Polymers General Properties of Polymers 1 2 3



















- Slides: 58

Polymers

General Properties of Polymers 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. Easy to mold Excellent surface finish can be obtained Produced with close dimensional tolerances Economical Low mechanical properties Poor temperature resistance Can be produced transparent or in different colors Resistant to chemicals & corrosion. Thermal and electrical insulators. Generally low density Can be processed in various ways

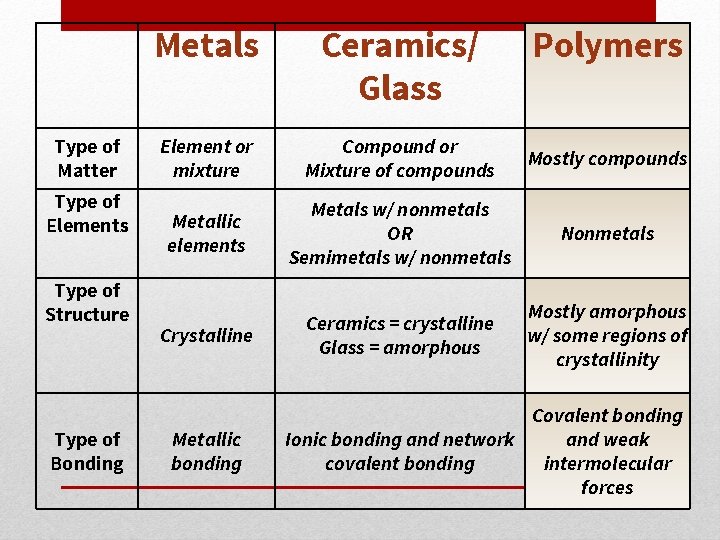
Type of Matter Type of Elements Type of Structure Type of Bonding Metals Ceramics/ Glass Element or mixture Compound or Mixture of compounds Mostly compounds Metallic elements Metals w/ nonmetals OR Semimetals w/ nonmetals Nonmetals Crystalline Ceramics = crystalline Glass = amorphous Mostly amorphous w/ some regions of crystallinity Metallic bonding Polymers Covalent bonding Ionic bonding and network and weak covalent bonding intermolecular forces

Synthetic Polymers Polypropylene “Everyday Polymers” Polypropylene Polystyrene Polyvinyl chloride Chloride Polyester Polyethylene Polystyrene Polyethylene

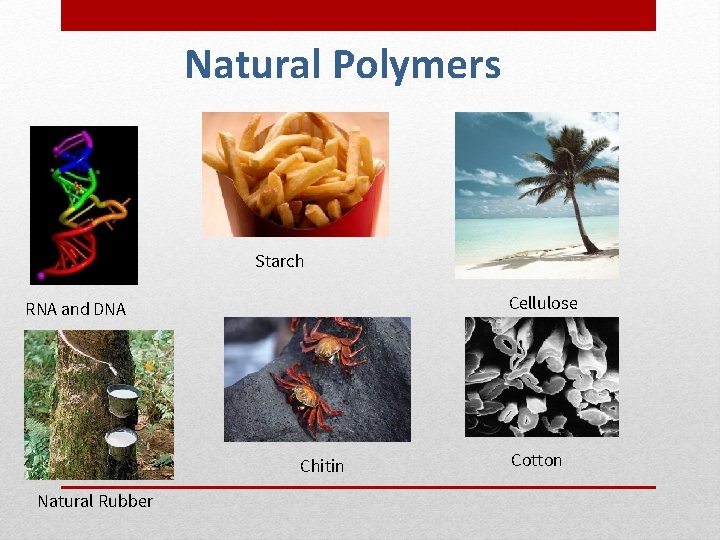
Natural Polymers Starch Cellulose RNA and DNA Chitin Natural Rubber Cotton

History of Polymers • 1850 ‘s – Celluloid - used to be widely used but not as much anymore—now used in table tennis balls and guitar picks • 1900 – 1920’s – Bakelite – chess pieces, containers – Rayon - Clothing, stockings – Cellophane - packaging, sticky tape – Polyvinyl chloride- PVC - Pipes, wire insulation

History of Polymers Continued. . • 1922 - Staudinger suggested polymers are long chains of atoms—won Nobel Prize • 1930 - 1940 ‘s – Polystyrene – Polyethylene Tetraborate - PETE – ‘Dacron’ and drinks bottles, toys, – Nylon engineering plastic and for clothing and stockings – Teflon – later used for non-stick pans

