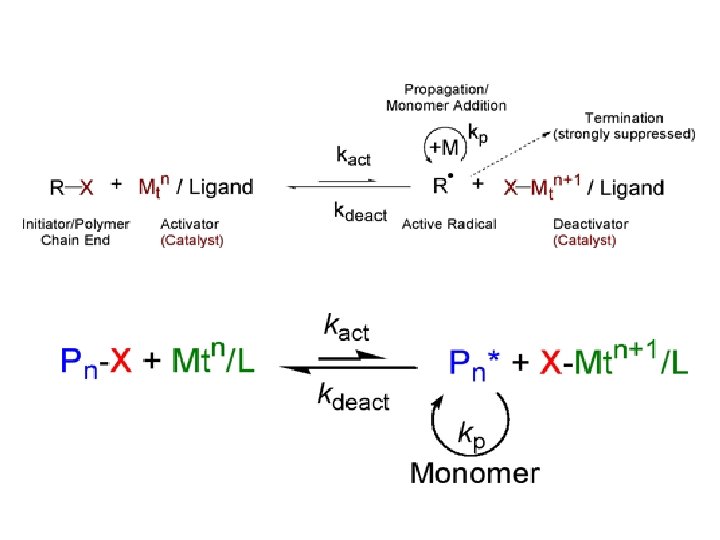
原子转移自由基聚合 Atom Transfer Radical Polymerization, ATRP or Atom Transfer Radical Polymerization is a Polymerization reaction involving free radicals. It was introduced as an extension to ATRA or Atom Transfer Radical Addition by Jinshan Wang and Matyjaszewski, (1995) and Sawamoto (1994/5).

Dr Krzysztof Matyjaszewski J. C. Warner Professor of Carnegie Mellon University Department of Chemistry 4400 Fifth Avenue Pittsburgh, PA 15213 Dr. Mistuo Sawamoto Department of Polymer Chemistry Graduate School of Engineering Kyoto University, Kyoto







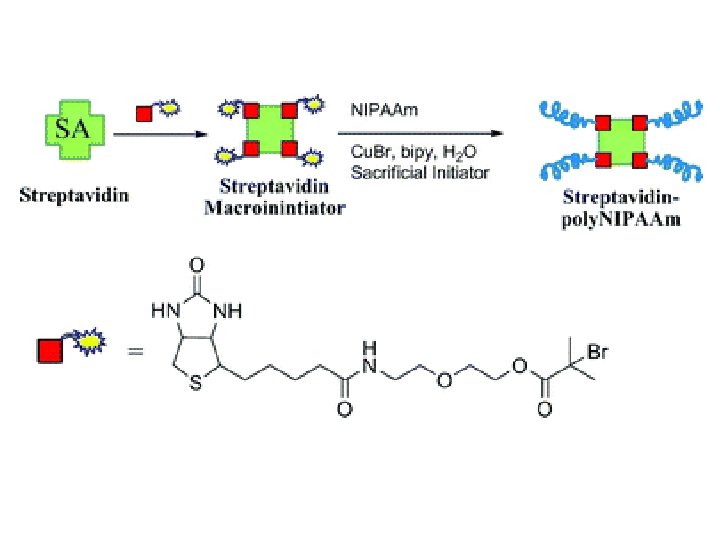
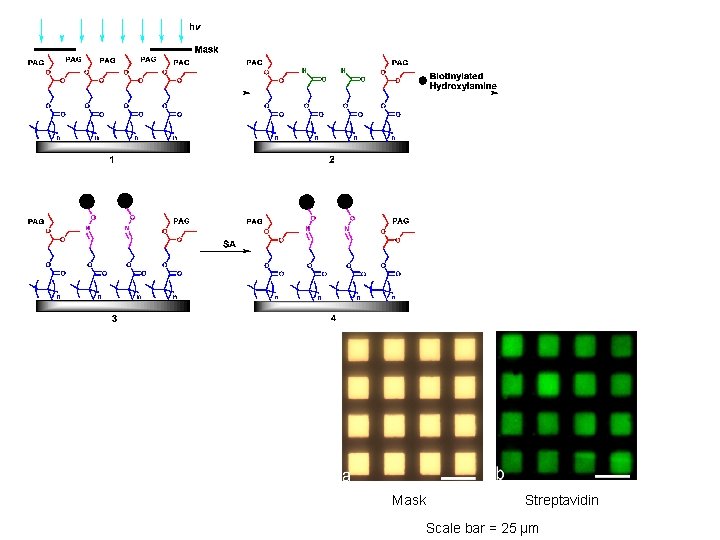
Mask Streptavidin Scale bar = 25 µm

