Whats coming up Oct 25 Oct 27 Oct









- Slides: 34

What’s coming up? ? ? • • • Oct 25 Oct 27 Oct 29 Nov 1 Nov 3, 5 Nov 8, 10 Nov 12 Nov 15 Nov 17 Nov 19 Nov 22 Nov 24 The atmosphere, part 1 Midterm … No lecture The atmosphere, part 2 Light, blackbodies, Bohr Postulates of QM, p-in-a-box Hydrogen and multi – e atoms Multi-electron atoms Periodic properties Valence-bond; Lewis structures VSEPR Hybrid orbitals; VSEPR Ch. 8 • Nov 26 Hybrid orbitals; MO theory Ch. 12 • • • MO theory bonding wrapup Review for exam Ch. 12 Ch. 11, 12 Nov 29 Dec 1 Dec 2 Ch. 8 Ch. 9, 10 Ch. 11, 12


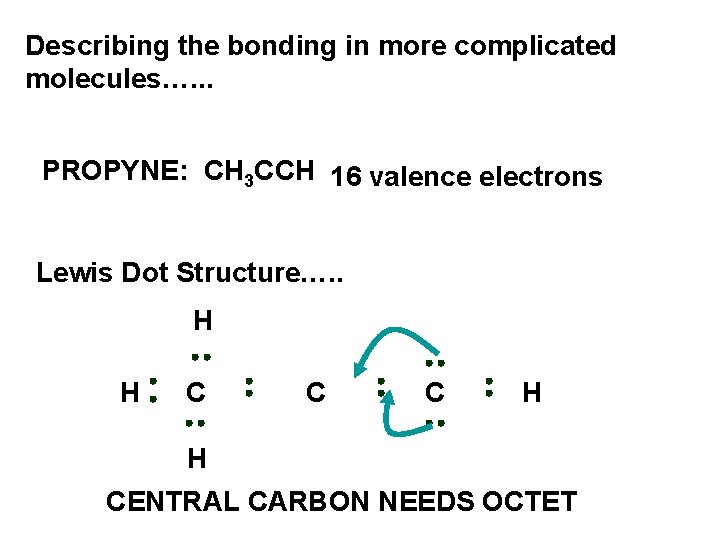
Describing the bonding in more complicated molecules…. . . PROPYNE: CH 3 CCH 16 valence electrons Lewis Dot Structure…. . H H C C C H H CENTRAL CARBON NEEDS OCTET

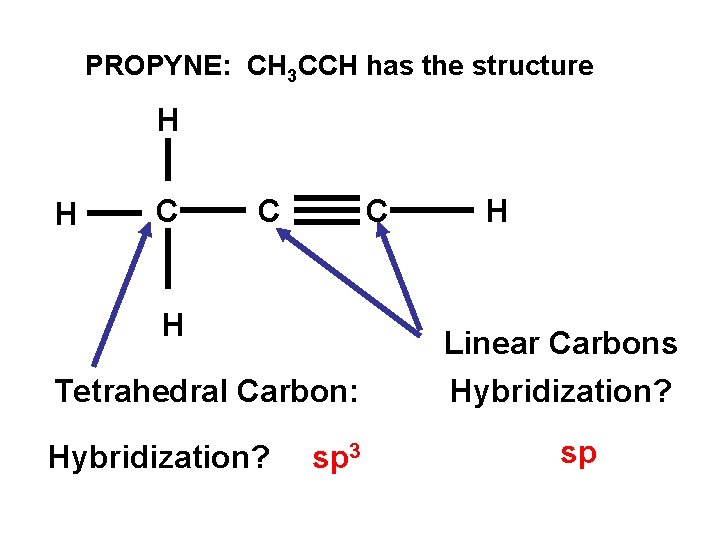
PROPYNE: CH 3 CCH has the structure H H C C C H Tetrahedral Carbon: Hybridization? sp 3 H Linear Carbons Hybridization? sp

