Anxiolytics and Hypnotics PRMC 610 9 Oct 2018














- Slides: 47

Anxiolytics and Hypnotics PRMC 610 9 Oct. 2018 Assoc. Prof. Gordon Mc. Carter Touro University – CA College of Pharmacy Biological Sciences 609

Lecture Outline • Definitions • Physiologic bases for the actions of GABAergic anxiolytics and hypnotics: neuronal inhibition • GABAergic drugs: benzodiazepines • GABAergic drugs: Z-drugs • GABAergic drugs: barbiturates • Other classes of anxiolytics and hypnotics Primary reference: Principles of Pharmacology, 4 e; Golan, Armstrong, & Armstrong. Chapter 13 – Pharmacology of GABAergic and glutamatergic neurotransmission.

Definitions • Anxiolytic – drug that reduces anxiety • Hypnotic – drug that induces or promotes sleep • Sedative – drug that reduces excitement, irritability, or agitation; usually equivalent to anxiolytic • Tranquilizer – same as sedative but sometimes includes anxiolytics; non-technical term • Minor tranquilizer – anxiolytic • Major tranquilizer – antipsychotic • Neuroleptic – major tranquilizer, antipsychotic • Mood stabilizer – drug for bipolar disorder; formerly just lithium but now applied to anticonvulsants and antipsychotics when used for BPD

2016 Top 200 drugs • • • #13 – alprazolam, Xanax #30 – zolpidem, Ambien #35 – clonazepam, Klonopin #40 – lorazepam, Ativan #79 – diazepam, Valium

Classes of anxiolytics and hypnotics • GABAergic drugs – Benzodiazepines – Barbiturates – “Z-drug” hypnotics • Serotonergic anxiolytics – Antidepressants and buspirone • Other classes – Melatonin agonists – Antihistamines

Physiologic bases for the actions of GABAergic anxiolytics and hypnotics: neuronal inhibition

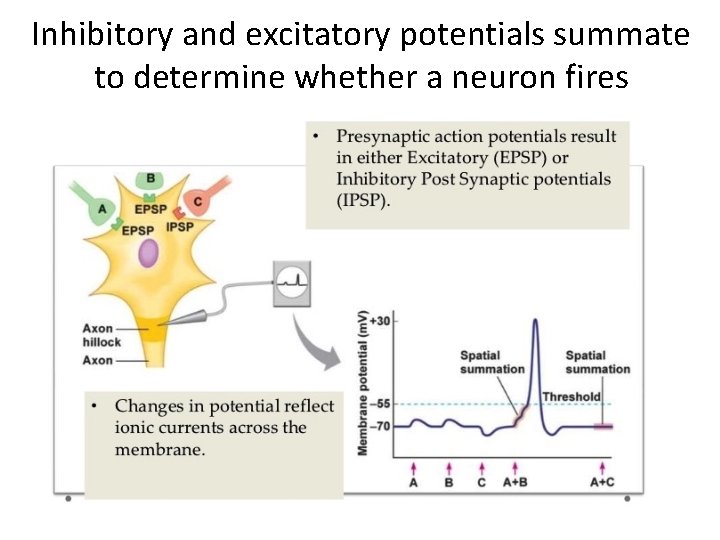
Inhibitory and excitatory potentials summate to determine whether a neuron fires

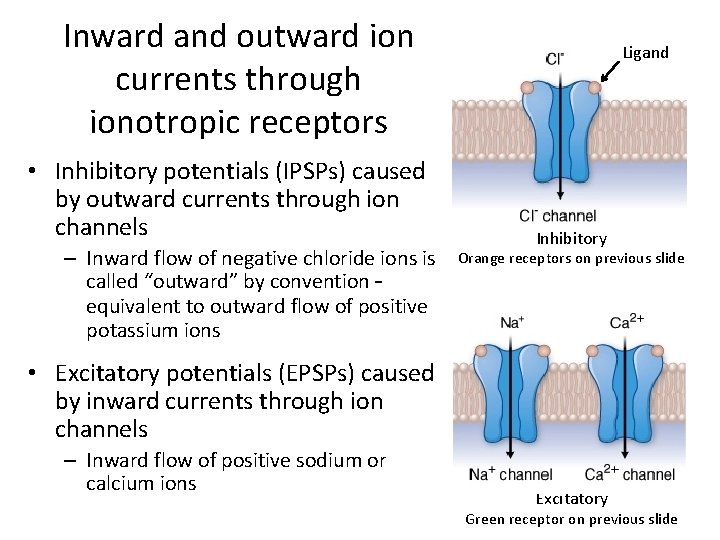
Inward and outward ion currents through ionotropic receptors • Inhibitory potentials (IPSPs) caused by outward currents through ion channels – Inward flow of negative chloride ions is called “outward” by convention – equivalent to outward flow of positive potassium ions Ligand Inhibitory Orange receptors on previous slide • Excitatory potentials (EPSPs) caused by inward currents through ion channels – Inward flow of positive sodium or calcium ions Excitatory Green receptor on previous slide

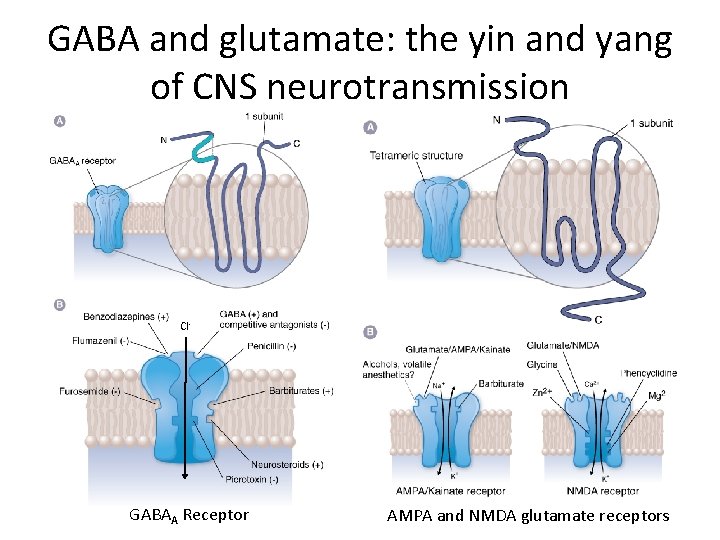
GABA and glutamate: the yin and yang of CNS neurotransmission Cl- GABAA Receptor AMPA and NMDA glutamate receptors