History of Polymers Continued. . • 1950 – 1960’s – Polyethylene- (Tupperware), Polypropylene , Styrofoam, Saran Wrap, Velcro – Bags containers, packaging film, ropes, fasteners • 1970 – 1990’s – PEEK (polyethylene terephthalate) biomaterial used in medical implants; – POM (Polyoxymethylene) for zippers, pumps, faucets. – Many engineering plastics for specialist applications, Mylar

History of Polymers Continued… • 1990 – Present – New materials • developing polymers for tissue repair, replacement and regeneration. • Making solar cells • engineering polymers to give specific, desired properties such as biodegradability or biocompatibility

Polymers • POLY- “many” • MERS- “units” • Polymers are long chain molecules made of thousands of repeated units called monomers

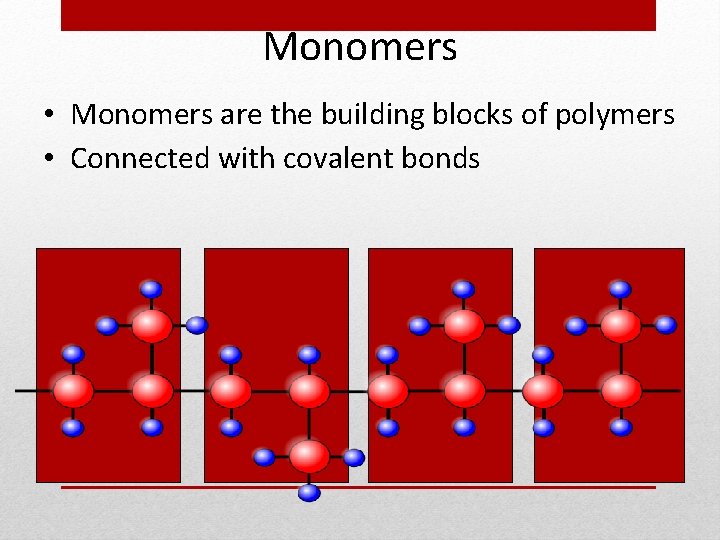
Monomers • Monomers are the building blocks of polymers • Connected with covalent bonds

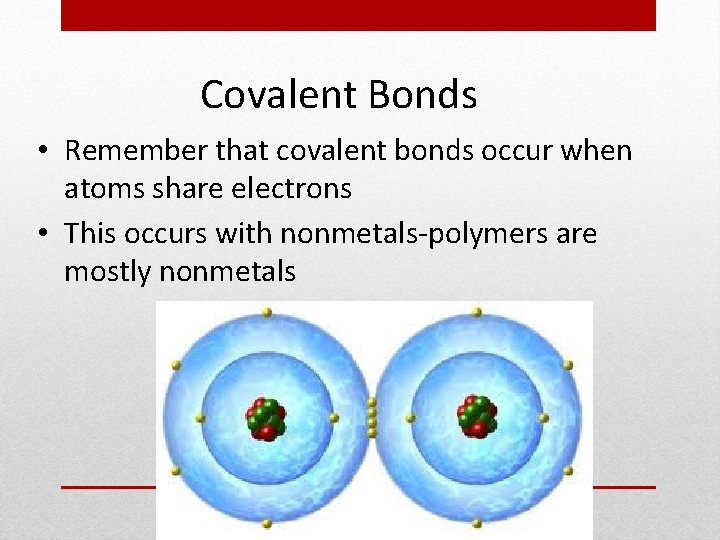
Covalent Bonds • Remember that covalent bonds occur when atoms share electrons • This occurs with nonmetals-polymers are mostly nonmetals

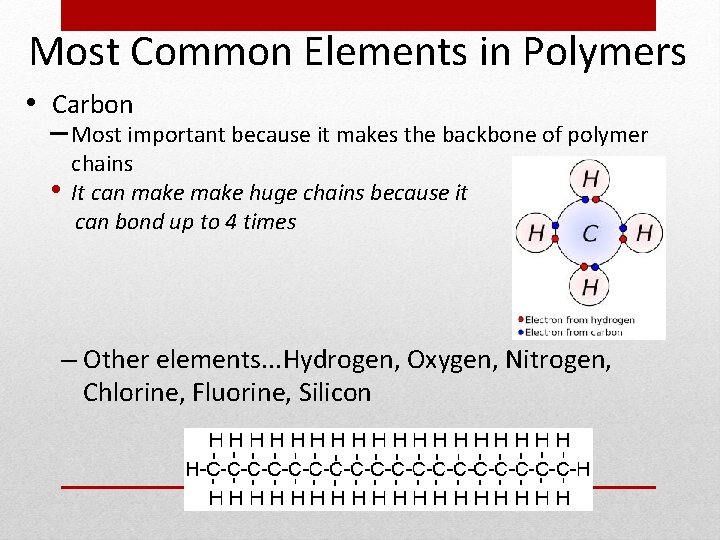
Most Common Elements in Polymers • Carbon – Most important because it makes the backbone of polymer • chains It can make huge chains because it can bond up to 4 times – Other elements. . . Hydrogen, Oxygen, Nitrogen, Chlorine, Fluorine, Silicon