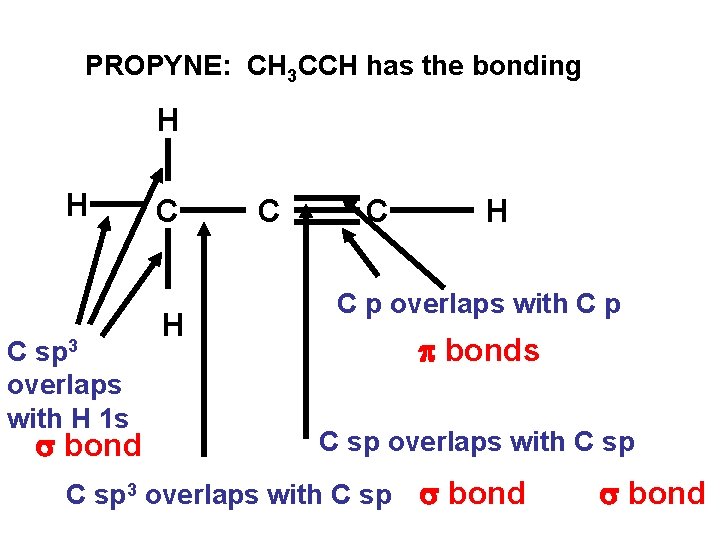
PROPYNE: CH 3 CCH has the bonding H H C sp 3 overlaps with H 1 s s bond C H C p overlaps with C p p bonds C sp overlaps with C sp 3 overlaps with C sp s bond

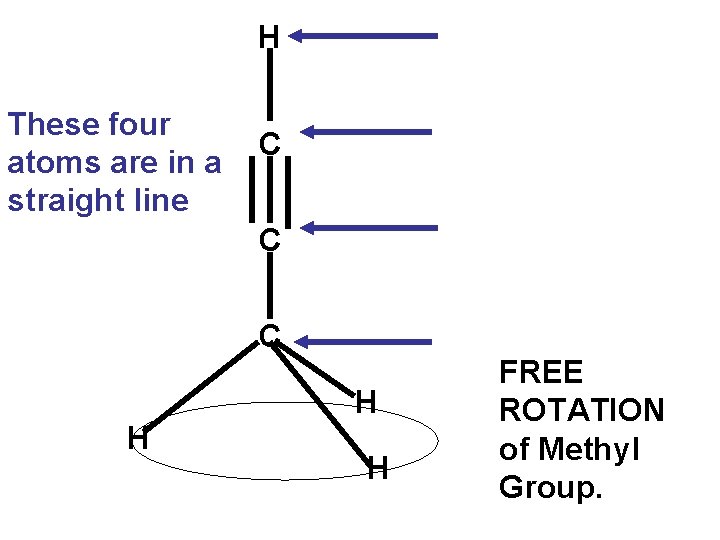
H These four atoms are in a straight line C C C H H H FREE ROTATION of Methyl Group.

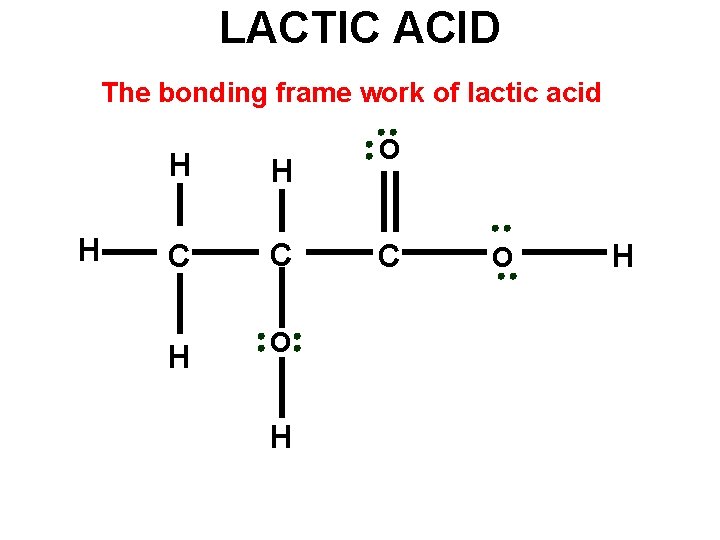
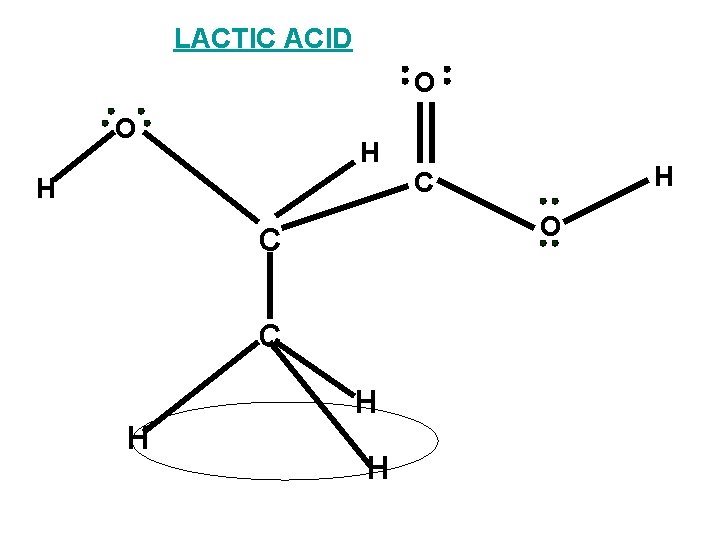
LACTIC ACID The bonding frame work of lactic acid H H H C C H O C O H