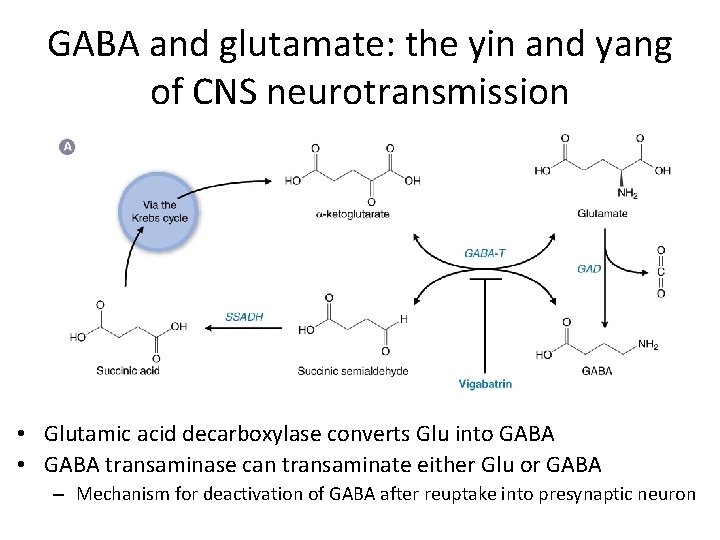
GABA and glutamate: the yin and yang of CNS neurotransmission • Glutamic acid decarboxylase converts Glu into GABA • GABA transaminase can transaminate either Glu or GABA – Mechanism for deactivation of GABA after reuptake into presynaptic neuron

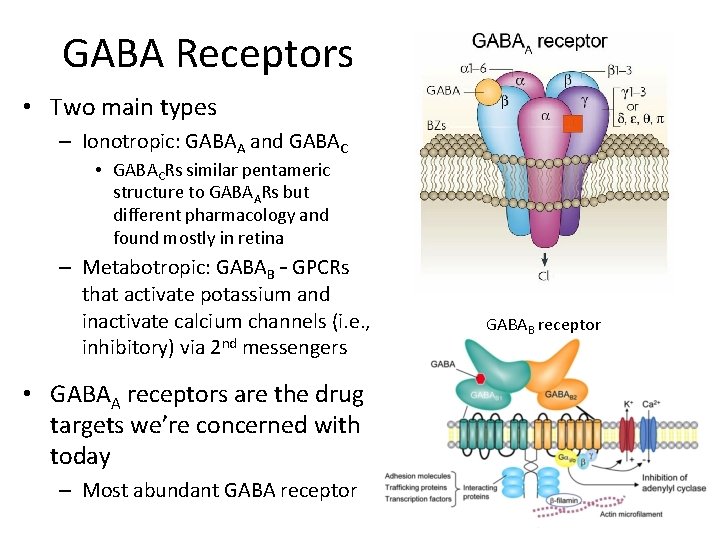
GABA Receptors • Two main types – Ionotropic: GABAA and GABAC • GABACRs similar pentameric structure to GABAARs but different pharmacology and found mostly in retina – Metabotropic: GABAB – GPCRs that activate potassium and inactivate calcium channels (i. e. , inhibitory) via 2 nd messengers • GABAA receptors are the drug targets we’re concerned with today – Most abundant GABA receptor GABAB receptor

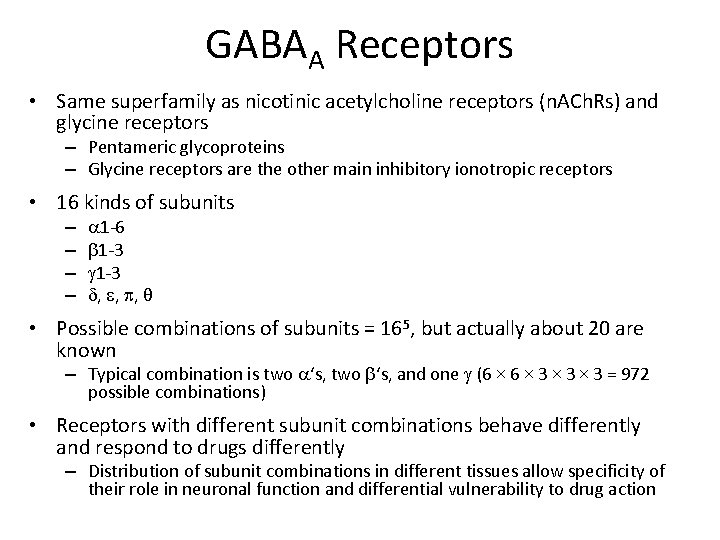
GABAA Receptors • Same superfamily as nicotinic acetylcholine receptors (n. ACh. Rs) and glycine receptors – Pentameric glycoproteins – Glycine receptors are the other main inhibitory ionotropic receptors • 16 kinds of subunits – – a 1 -6 b 1 -3 g 1 -3 d, e, p, θ • Possible combinations of subunits = 165, but actually about 20 are known – Typical combination is two a‘s, two b‘s, and one g (6 × 3 × 3 = 972 possible combinations) • Receptors with different subunit combinations behave differently and respond to drugs differently – Distribution of subunit combinations in different tissues allow specificity of their role in neuronal function and differential vulnerability to drug action