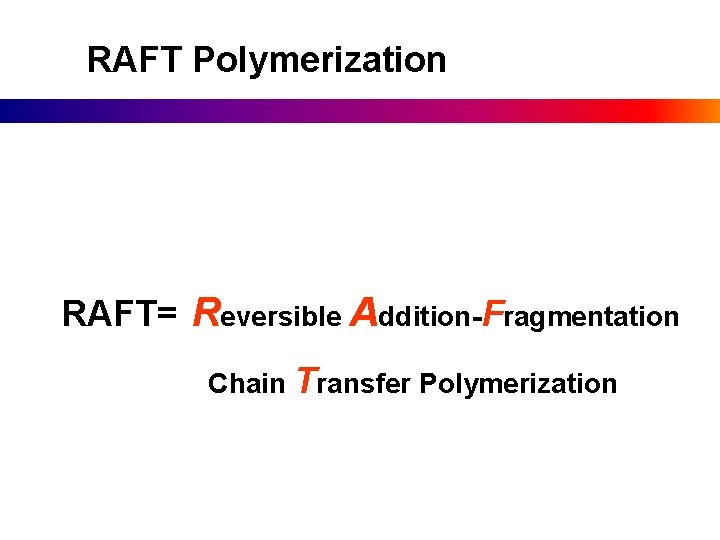
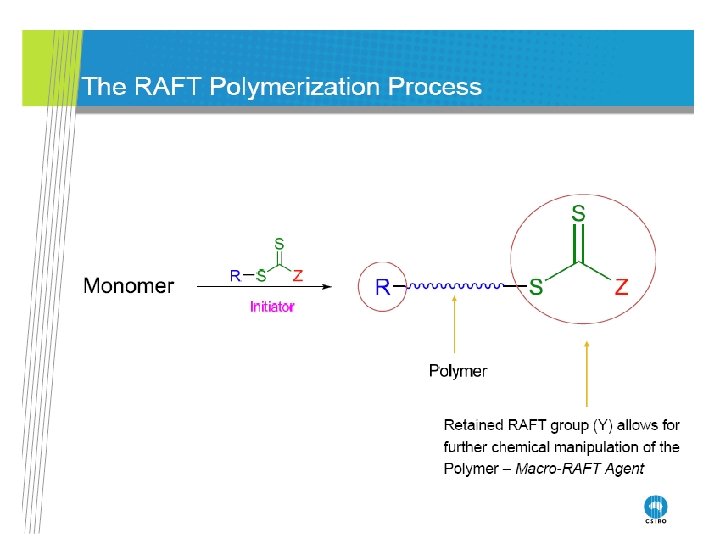
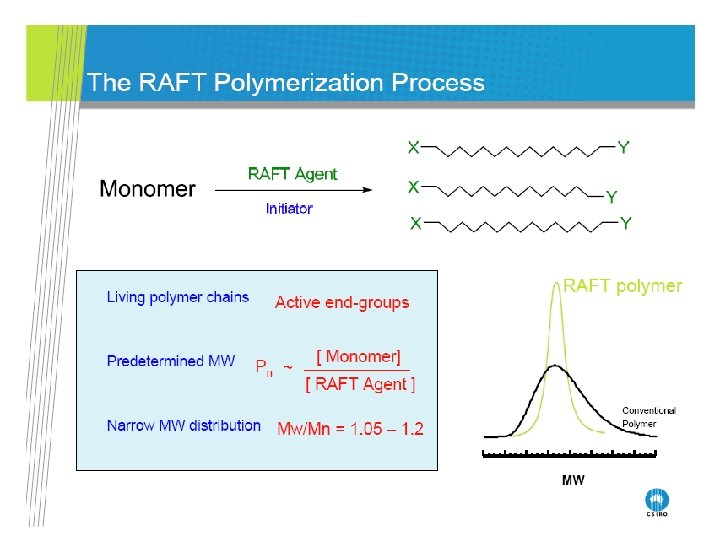
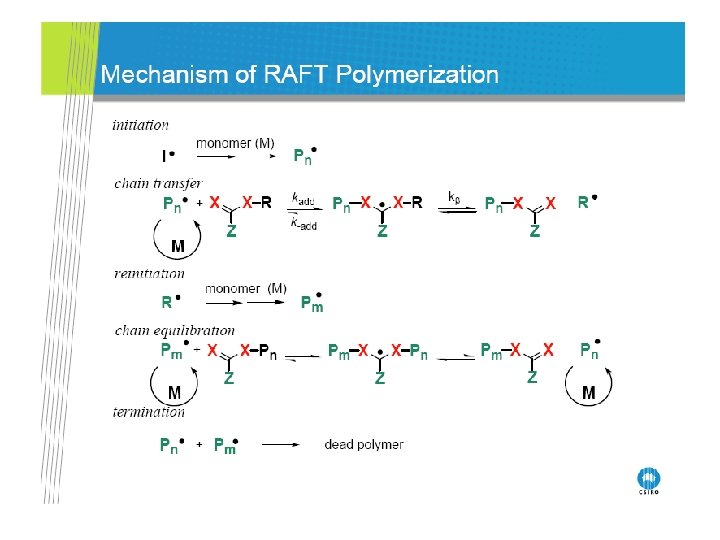
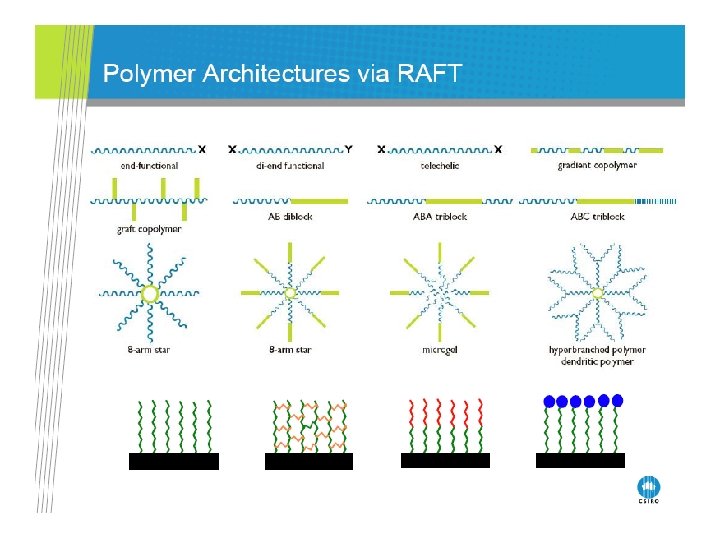
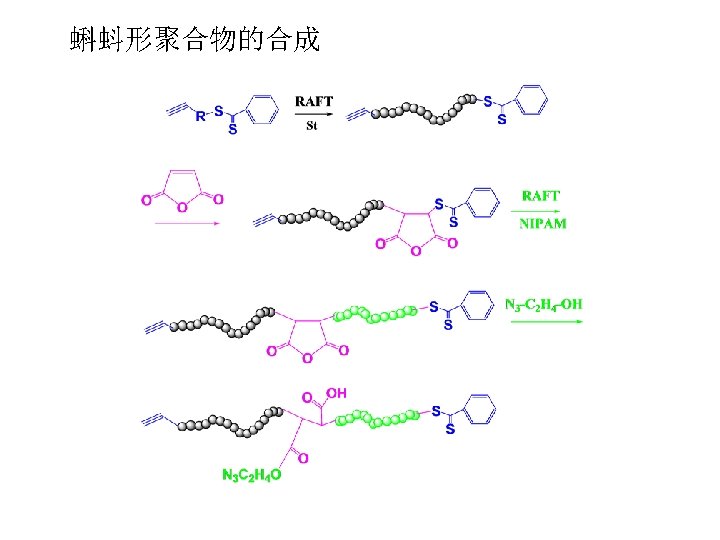
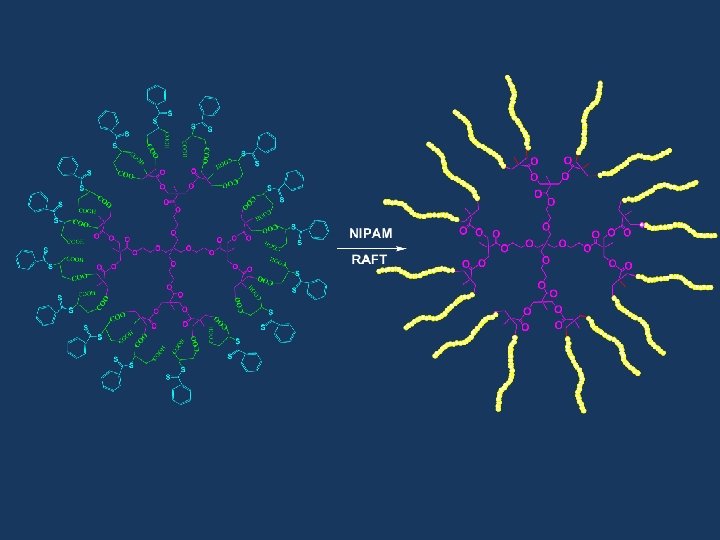
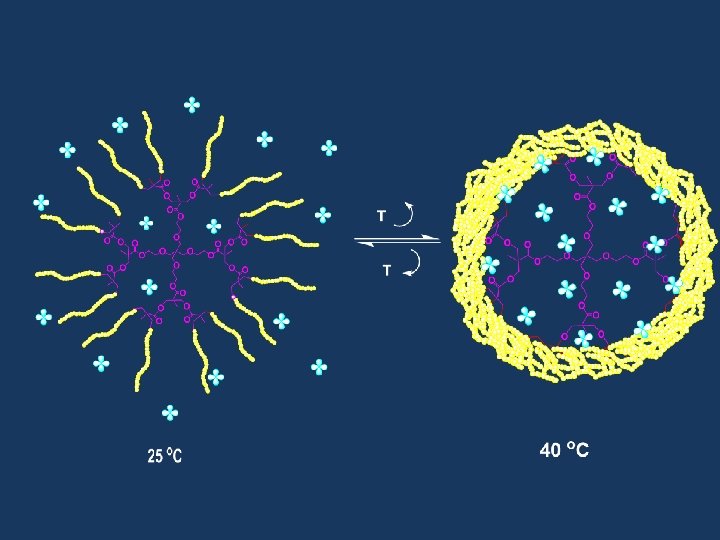
RAFT Polymerization RAFT= Reversible Addition-Fragmentation Chain Transfer Polymerization