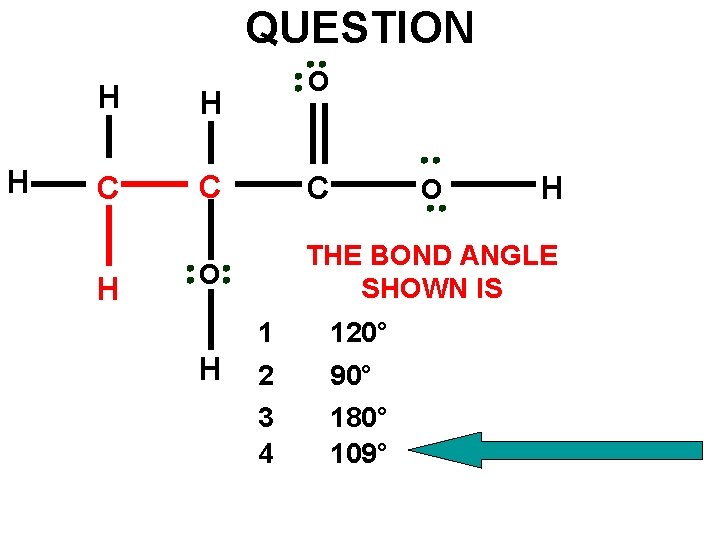
QUESTION H O H H C C C O THE BOND ANGLE SHOWN IS H H 1 120° 2 3 4 90° 180° 109° O H

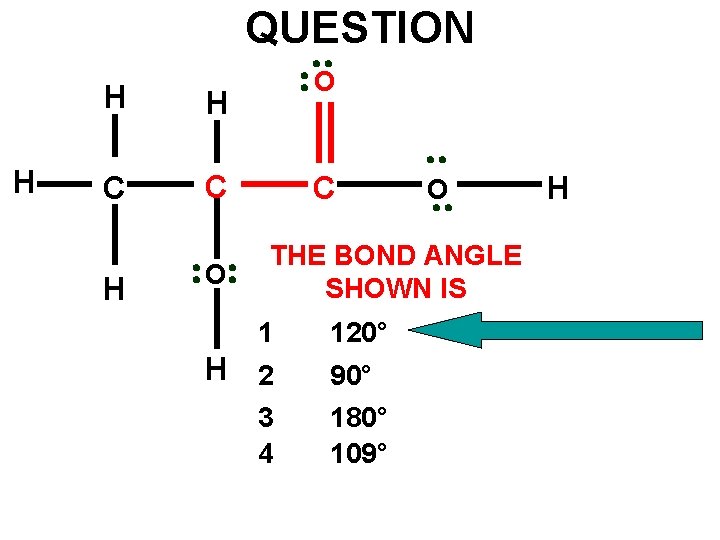
QUESTION H H H C C H O O C O THE BOND ANGLE SHOWN IS 1 120° H 2 90° 180° 109° 3 4 H