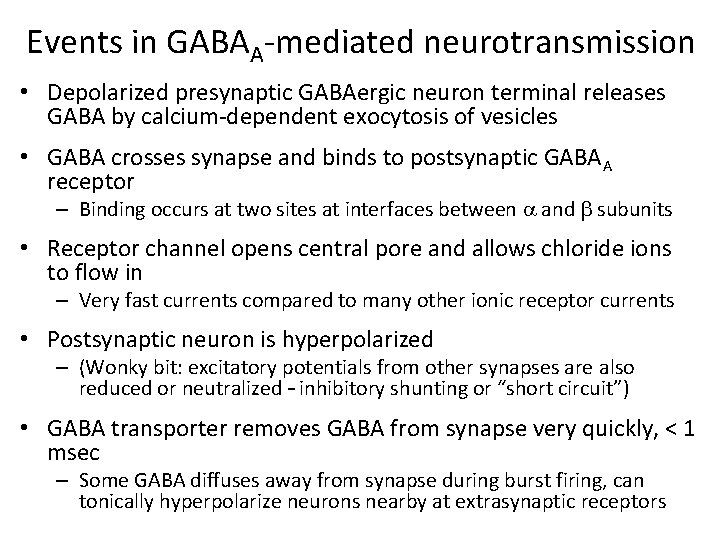
Events in GABAA-mediated neurotransmission • Depolarized presynaptic GABAergic neuron terminal releases GABA by calcium-dependent exocytosis of vesicles • GABA crosses synapse and binds to postsynaptic GABAA receptor – Binding occurs at two sites at interfaces between a and b subunits • Receptor channel opens central pore and allows chloride ions to flow in – Very fast currents compared to many other ionic receptor currents • Postsynaptic neuron is hyperpolarized – (Wonky bit: excitatory potentials from other synapses are also reduced or neutralized – inhibitory shunting or “short circuit”) • GABA transporter removes GABA from synapse very quickly, < 1 msec – Some GABA diffuses away from synapse during burst firing, can tonically hyperpolarize neurons nearby at extrasynaptic receptors

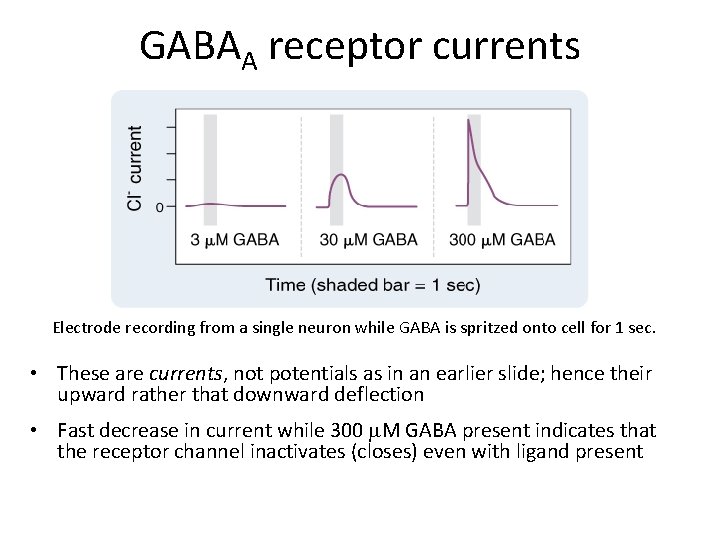
GABAA receptor currents Electrode recording from a single neuron while GABA is spritzed onto cell for 1 sec. • These are currents, not potentials as in an earlier slide; hence their upward rather that downward deflection • Fast decrease in current while 300 m. M GABA present indicates that the receptor channel inactivates (closes) even with ligand present

GABAergic drugs: benzodiazepines

GABAergic drug actions in the CNS • Inhibiting GABAA receptors by drugs can cause seizures – out of control firing of neurons – Shows that neurons are constantly being inhibited by background GABA activity • Increasing GABAA receptor activity reduces excitability of many neurons, inhibits many CNS functions (today’s topic) • Metabolites of steroid hormones can modulate GABAA receptors – E. g. , pregnenolone, dihydroepiandosterone (DHEA) – These neurosteroids do not act through regular nuclear steroid receptors, have unique binding site on GABAA receptor • Oleamide accumulates in CSF during sleep deprivation, induces sleep – Endogenous GABAA receptor modulator, binds beta 3 subunit

Drugs that act on GABAergic synapses GABA transporter (GAT-1) inhibitor – tiagabine (Gabitril) Glutamic acid decarboxylase inhibitor – allylglycine GABA transaminase inhibitor – vigabatrin (Sabril) GABAA receptor agonist – muscimol (Amanita muscaria mushrooms) • GABAA receptor antagonist – bicuculline, gabazine, picrotoxin • GABAA receptor positive allosteric modulator – benzodiazepines, barbiturates • GABAB receptor agonist – baclofen (Lioresal – muscle relaxant) • • Predict how each of these drug classes will affect general neuronal activity – i. e. , convulsant or anticonvulsant

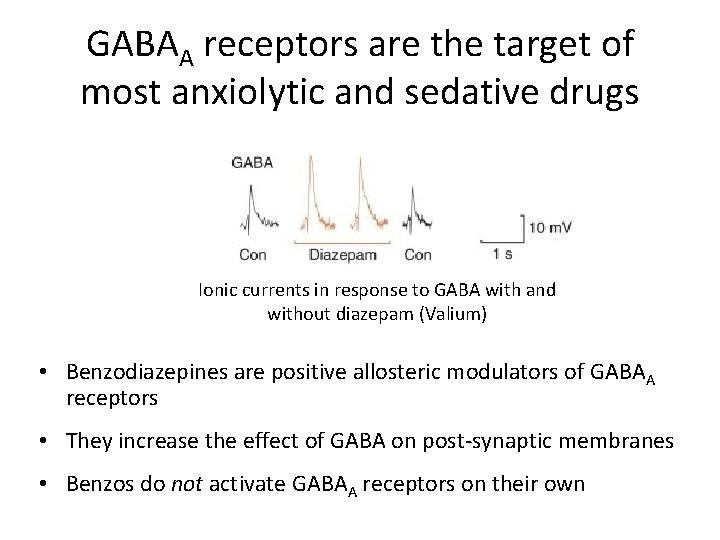
GABAA receptors are the target of most anxiolytic and sedative drugs Ionic currents in response to GABA with and without diazepam (Valium) • Benzodiazepines are positive allosteric modulators of GABAA receptors • They increase the effect of GABA on post-synaptic membranes • Benzos do not activate GABAA receptors on their own

Benzodiazepines – BZDs or “benzos” • Prototype (chlordiazepoxide, Librium) discovered by accident in 1961 • Core structure is a 7 -membered, 2 -nitrogen ring (diazepine) fused to benzene ring with an aryl group attached • 10 -20 family members all have anxiolytic, hypnotic, anticonvulsant, muscle-relaxant, and amnesic actions – Some differences in which actions are strongest – Main clinical differences are in pharmacokinetics (halflife)