Dr. Graeme Moad CSIRO Molecular and Health Technologies, Bayview Avenue, Clayton, Victoria 3168, Australia He joined CSIRO as a research scientist in 1979 and is currently a chief research scientist. Dr Moad is coauthor of the book ‘The Chemistry of Free Radical Polymerization’ which appeared as a second edition in 2006. His research interests lie in the fields of polymer design and synthesis (free radical polymerization, reactive extrusion), polymerization kinetics and mechanism, and most recently polymer nanocomposites.

Dr. Ezio Rizzardo CSIRO Molecular and Health Technologies, Bayview Avenue, Clayton, Victoria 3168, Australia Ezio Rizzardo is a graduate of the University of New South Wales and received his Ph. D. from the University of Sydney for his studies on the photochemistry of nitro compounds. He joined CSIRO in 1976 after a postdoc at Rice University, RIMAC, and the Australian National University. His CSIRO research has focused on developing methods for controlling free radical polymerization. For this he has received a number of awards including the RACI Australian Polymer Medal and the CSIRO Chairman’s Gold Medal. Currently he is a CSIRO Fellow and a Fellow of the Australian Academy of Science.

Dr. San H. Thang CSIRO Molecular and Health Technologies, Bayview Avenue, Clayton, Victoria 3168, Australia San H. Thang was born in Saigon, Vietnam, in 1954 and came to Australia in 1979 as a refugee. He completed his B. Sc. (Hons) degree in 1983 and Ph. D. in 1987 from Griffith University. He joined CSIRO in 1986 as a research fellow. He then moved to ICI Australia in late 1987 to undertake the challenge of industrial research. He returned to CSIRO in late 1990, and in 1995 he was co-inventor of the RAFT Process. He is currently a senior principal research scientist at CSIRO Molecular and Health Technologies where his research focuses on the interface between organic and polymer chemistry.





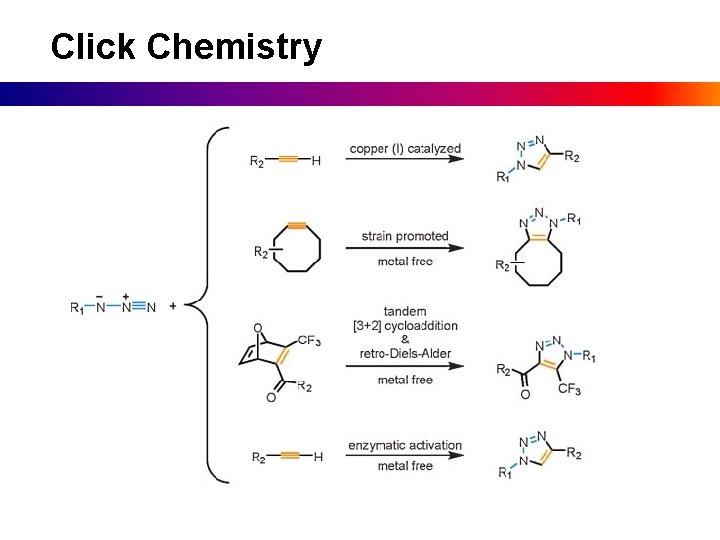
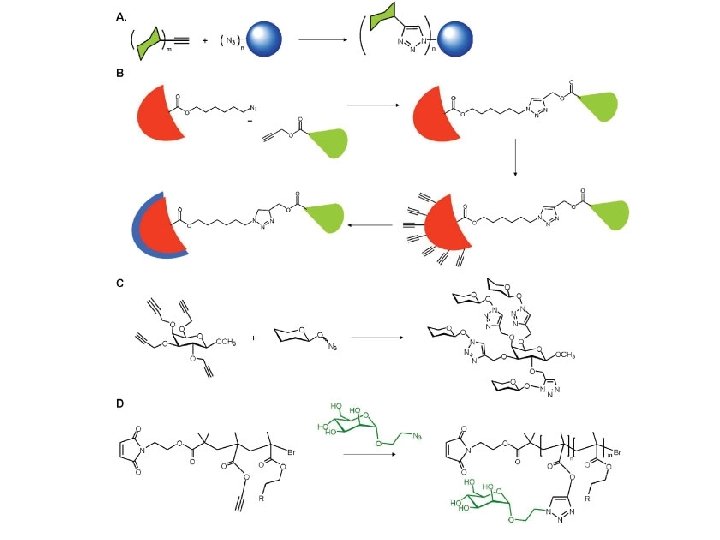
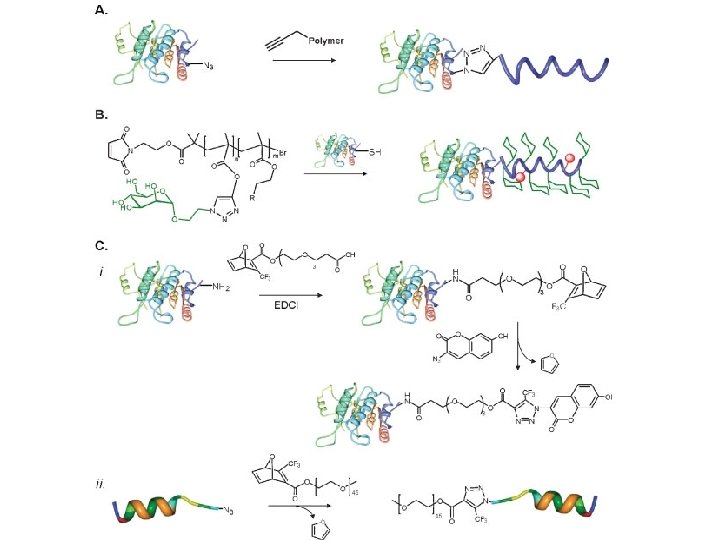
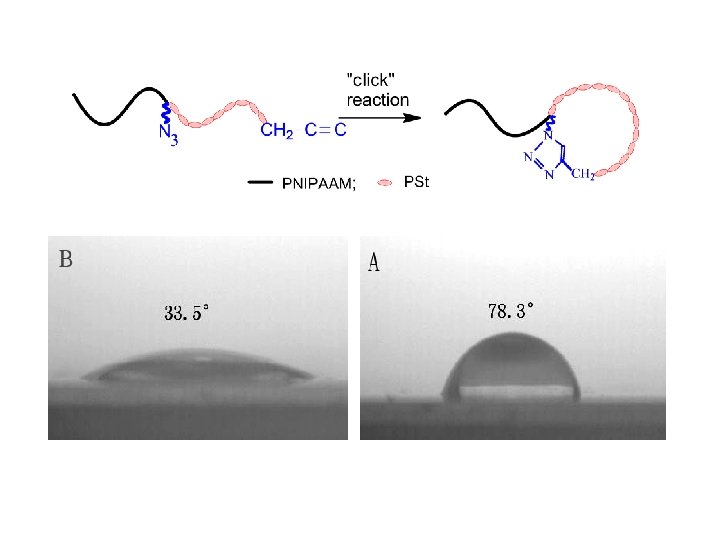
Click Chemistry