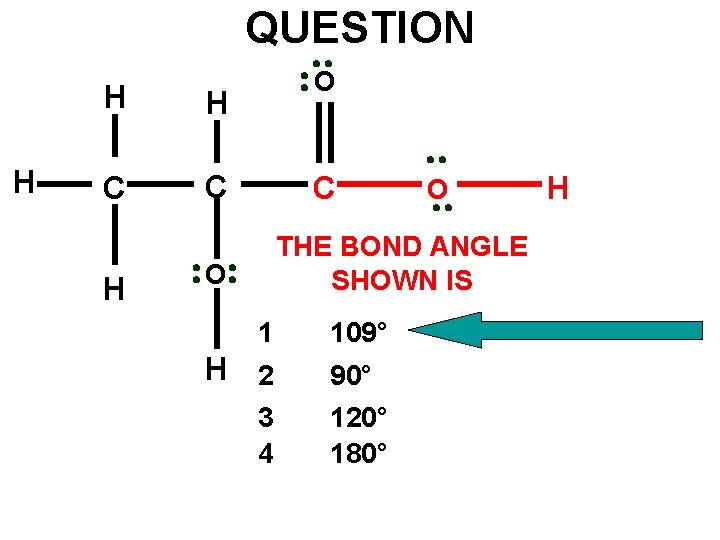
QUESTION H H H C C H O C O THE BOND ANGLE SHOWN IS O 1 109° H 2 90° 120° 180° 3 4 H

LACTIC ACID O O H H O C C H H H C H


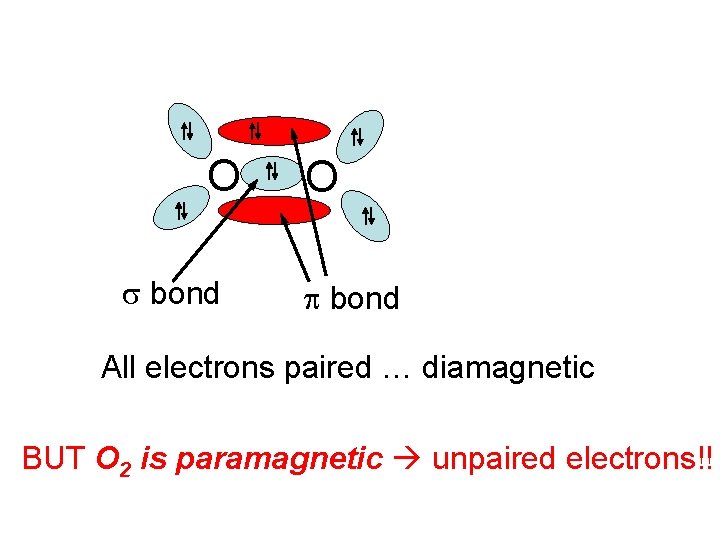
OK, so we have it all figured out! … or do we? ? Let’s think about oxygen, O 2 12 electrons … Lewis picture gives : O: . . 3 electron groups around each … sp 2 and 1200

O s bond O p bond All electrons paired … diamagnetic BUT O 2 is paramagnetic unpaired electrons!!

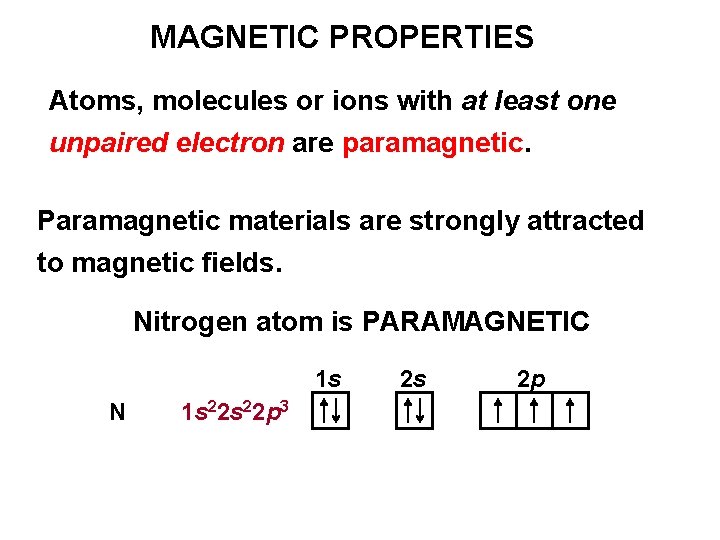
MAGNETIC PROPERTIES Atoms, molecules or ions with at least one unpaired electron are paramagnetic. Paramagnetic materials are strongly attracted to magnetic fields. Nitrogen atom is PARAMAGNETIC 1 s N 1 s 22 p 3 2 s 2 p