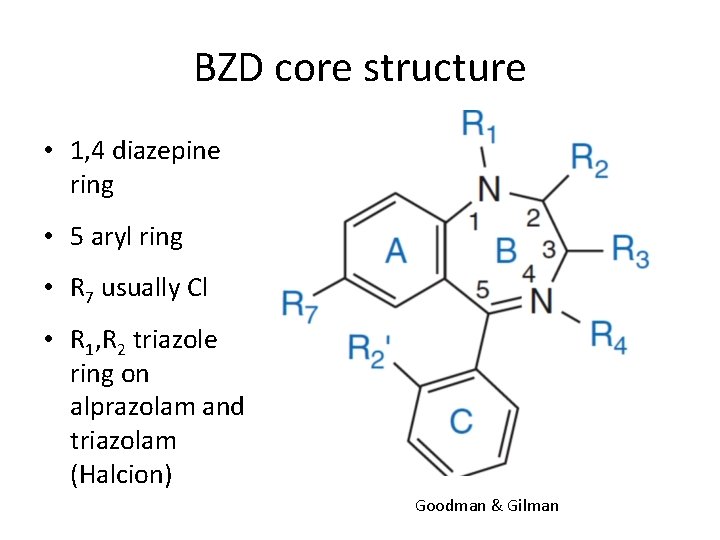
BZD core structure • 1, 4 diazepine ring • 5 aryl ring • R 7 usually Cl • R 1, R 2 triazole ring on alprazolam and triazolam (Halcion) Goodman & Gilman

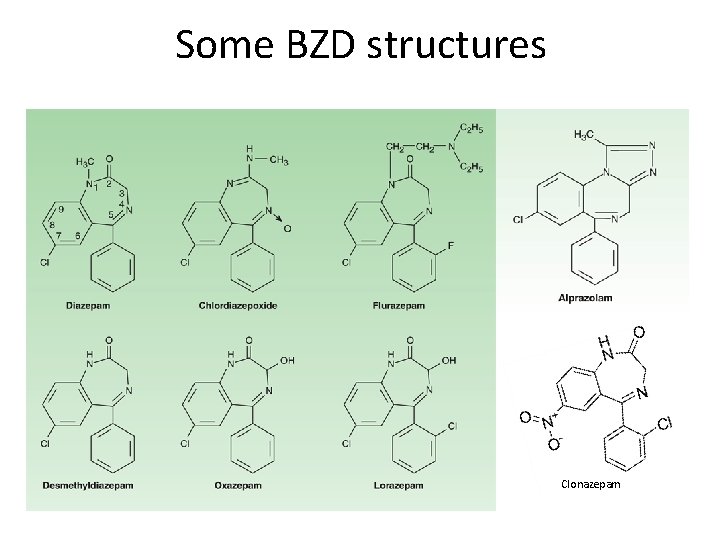
Some BZD structures Clonazepam

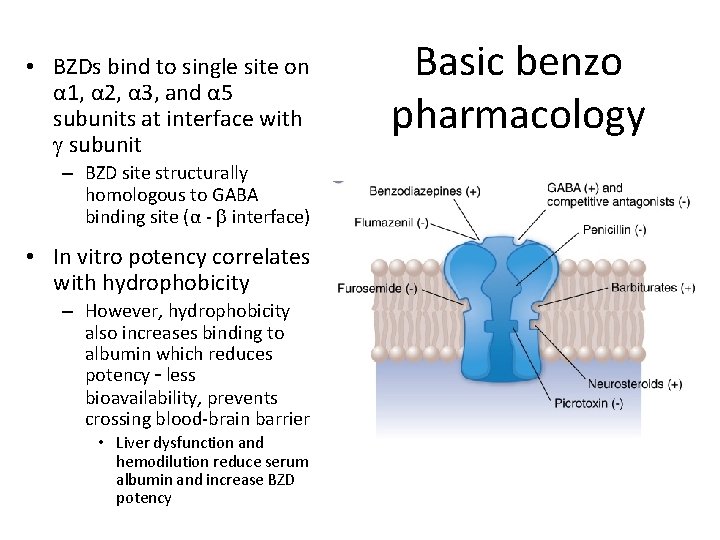
• BZDs bind to single site on α 1, α 2, α 3, and α 5 subunits at interface with g subunit – BZD site structurally homologous to GABA binding site (α - b interface) • In vitro potency correlates with hydrophobicity – However, hydrophobicity also increases binding to albumin which reduces potency – less bioavailability, prevents crossing blood-brain barrier • Liver dysfunction and hemodilution reduce serum albumin and increase BZD potency Basic benzo pharmacology

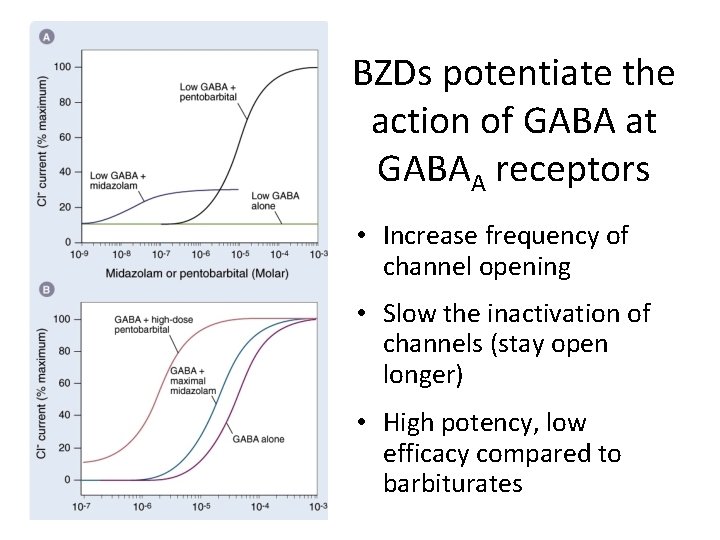
(10 m. M) BZDs potentiate the action of GABA at GABAA receptors • Increase frequency of channel opening • Slow the inactivation of channels (stay open longer) • High potency, low efficacy compared to barbiturates