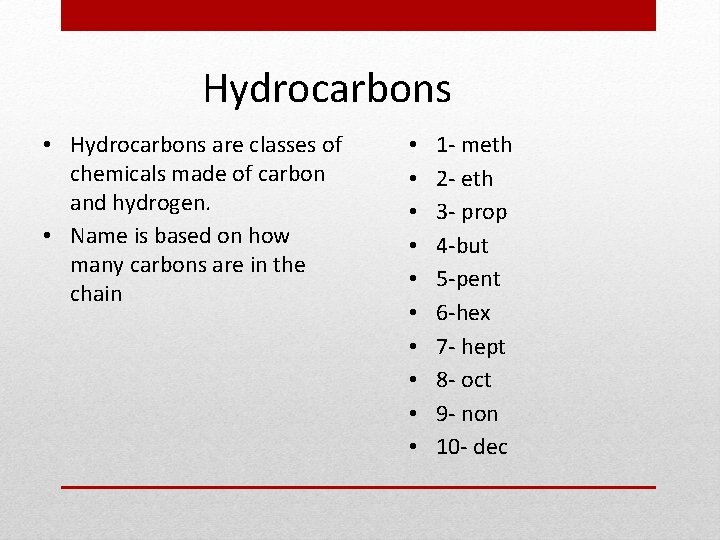
Hydrocarbons • Hydrocarbons are classes of chemicals made of carbon and hydrogen. • Name is based on how many carbons are in the chain • • • 1 - meth 2 - eth 3 - prop 4 -but 5 -pent 6 -hex 7 - hept 8 - oct 9 - non 10 - dec

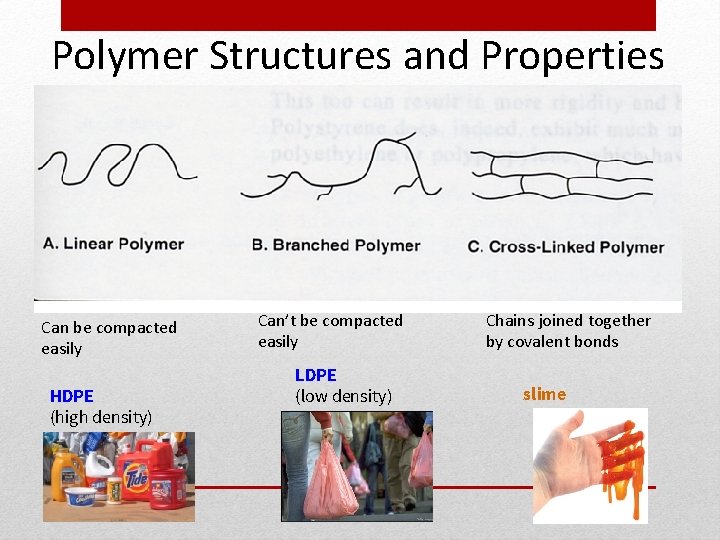
Polymer Structures and Properties Can be compacted easily HDPE (high density) Can’t be compacted easily LDPE (low density) Chains joined together by covalent bonds slime