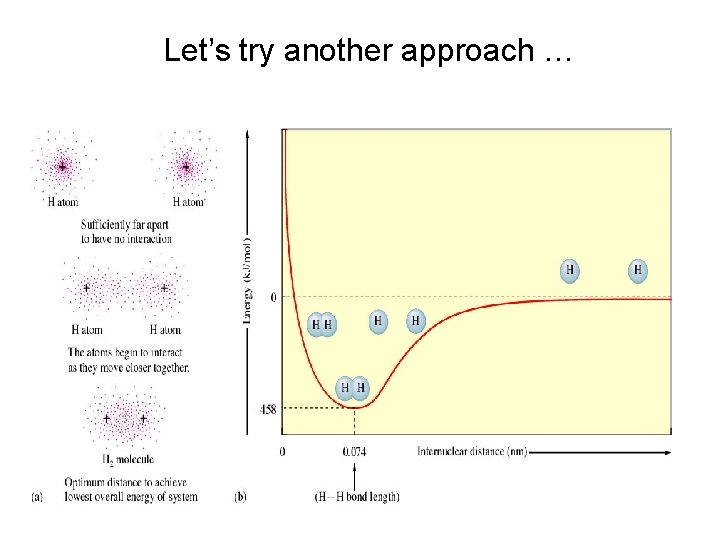
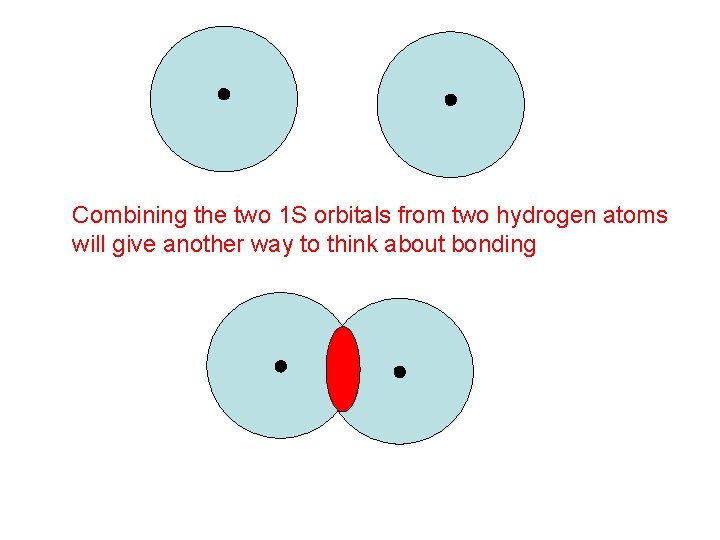
Let’s try another approach …

Combining the two 1 S orbitals from two hydrogen atoms will give another way to think about bonding