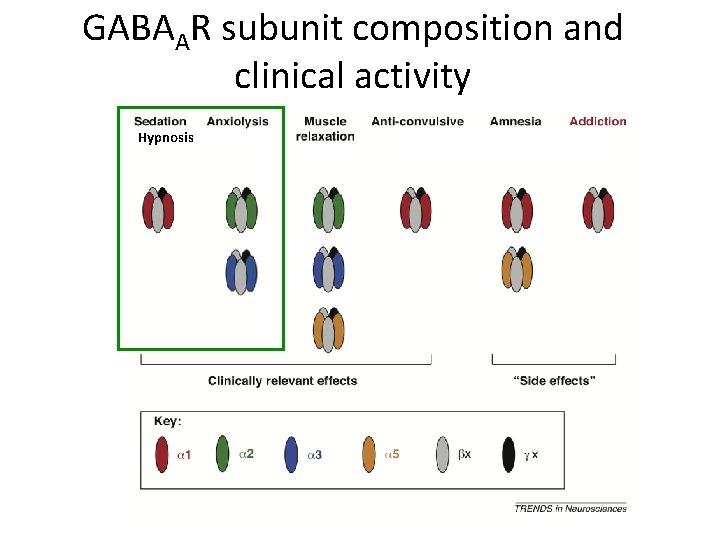
GABAAR subunit composition and clinical activity Hypnosis

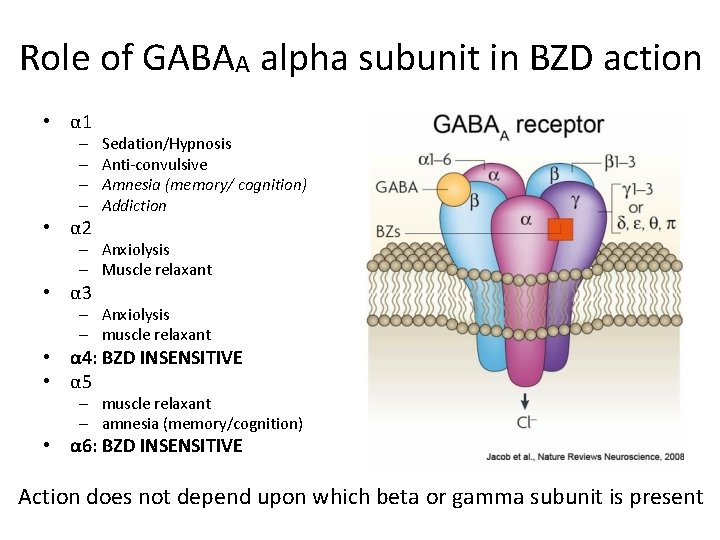
Role of GABAA alpha subunit in BZD action • α 1 – – • α 2 Sedation/Hypnosis Anti-convulsive Amnesia (memory/ cognition) Addiction – Anxiolysis – Muscle relaxant • α 3 – Anxiolysis – muscle relaxant • α 4: BZD INSENSITIVE • α 5 – muscle relaxant – amnesia (memory/cognition) • α 6: BZD INSENSITIVE Action does not depend upon which beta or gamma subunit is present

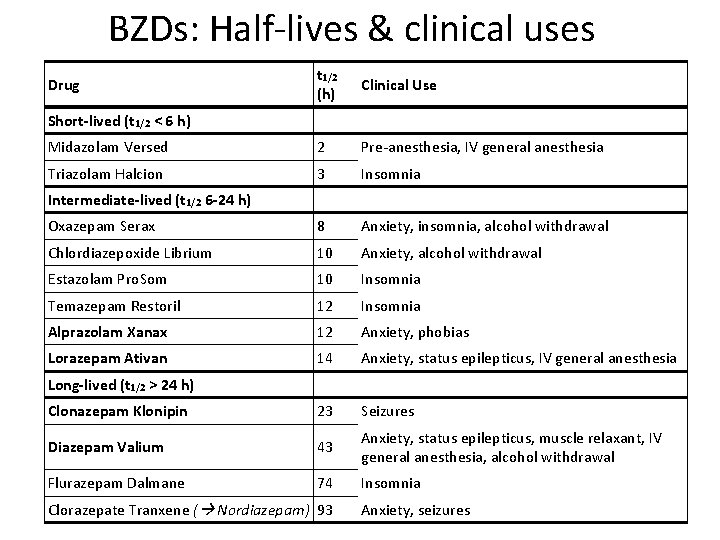
BZDs: Half-lives & clinical uses t 1/2 (h) Clinical Use Midazolam Versed 2 Pre-anesthesia, IV general anesthesia Triazolam Halcion 3 Insomnia Oxazepam Serax 8 Anxiety, insomnia, alcohol withdrawal Chlordiazepoxide Librium 10 Anxiety, alcohol withdrawal Estazolam Pro. Som 10 Insomnia Temazepam Restoril 12 Insomnia Alprazolam Xanax 12 Anxiety, phobias Lorazepam Ativan 14 Anxiety, status epilepticus, IV general anesthesia Clonazepam Klonipin 23 Seizures Diazepam Valium 43 Anxiety, status epilepticus, muscle relaxant, IV general anesthesia, alcohol withdrawal Flurazepam Dalmane 74 Insomnia Drug Short-lived (t 1/2 < 6 h) Intermediate-lived (t 1/2 6 -24 h) Long-lived (t 1/2 > 24 h) Clorazepate Tranxene ( Nordiazepam) 93 Anxiety, seizures

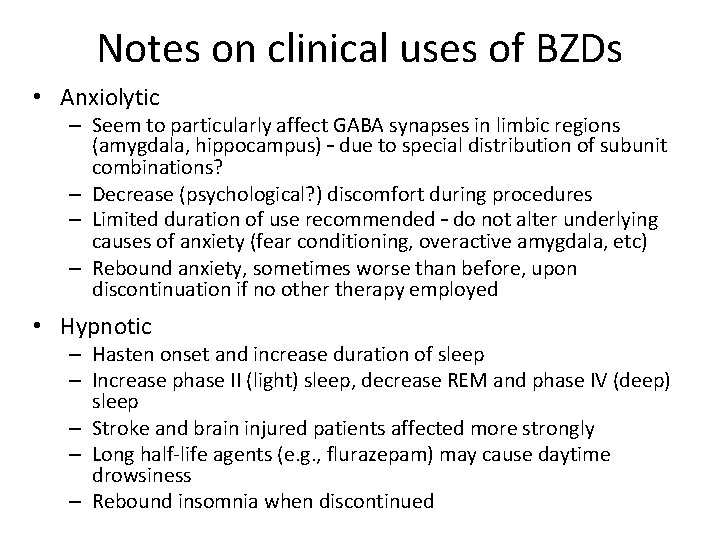
Notes on clinical uses of BZDs • Anxiolytic – Seem to particularly affect GABA synapses in limbic regions (amygdala, hippocampus) – due to special distribution of subunit combinations? – Decrease (psychological? ) discomfort during procedures – Limited duration of use recommended – do not alter underlying causes of anxiety (fear conditioning, overactive amygdala, etc) – Rebound anxiety, sometimes worse than before, upon discontinuation if no otherapy employed • Hypnotic – Hasten onset and increase duration of sleep – Increase phase II (light) sleep, decrease REM and phase IV (deep) sleep – Stroke and brain injured patients affected more strongly – Long half-life agents (e. g. , flurazepam) may cause daytime drowsiness – Rebound insomnia when discontinued