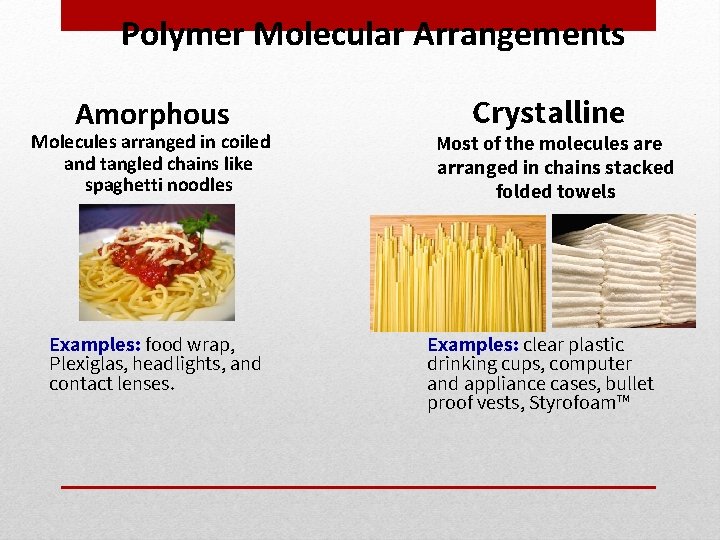
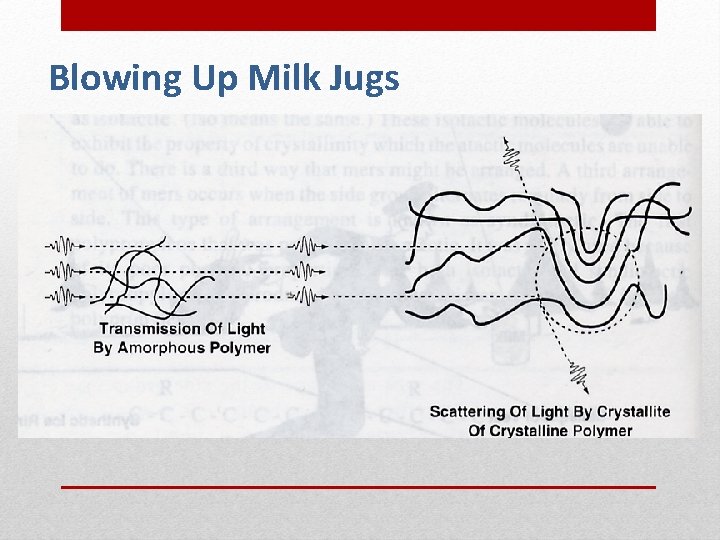
Polymer Molecular Arrangements Amorphous Molecules arranged in coiled and tangled chains like spaghetti noodles Examples: food wrap, Plexiglas, headlights, and contact lenses. Crystalline Most of the molecules are arranged in chains stacked folded towels Examples: clear plastic drinking cups, computer and appliance cases, bullet proof vests, Styrofoam™

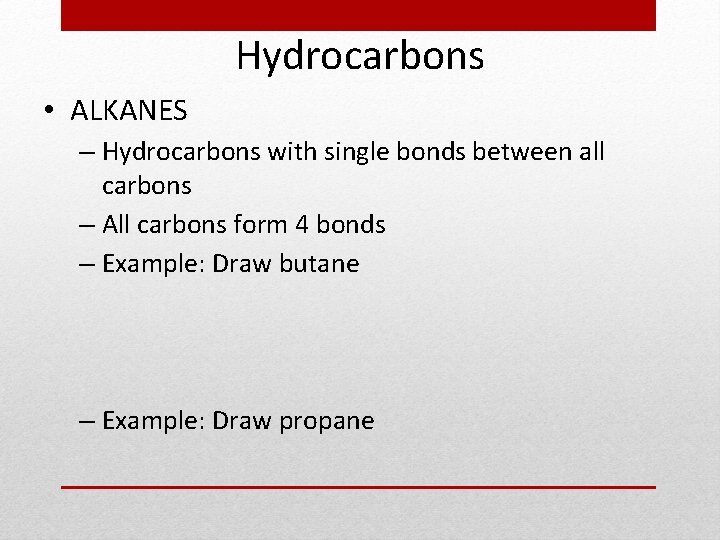
Hydrocarbons • ALKANES – Hydrocarbons with single bonds between all carbons – All carbons form 4 bonds – Example: Draw butane – Example: Draw propane

Hydrocarbons • ALKENES – Hydrocarbons with double bonds between at least 2 of the carbons • Double bonds are stronger and more reactive than single bonds • Example: Draw hexene

Polymerization • Polymerization- the process of forming polymer chains by linking smaller monomers together • 2 methods of polymerization – Addition – Condensation

Addition Polymerization • Occurs when monomers are added to one another usually by breaking double bonds – What is an Initiator? • Starts the polymerization process – What happens to the length of chains as there are more initiators? • More but shorter chains • http: //www 1. biologie. uni-hamburg. de/bonline/library/newton/Chy 251_253/Lectures/Polymers/Po lymers. I. html