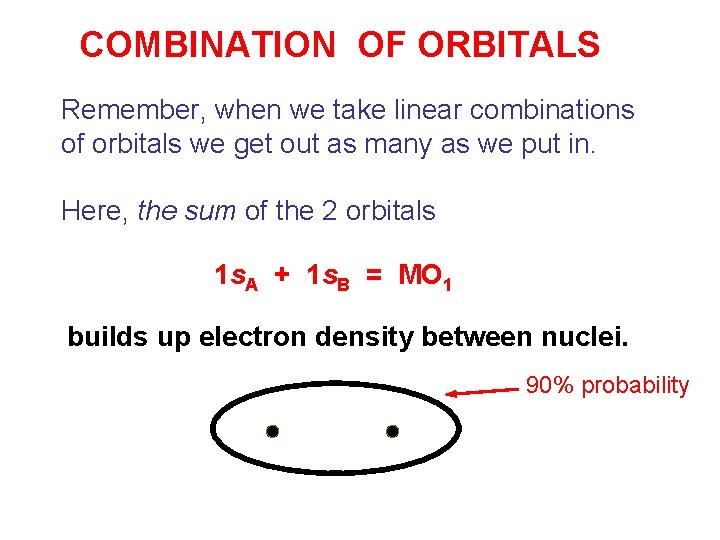
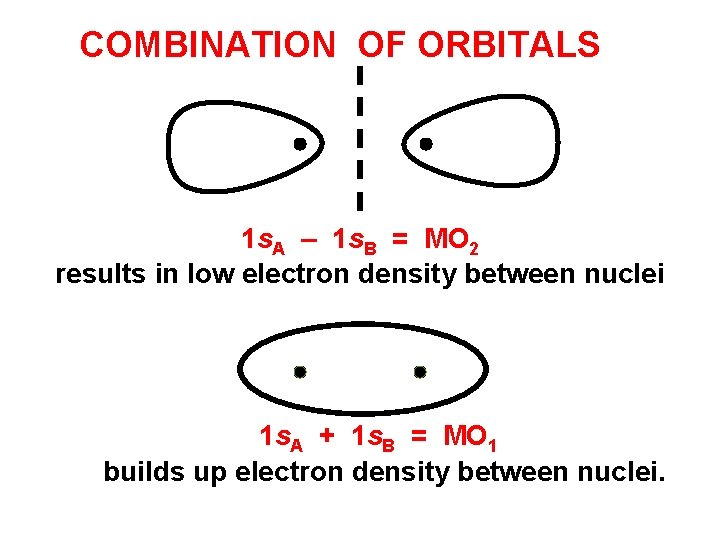
COMBINATION OF ORBITALS Remember, when we take linear combinations of orbitals we get out as many as we put in. Here, the sum of the 2 orbitals 1 s. A + 1 s. B = MO 1 builds up electron density between nuclei. 90% probability

COMBINATION OF ORBITALS 1 s. A – 1 s. B = MO 2 results in low electron density between nuclei 1 s. A + 1 s. B = MO 1 builds up electron density between nuclei.

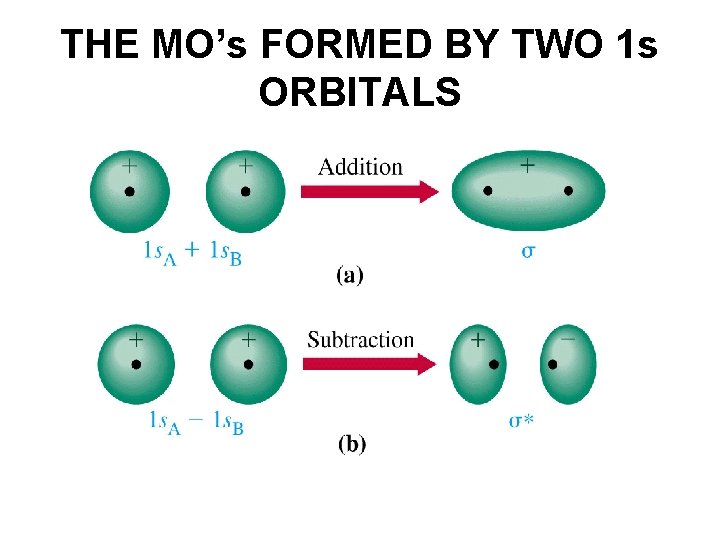
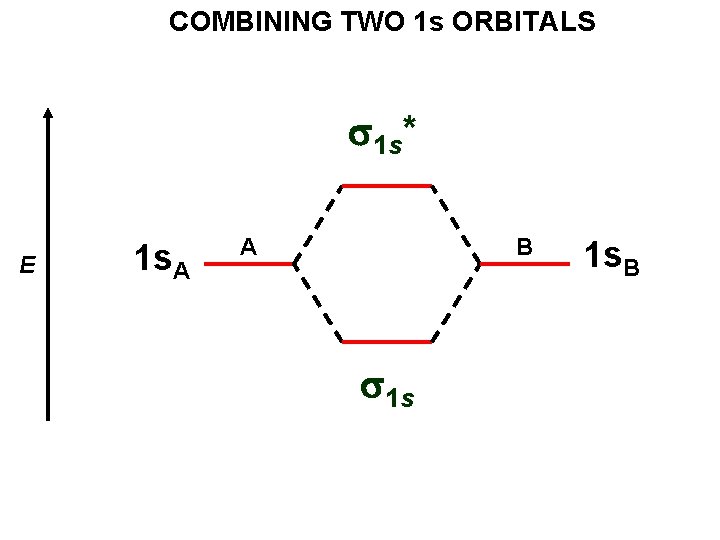
THE MO’s FORMED BY TWO 1 s ORBITALS