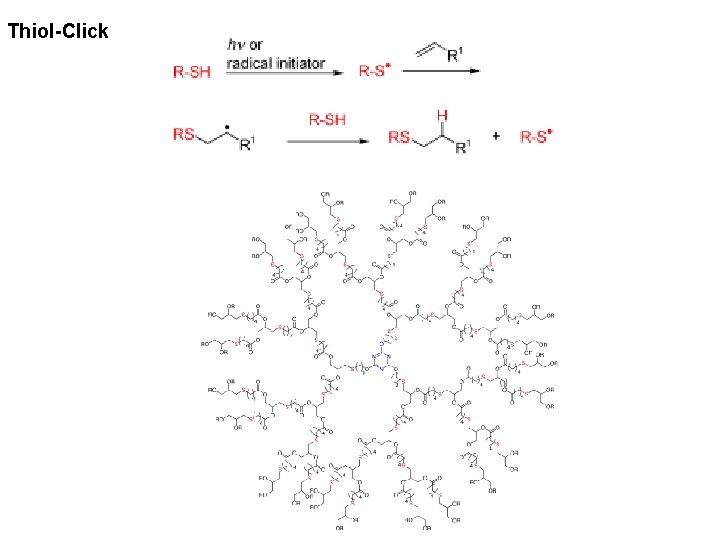
Thiol-Click

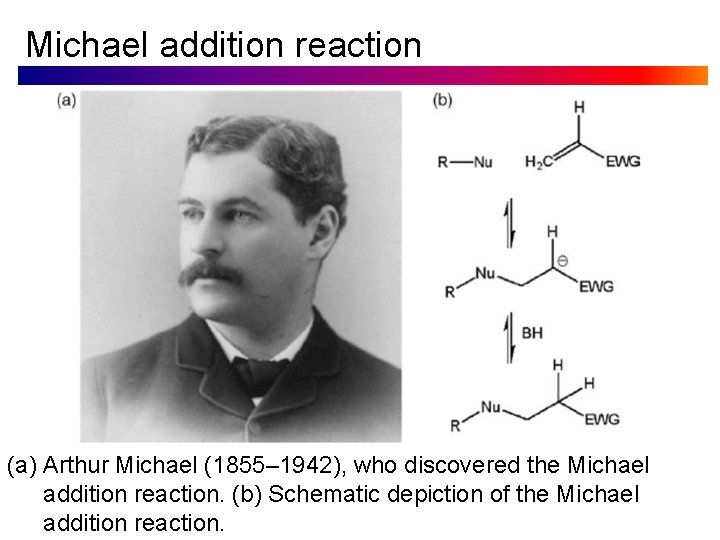
Michael addition reaction (a) Arthur Michael (1855– 1942), who discovered the Michael addition reaction. (b) Schematic depiction of the Michael addition reaction.

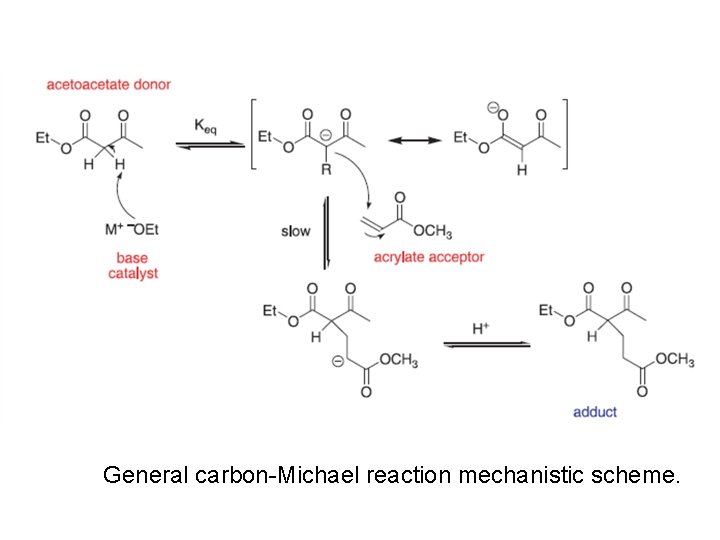
General carbon-Michael reaction mechanistic scheme.

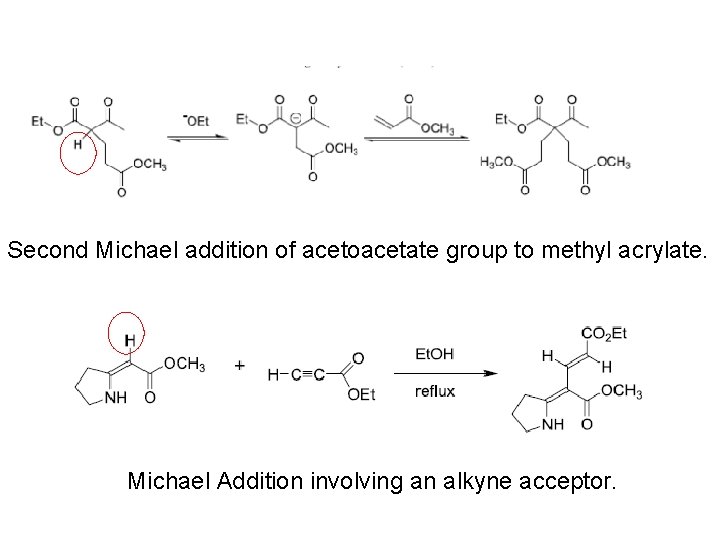
Second Michael addition of acetoacetate group to methyl acrylate. Michael Addition involving an alkyne acceptor.