CLASS DEMONSTRATION

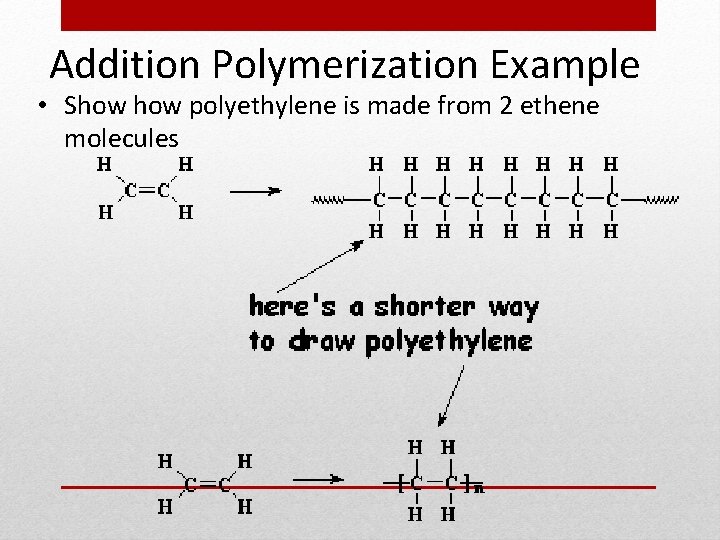
Addition Polymerization Example • Show polyethylene is made from 2 ethene molecules

Condensation Polymerization • Method of making a polymer that often releases a water molecule or small molecule as a by product • Examples: – silk – polyesters – nylon