ANTI-BONDING s 1 s* 1 s. A – 1 s. B = MO 2 BONDING Each orbital can hold up to two electrons. s 1 s 1 s. A + 1 s. B = MO 1

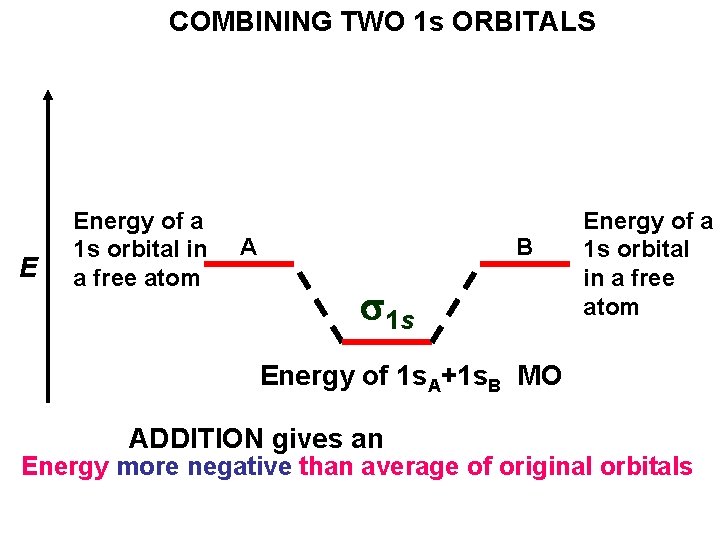
COMBINING TWO 1 s ORBITALS E Energy of a 1 s orbital in a free atom A B Energy of a 1 s orbital in a free atom

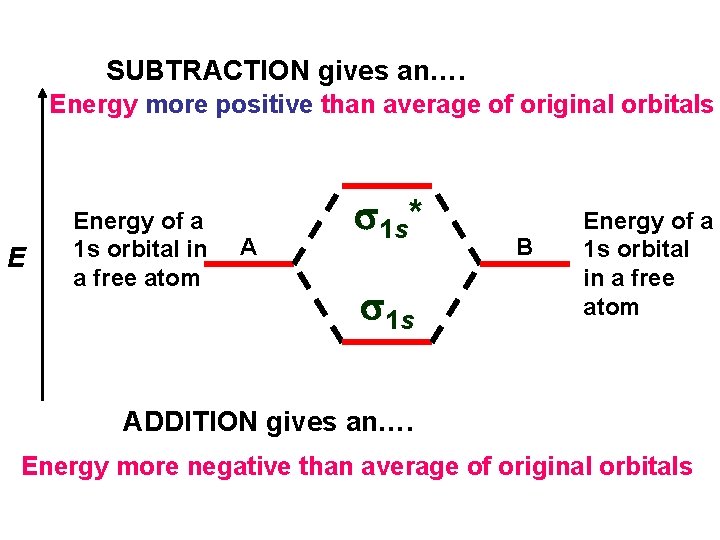
COMBINING TWO 1 s ORBITALS E Energy of a 1 s orbital in a free atom A B s 1 s Energy of a 1 s orbital in a free atom Energy of 1 s. A+1 s. B MO ADDITION gives an Energy more negative than average of original orbitals

SUBTRACTION gives an…. Energy more positive than average of original orbitals E Energy of a 1 s orbital in a free atom A s 1 s* s 1 s B Energy of a 1 s orbital in a free atom ADDITION gives an…. Energy more negative than average of original orbitals