Notes on clinical uses of BZDs • Anesthesia – Not anesthetic themselves but potentiate effect of anesthetic agents – Short acting midazolam most common for this use – Often a component of Monitored Anesthesia Care (MAC) for surgical procedures – light sedation with opioid and local anesthetic (for example) – Amnesic action a benefit here • Muscle relaxant – Decrease muscle spasticity after injury or in MS, etc. – Increase activity of spinal cord inhibitory interneurons

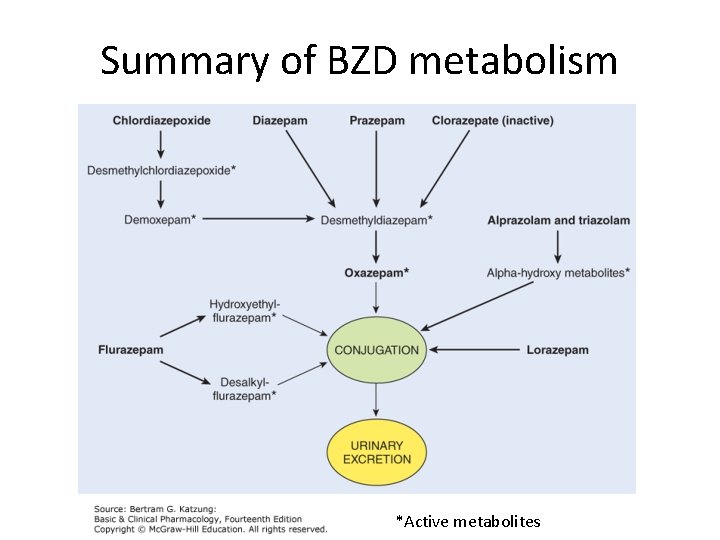
Benzodiazepines ADME • Absorption – Usually oral but also transmucosal, IV, and IM forms – Lipid solubility is a major determinant of how rapidly a particular BZD enters CNS – High protein binding (albumin), complete absorption • Metabolism – Metabolic transformation to more water-soluble metabolites is necessary for clearance of most sedative-hypnotics from body – Most BZD metabolism occurs in the liver, usually via CYP 3 A 4 – Phase I & II reactions to form glucuronides that are excreted in urine – However, MOST phase I metabolites of BZDs are pharmacologically active, with LONG half-lives • Distribution – Redistribution from muscle and fat can extend even further extend the time these drugs will be detected

Summary of BZD metabolism *Active metabolites

Benzodiazepine adverse effects • Additive effects – Overdoses rarely lethal alone but sedation is additive with other CNS sedatives (eg. alcohol, barbiturates, anti-histamines) – Cumulative effects of doses are especially problematic for those BZDs in which the parent drug (or active metabolites) have long half-lives • Unwanted side-effects – Drowsiness, impaired judgement, diminished motor skills – May also cause anterograde amnesia • eg. flunitrazepam (rohypnol), zolpidem Synergistic effects with alcohol – why? – same target (GABAAR)

Benzodiazepine adverse effects • Tolerance and dependence – Tolerance hypothesized to be due to decreased GABAAR expression with prolonged exposure – Physical dependence develops when used at high doses or for prolonged time • Withdrawal symptoms (anxiety, agitation, irritability, confusion) may occur when medication stopped abruptly – Classified as controlled substances: Class IV (abuse potential – “downers”) • Geriatric use discouraged – significant increase in risk for falls – Falls are leading cause of ED visits and accidental death in population over 65 years of age

BZD and Z-drug antagonist: flumazenil • Romazicon® - only BZD site antagonist • Used to reverse effects of BZD overdoses, and used to hasten recovery following use of BZDs in anesthetic & diagnostic procedures • Also works for Z-drug overdose • Given IV, acts rapidly; short half life (1 h) due to rapid liver clearance, so requires repeated administration • Does not antagonize actions of barbiturates or ethanol Why does flumazenil antagonize BZDs and Z-drugs but not barbiturates and alcohol?
GABAergic drugs: Z-drugs

Nonbenzodiazepine “Z-Drug” hypnotics • Structurally distinct from benzodiazapines but act at BZD receptor Zaleplon Zopiclone Zolpidem Eszopiclone • Zolpidem, zaleplon, zopiclone – More alpha 1 selective • "Fewer" SEs than BZDs – Lipophilic -- rapid absorption – Short t 1/2 • Eszopiclone – Active (S) enantiomer of zopiclone (zopiclone is a racemic mixture) • Claimed minimal abuse potential • Claimed minimal rebound insomnia 3 5