MAKING NYLON DEMO

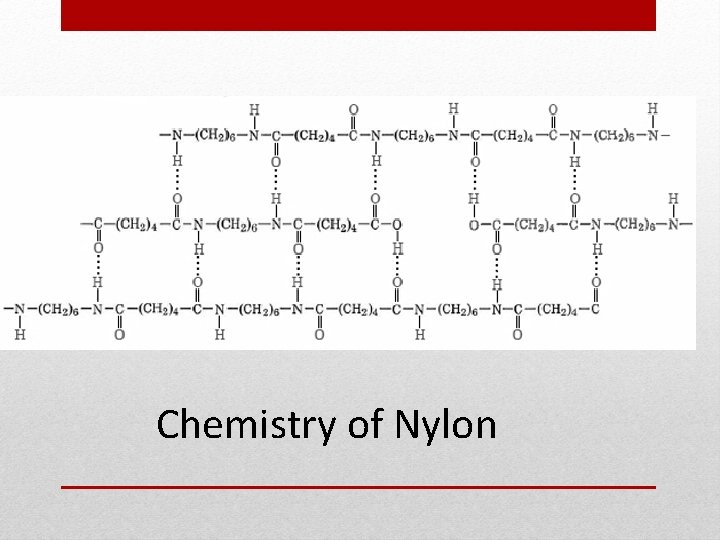
Chemistry of Nylon

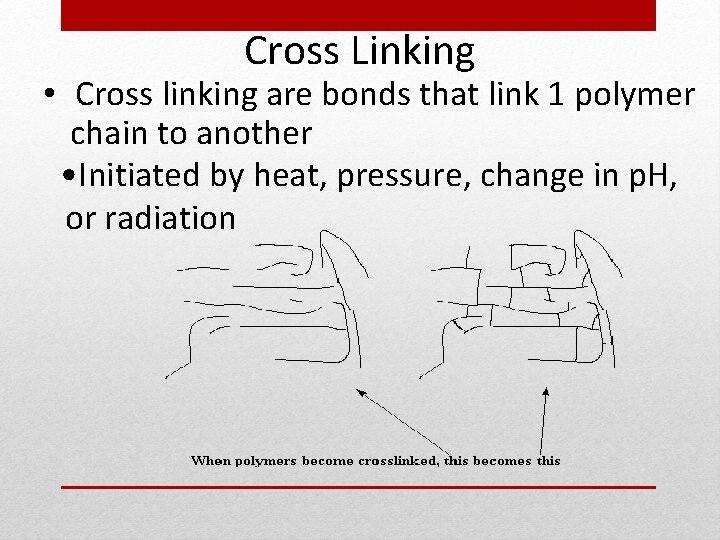
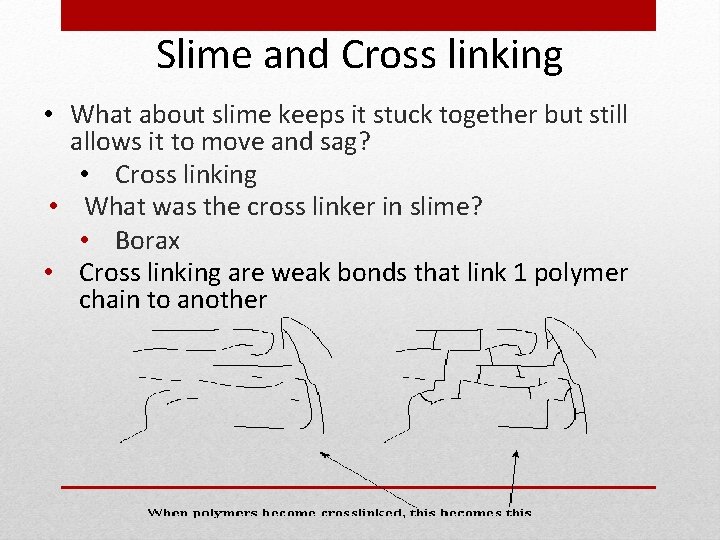
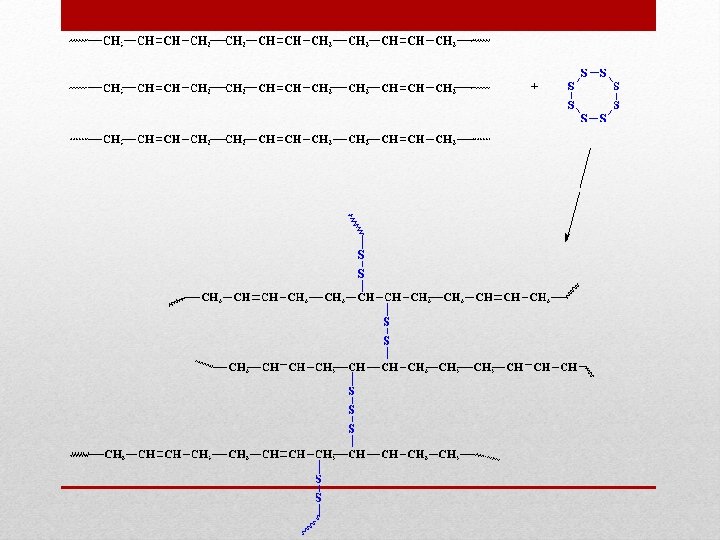
Cross Linking • Cross linking are bonds that link 1 polymer chain to another • Initiated by heat, pressure, change in p. H, or radiation

Cross Linking Properties Cross-Linking Provides: • Higher tensile strength • More of a solid under high temps • Better crush resistance • More elasticity • More rigid, not as flexible • Resistance to stress cracking • Improved behavior at higher temps • Slightly better flame resistance • No more class

PVA • Polyvinyl alcohol • Uses • 3 D printing • Laundry detergent pods • Hospital laundry bags • Fishing baits

SLIME LAB

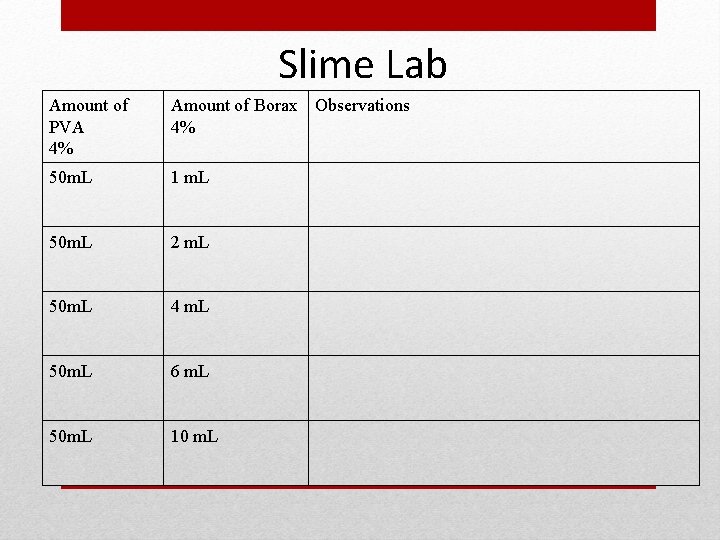
Slime Lab Amount of PVA 4% Amount of Borax Observations 4% 50 m. L 1 m. L 50 m. L 2 m. L 50 m. L 4 m. L 50 m. L 6 m. L 50 m. L 10 m. L

Slime and Cross linking • What about slime keeps it stuck together but still allows it to move and sag? • Cross linking • What was the cross linker in slime? • Borax • Cross linking are weak bonds that link 1 polymer chain to another

Cross Linking and Rubber • Vulcanization – Process of creating crosslinking in rubber – If the chains were not linked together rubber would remain deformed because individual chains would straighten out – This cross linking gives the chains of molecules stability, ensuring the ball returns to its spherical shape. – Changes rubber to hard, durable material (like in tires, shoe soles, hoses, and hockey pucks) • Uses sulfur and heat • Named after Vulcan Roman God of Fire • Often called sulfur curing


Classification of Polymers 1. Thermoplastic – Soften when heated and resolidify when cooled – Primary types of used for recycling – Formed/shaped when in softened state.