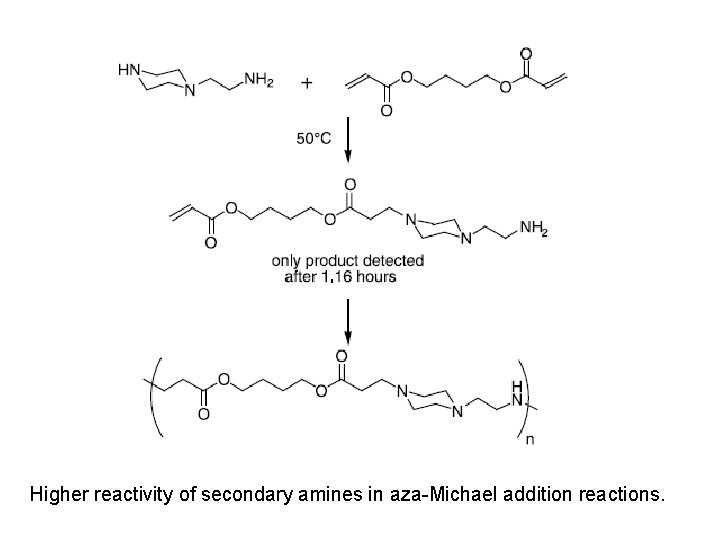
Higher reactivity of secondary amines in aza-Michael addition reactions.


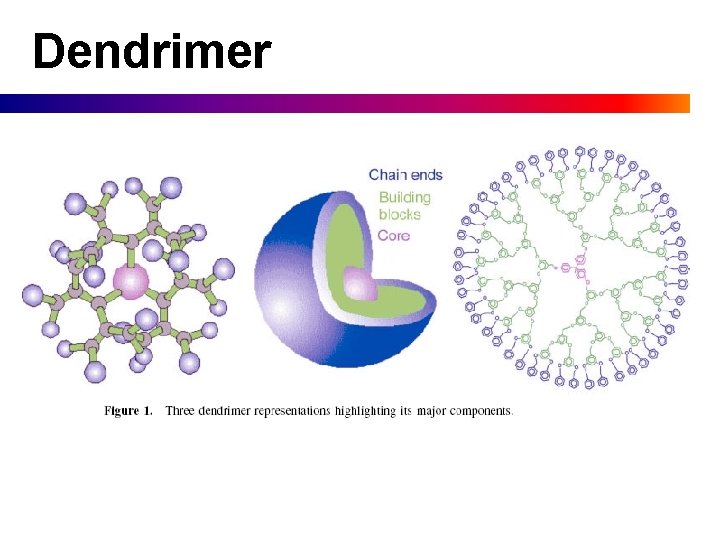
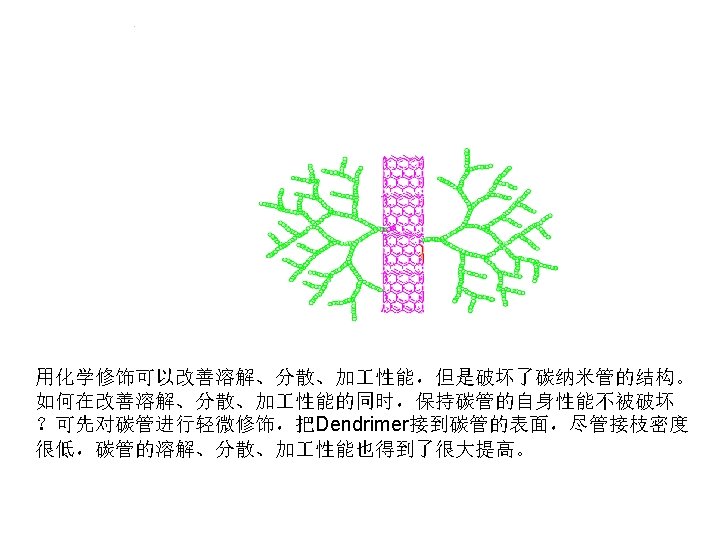
Dendrimer


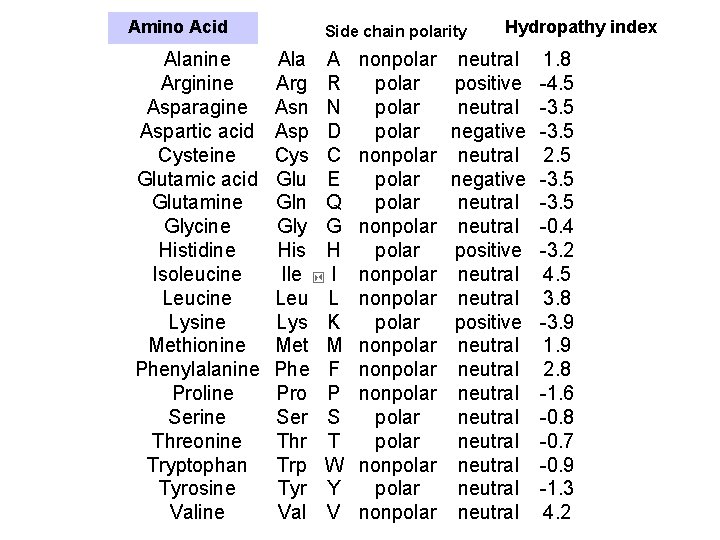
Amino Acid Alanine Arginine Asparagine Aspartic acid Cysteine Glutamic acid Glutamine Glycine Histidine Isoleucine Lysine Methionine Phenylalanine Proline Serine Threonine Tryptophan Tyrosine Valine Side chain polarity Ala Arg Asn Asp Cys Glu Gln Gly His Ile Leu Lys Met Phe Pro Ser Thr Trp Tyr Val A R N D C E Q G H I L K M F P S T W Y V nonpolar polar nonpolar polar nonpolar Hydropathy index neutral positive neutral negative neutral positive neutral neutral 1. 8 -4. 5 -3. 5 2. 5 -3. 5 -0. 4 -3. 2 4. 5 3. 8 -3. 9 1. 9 2. 8 -1. 6 -0. 8 -0. 7 -0. 9 -1. 3 4. 2