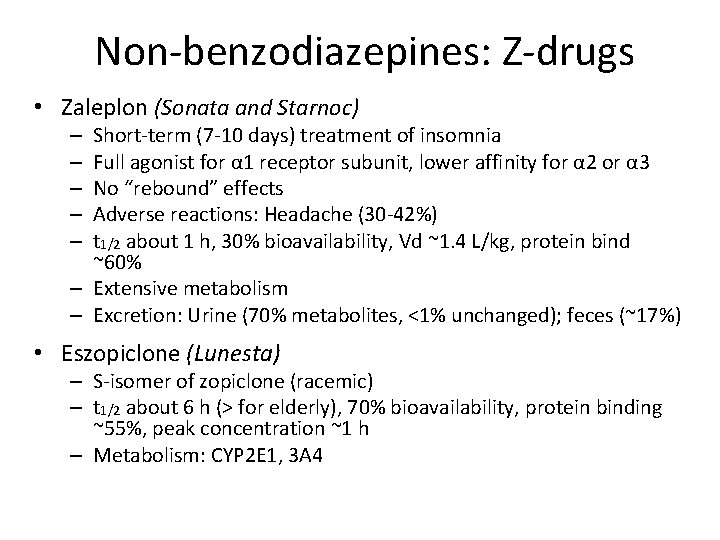
Non-benzodiazepines: Z-drugs • Zaleplon (Sonata and Starnoc) Short-term (7 -10 days) treatment of insomnia Full agonist for α 1 receptor subunit, lower affinity for α 2 or α 3 No “rebound” effects Adverse reactions: Headache (30 -42%) t 1/2 about 1 h, 30% bioavailability, Vd ~1. 4 L/kg, protein bind ~60% – Extensive metabolism – Excretion: Urine (70% metabolites, <1% unchanged); feces (~17%) – – – • Eszopiclone (Lunesta) – S-isomer of zopiclone (racemic) – t 1/2 about 6 h (> for elderly), 70% bioavailability, protein binding ~55%, peak concentration ~1 h – Metabolism: CYP 2 E 1, 3 A 4

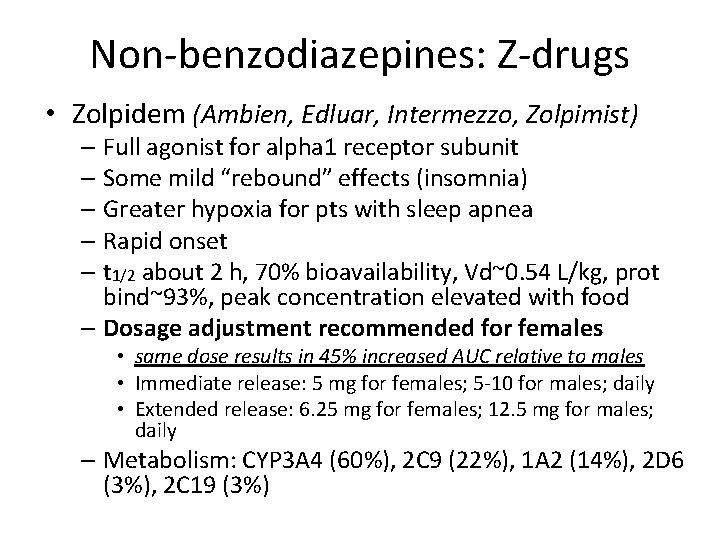
Non-benzodiazepines: Z-drugs • Zolpidem (Ambien, Edluar, Intermezzo, Zolpimist) – Full agonist for alpha 1 receptor subunit – Some mild “rebound” effects (insomnia) – Greater hypoxia for pts with sleep apnea – Rapid onset – t 1/2 about 2 h, 70% bioavailability, Vd~0. 54 L/kg, prot bind~93%, peak concentration elevated with food – Dosage adjustment recommended for females • same dose results in 45% increased AUC relative to males • Immediate release: 5 mg for females; 5 -10 for males; daily • Extended release: 6. 25 mg for females; 12. 5 mg for males; daily – Metabolism: CYP 3 A 4 (60%), 2 C 9 (22%), 1 A 2 (14%), 2 D 6 (3%), 2 C 19 (3%)

Z-Drugs: Side effects • Abnormal thinking and behavioral changes – decreased inhibition – aggression – agitation – hallucinations • CNS depression • Behaviors during sleep – sleep-driving – sleep-cooking – sleep-eating – sleep-sexing – sleep-talking on the phone 3 8
GABAergic drugs: barbiturates

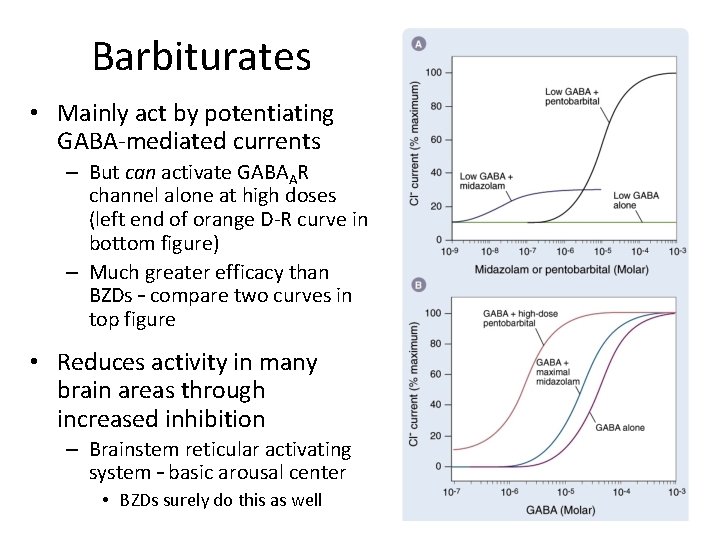
Barbiturates • Mainly act by potentiating GABA-mediated currents – But can activate GABAAR channel alone at high doses (left end of orange D-R curve in bottom figure) – Much greater efficacy than BZDs – compare two curves in top figure • Reduces activity in many brain areas through increased inhibition – Brainstem reticular activating system – basic arousal center • BZDs surely do this as well

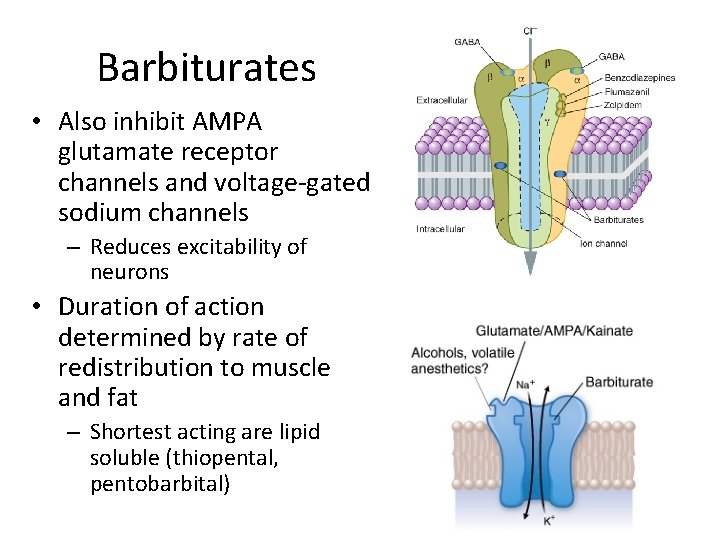
Barbiturates • Also inhibit AMPA glutamate receptor channels and voltage-gated sodium channels – Reduces excitability of neurons • Duration of action determined by rate of redistribution to muscle and fat – Shortest acting are lipid soluble (thiopental, pentobarbital)