Blowing Up Milk Jugs

Shrinkidinks Demo: Milk Jug

Classification of Polymers Continued. . 2. Thermoset – cross linked polymers – Hard – set into a solid permanently • can’t be softened by heat (they simply burn instead) – therefore it is very hard or impossible to recycle – tends to be stronger than a thermoplastic.

Eurocast

Classification of Polymers Continued… 3. Elastomer – Polymers that show a high degree of elasticity like rubber – Are amorphous polymers – Helps make seals, adhesives, molded flexible parts 4. Other types of Polymers – Fibers, Coatings, Films, Foams

Latex Ball

Packing Peanuts

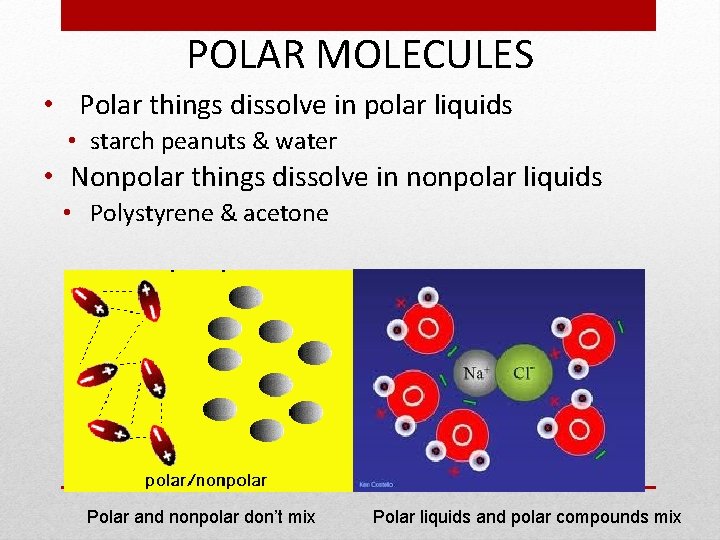
POLAR MOLECULES • Polar things dissolve in polar liquids • starch peanuts & water • Nonpolar things dissolve in nonpolar liquids • Polystyrene & acetone Polar and nonpolar don’t mix Polar liquids and polar compounds mix

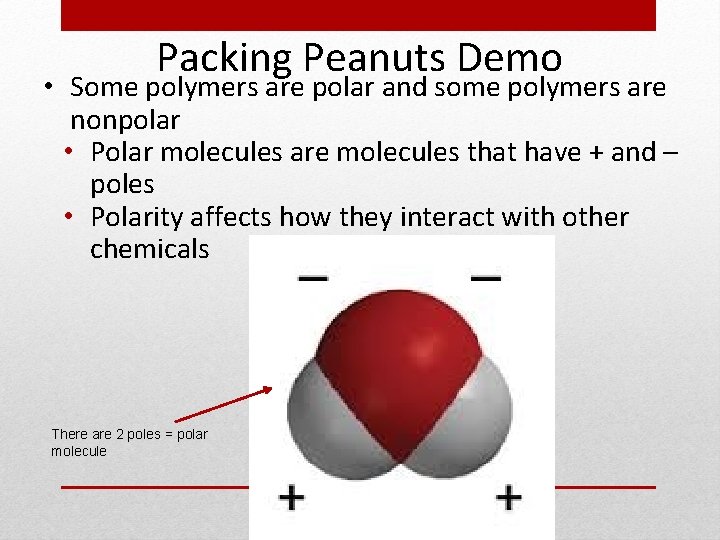
Packing Peanuts Demo • Some polymers are polar and some polymers are nonpolar • Polar molecules are molecules that have + and – poles • Polarity affects how they interact with other chemicals There are 2 poles = polar molecule

Polyacrylate & HDPE Fortune Fish

Hydrophilic vs Hydrophobic Polymers • Fortune Fish Activity • Hydrophobic: water loving • Hydrophilic: water hating