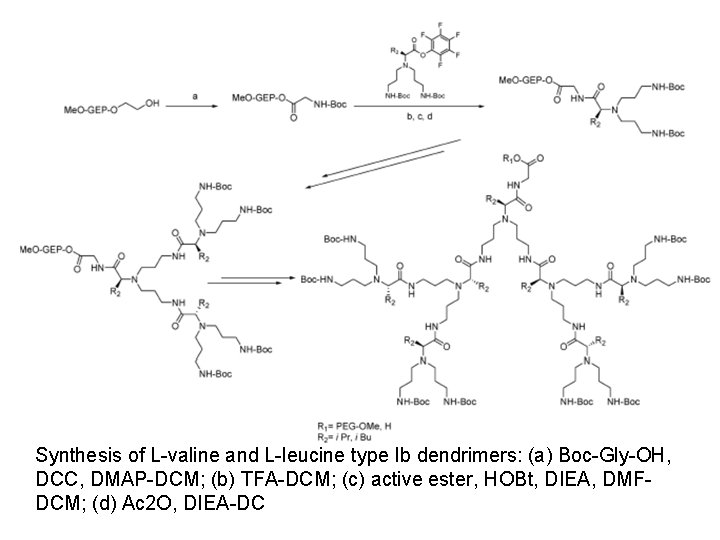
Synthesis of L-valine and L-leucine type Ib dendrimers: (a) Boc-Gly-OH, DCC, DMAP-DCM; (b) TFA-DCM; (c) active ester, HOBt, DIEA, DMFDCM; (d) Ac 2 O, DIEA-DC

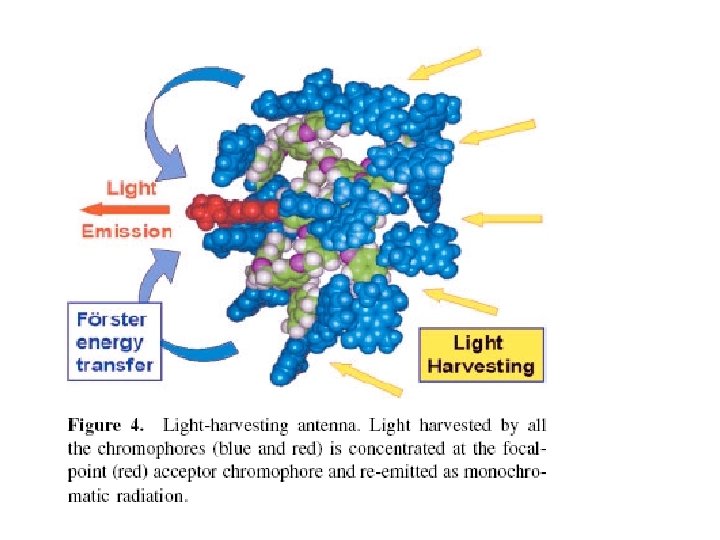
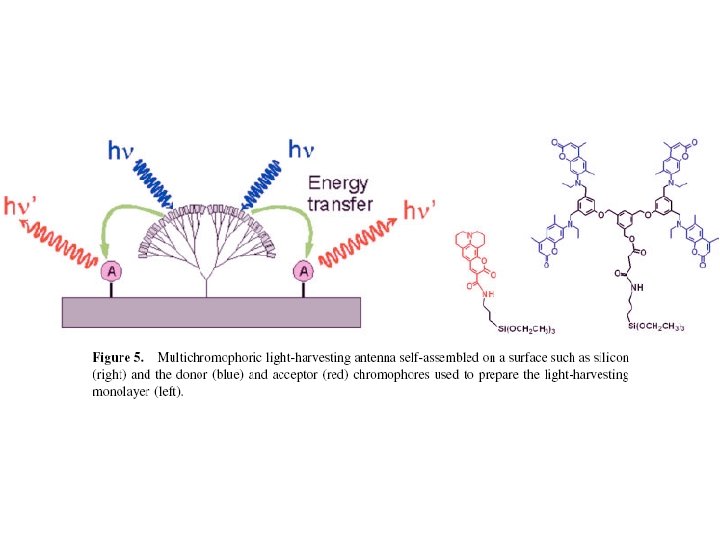
Is Dendrimer an Antenna? Photon

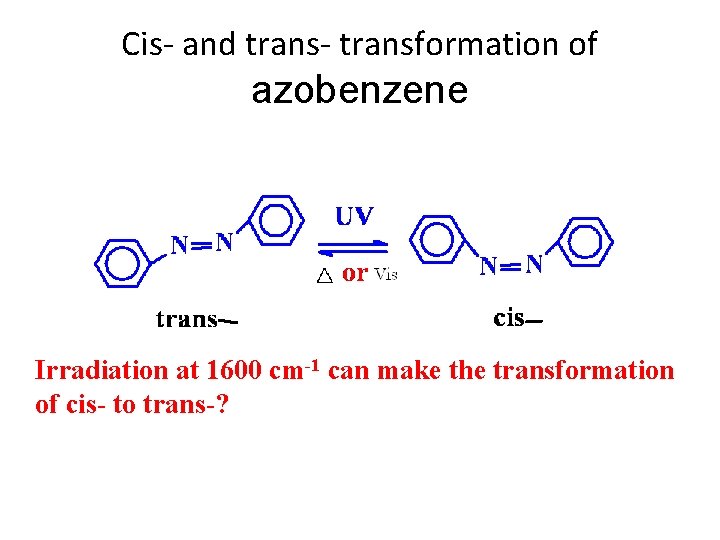
Cis- and trans- transformation of azobenzene Irradiation at 1600 cm-1 can make the transformation of cis- to trans-?

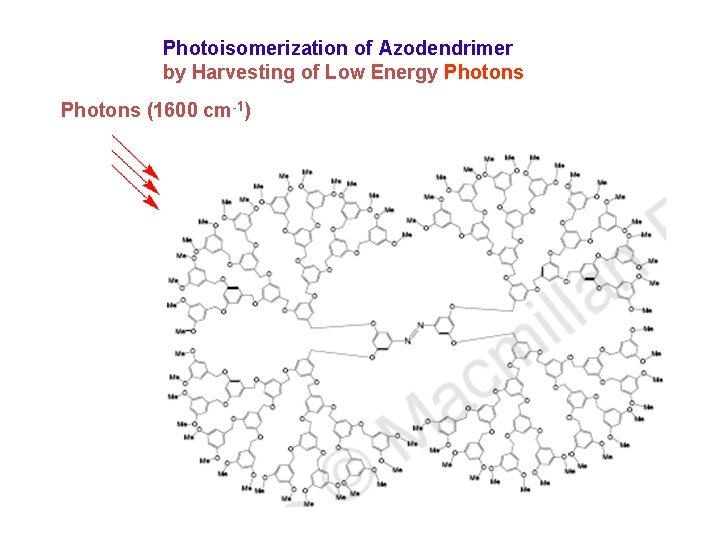
Photoisomerization of Azodendrimer by Harvesting of Low Energy Photons (1600 cm-1)