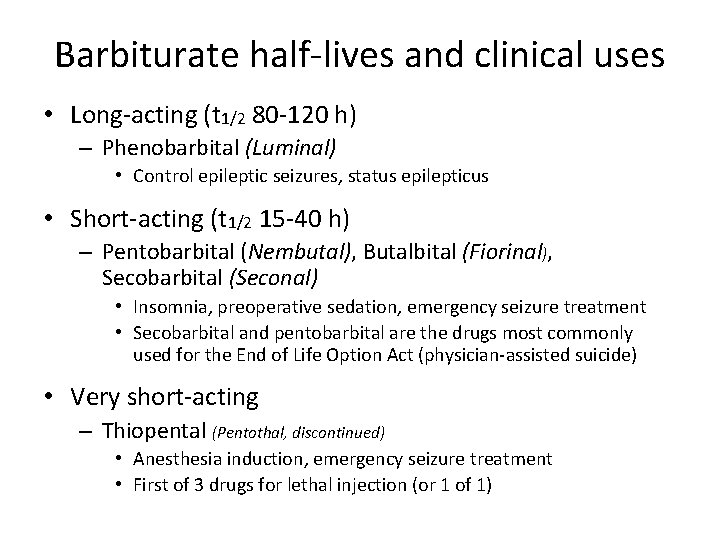
Barbiturate half-lives and clinical uses • Long-acting (t 1/2 80 -120 h) – Phenobarbital (Luminal) • Control epileptic seizures, status epilepticus • Short-acting (t 1/2 15 -40 h) – Pentobarbital (Nembutal), Butalbital (Fiorinal), Secobarbital (Seconal) • Insomnia, preoperative sedation, emergency seizure treatment • Secobarbital and pentobarbital are the drugs most commonly used for the End of Life Option Act (physician-assisted suicide) • Very short-acting – Thiopental (Pentothal, discontinued) • Anesthesia induction, emergency seizure treatment • First of 3 drugs for lethal injection (or 1 of 1)

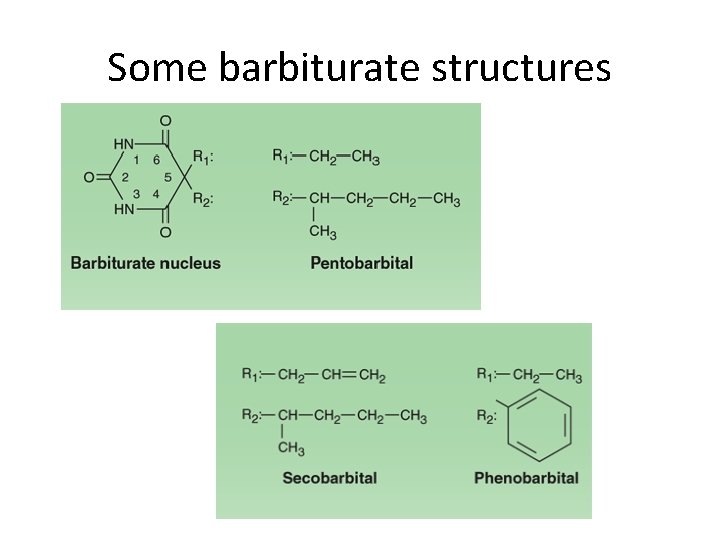
Some barbiturate structures

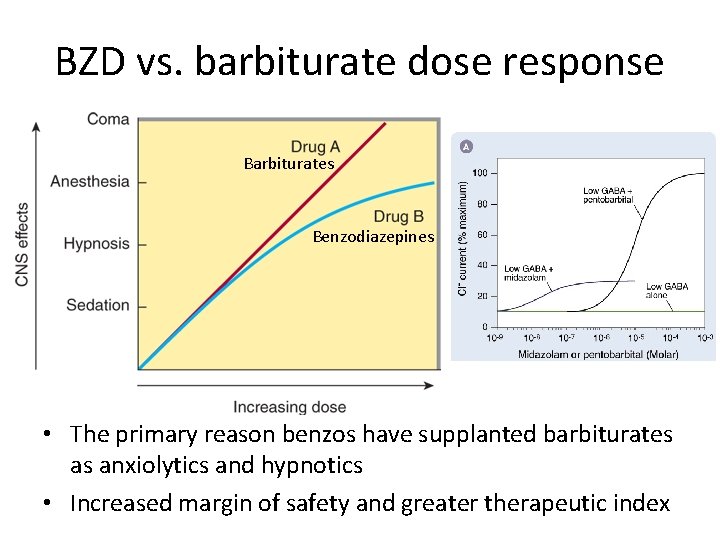
BZD vs. barbiturate dose response Barbiturates Benzodiazepines • The primary reason benzos have supplanted barbiturates as anxiolytics and hypnotics • Increased margin of safety and greater therapeutic index

Other classes of anxiolytics and hypnotics

Other anxiolytics and hypnotics • Buspirone (Buspar) – Anxiolytic with no sedative, hypnotic, anticonvulsant effects – no abuse potential – Usually for GAD – Partial 5 -HT 1 A agonist, presynaptic (autoinhibitory) D 2 antagonist – Inhibits locus ceruleus – No withdrawal effects (rebound, etc. ), no psychomotor impairment – Takes 3 – 4 weeks for effect – Efficacy – meh?

Other anxiolytics and hypnotics • Melatonin receptor agonists – Ramelteon and Tasimelteon – MT 1 and MT 2 receptor in suprachiasmatic nucleus – controls circadian rhythms – Supposed to hasten onset of sleep (i. e. , for initial insomnia vs. maintenance insomnia) – No effect on sleep architecture (duration of phases – I, IV, REM, etc. ) – Why not just use melatonin? Melatonin Ramelteon