Sodium Polyacrylate (Water Lock) • Why did the sodium polyacrylate absorb so much water? • When water is added the sodium leaves (dissociates) leaving room for water to bond • Can absorb 800 x mass in water • Why did salt reduce how much water it absorbed? • adding more salt occupies some of the sites where water “wants” to bond

APPLICATIONS OF WATER LOCK • • DIAPER FIREPROOFING FROG TAPE Thickening agents

Sodium Polyacrylate (Poly. Snow) • How is this sodium polyacrylate (polysnow) different than sodium polyacrylate (Water lock)? • Water lock: less cross linked, water absorbed more on the outside of polymer chains • Polysnow: more cross linked, water absorbed in middle of polymer chains

Types of Polymer Manufacturing

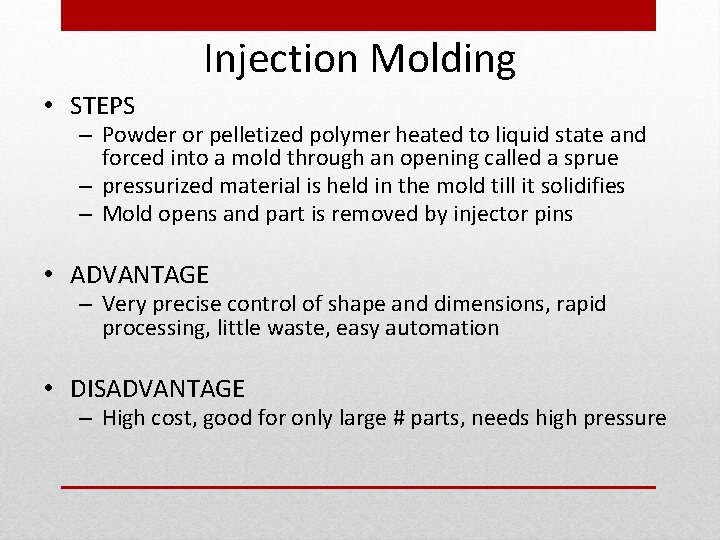
Injection Molding • STEPS – Powder or pelletized polymer heated to liquid state and forced into a mold through an opening called a sprue – pressurized material is held in the mold till it solidifies – Mold opens and part is removed by injector pins • ADVANTAGE – Very precise control of shape and dimensions, rapid processing, little waste, easy automation • DISADVANTAGE – High cost, good for only large # parts, needs high pressure

Injection Molding

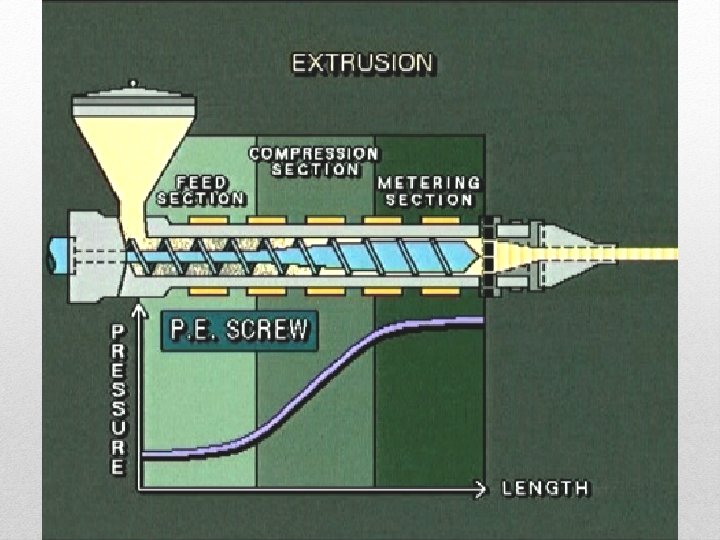
Extrusion • STEPS – Polymer pellets are put in hopper (barrel with continuous feed screw) – Material is heated and forced through die – Material is cooled carefully • Advantage – Best suited for parts of constant cross sections like pipes and rods, used for wraps, films, or long continuous parts • Disadvantage – Needs to be cooled to keep polymer stable

Extrusion • This continuous process is used to produce films, sheet, profiles, tubes, and pipes.


Blow Molding • STEPS – Softened plastic tube is extruded – Tube is clamped at one end and inflated to fill a mold – Solid shell • Advantages – Makes hollow parts like bottles, fast cycle, not labor intensive, and relatively cheap • Disadvantages – Can’t control wall thickness well, can’t mold details with good precision, need polymers with high melting points

Blow Molding

Rigid vs. Flexible Foam

Recycling Project