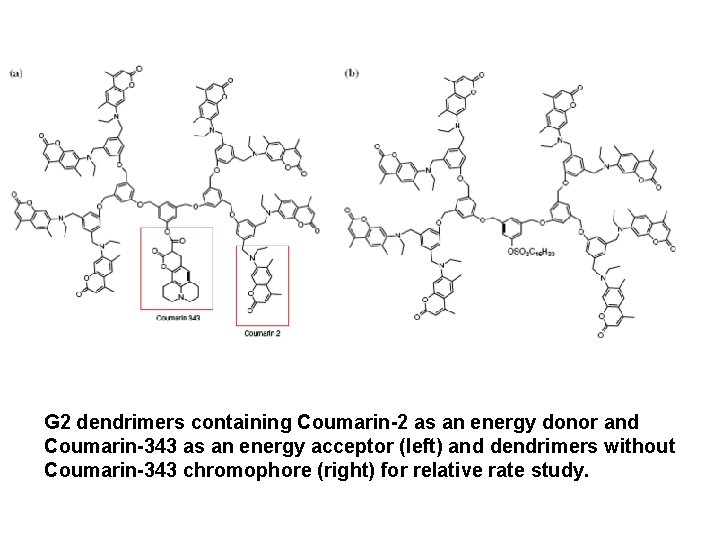
G 2 dendrimers containing Coumarin-2 as an energy donor and Coumarin-343 as an energy acceptor (left) and dendrimers without Coumarin-343 chromophore (right) for relative rate study.


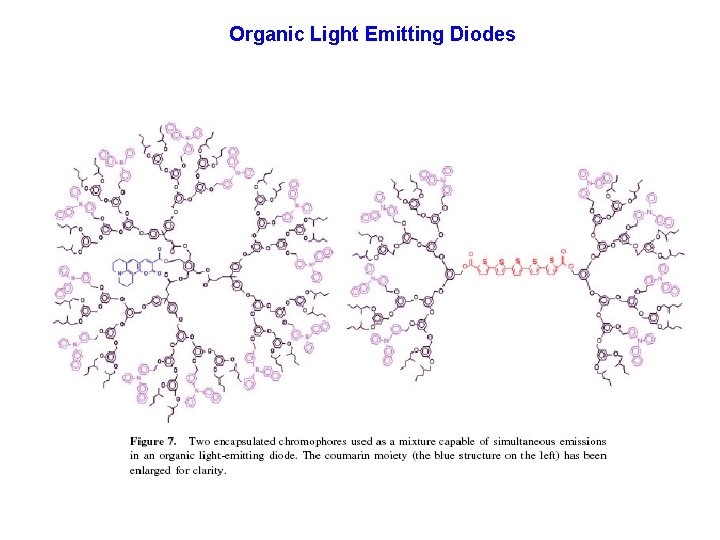
Organic Light Emitting Diodes

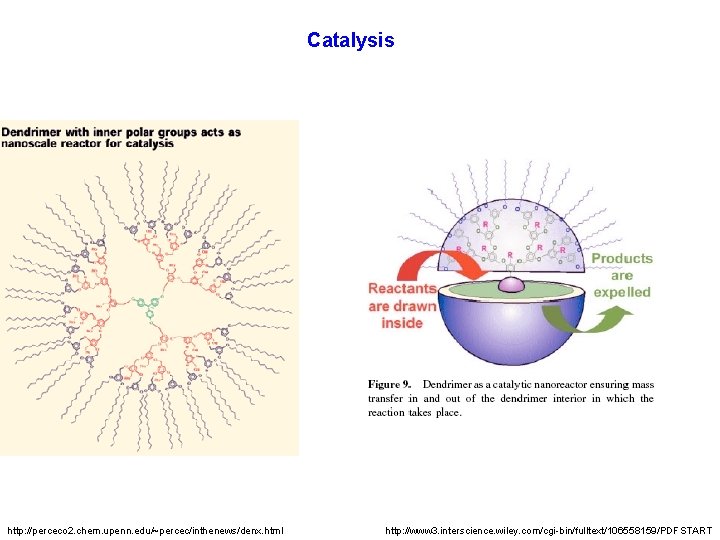
Catalysis http: //perceco 2. chem. upenn. edu/~percec/inthenews/denx. html http: //www 3. interscience. wiley. com/cgi-bin/fulltext/106558159/PDFSTART

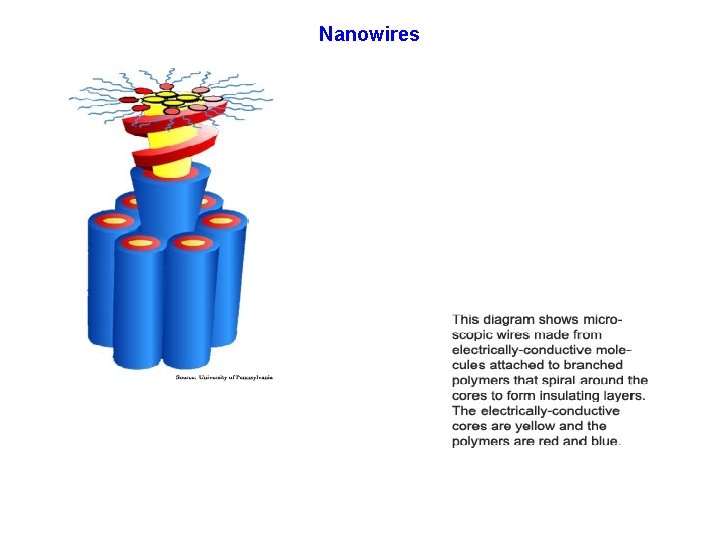
Nanowires

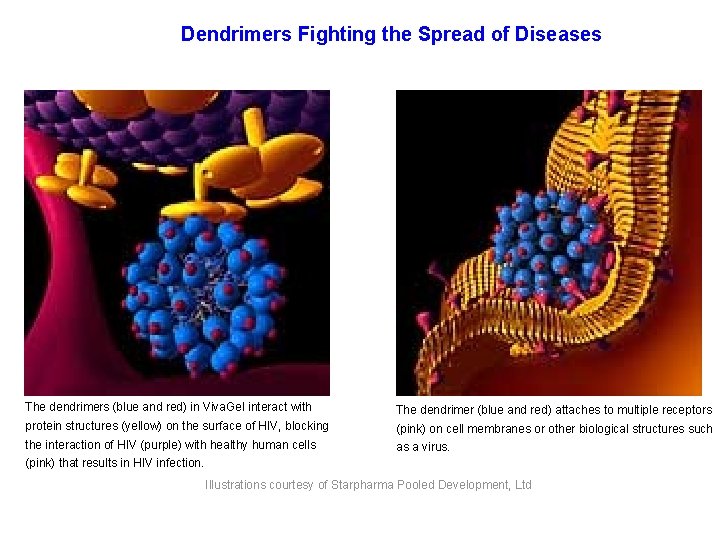
Dendrimers Fighting the Spread of Diseases The dendrimers (blue and red) in Viva. Gel interact with The dendrimer (blue and red) attaches to multiple receptors protein structures (yellow) on the surface of HIV, blocking (pink) on cell membranes or other biological structures such the interaction of HIV (purple) with healthy human cells as a virus. (pink) that results in HIV infection. Illustrations courtesy of Starpharma Pooled Development, Ltd





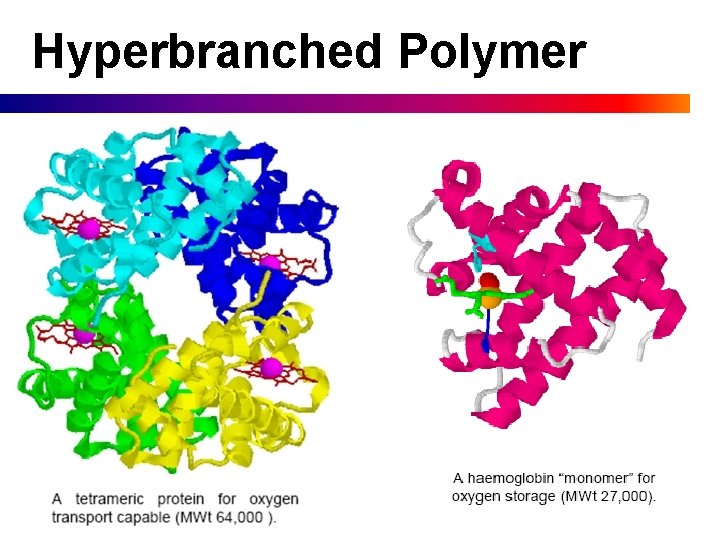
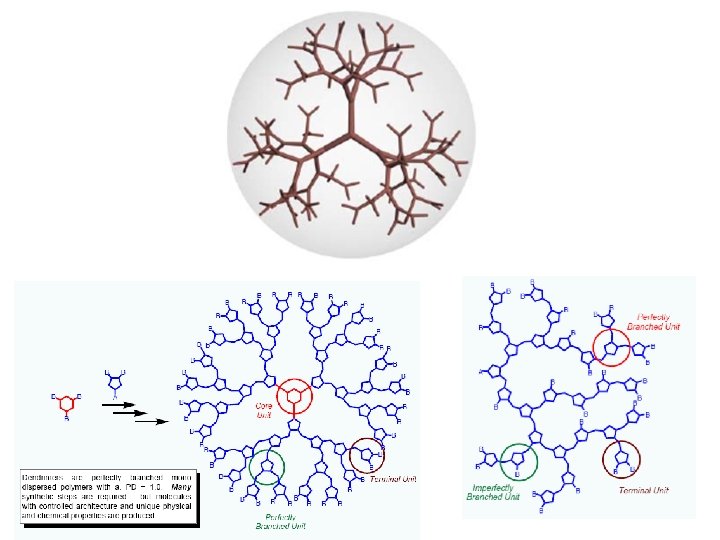
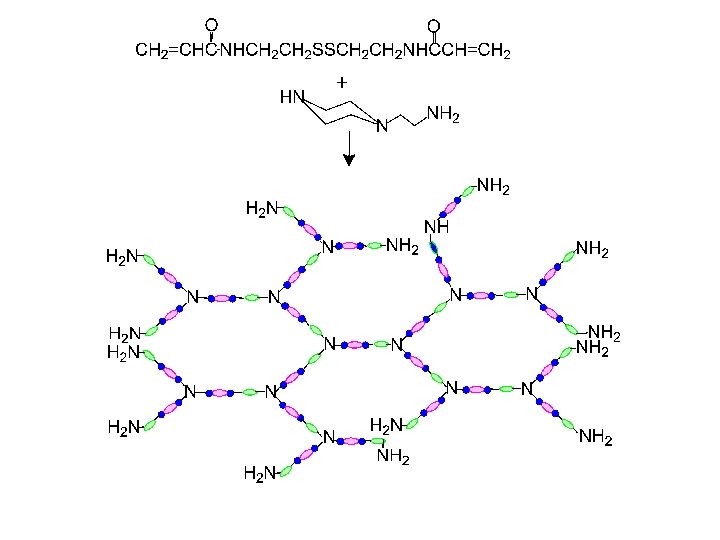
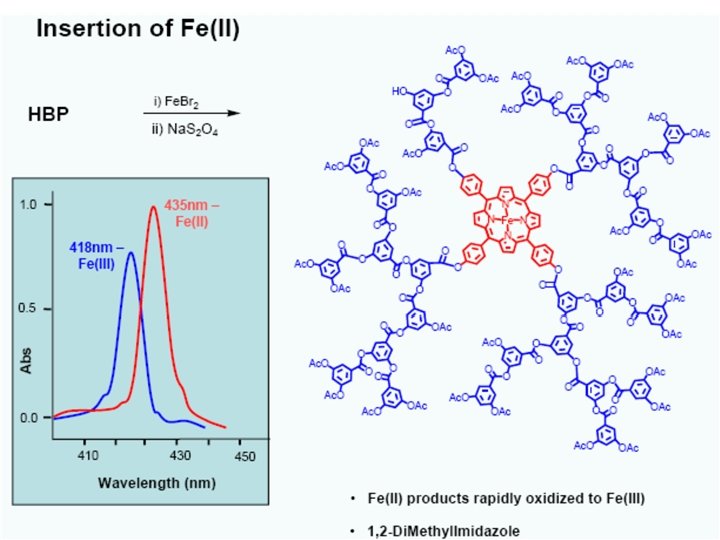
Hyperbranched Polymer


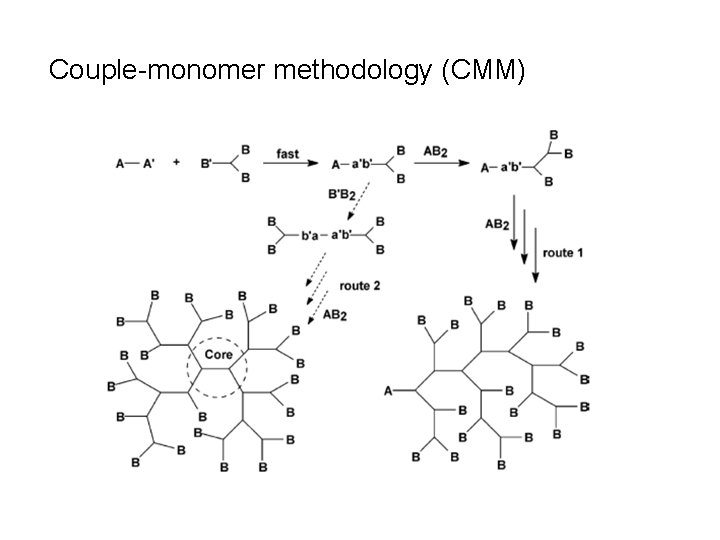
Couple-monomer methodology (CMM)


No filter WU (330~3 85 nm) WB (460~4 90 nm) WG (510~5 50 nm)






-The End- Thank You!