COMBINING TWO 1 s ORBITALS s 1 s* E 1 s. A A B s 1 s 1 s. B

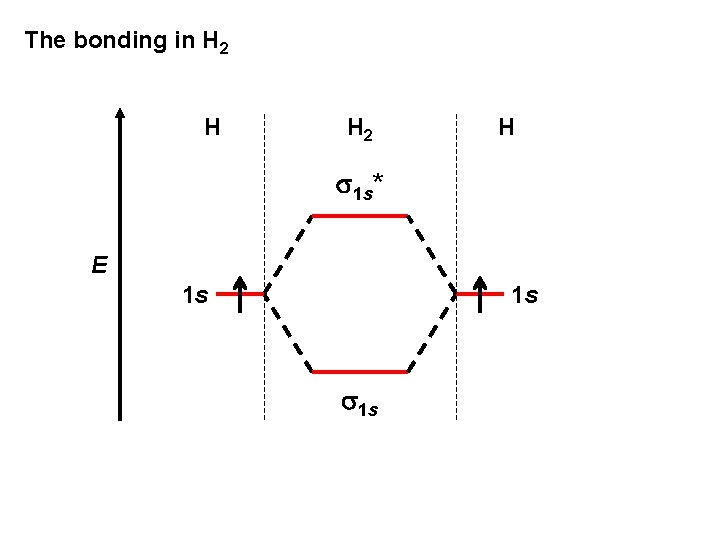
The bonding in H 2 H s 1 s* E 1 s 1 s

H H 2 H s 1 s* E 1 s 1 s H 2: (s 1 s)2

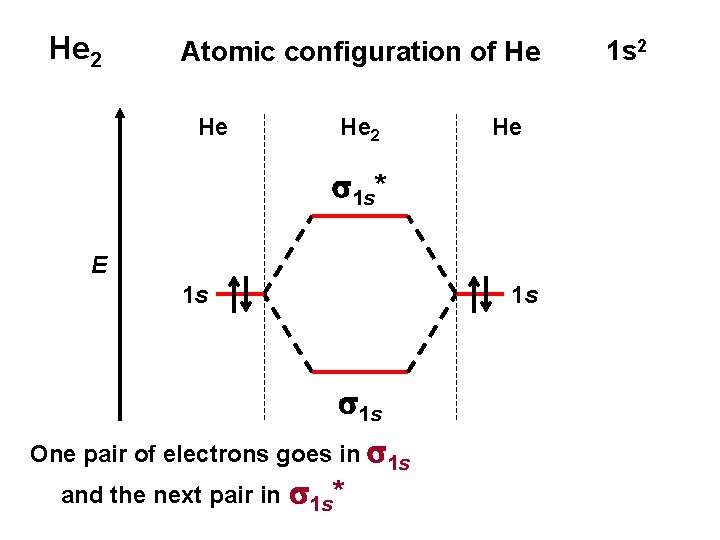
He 2 Atomic configuration of He He He 2 He s 1 s* E 1 s 1 s One pair of electrons goes in s 1 s and the next pair in s 1 s* 1 s 2

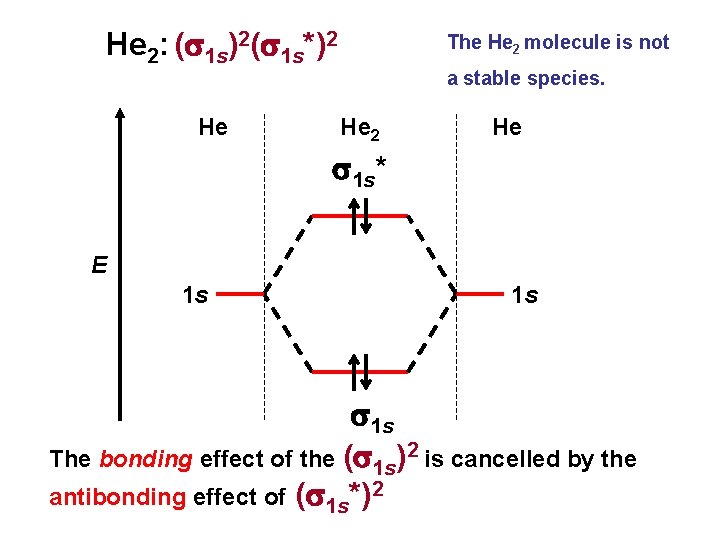
He 2: (s 1 s)2(s 1 s*)2 He The He 2 molecule is not a stable species. He 2 He s 1 s* E 1 s 1 s The bonding effect of the (s 1 s)2 is cancelled by the antibonding effect of (s 1 s*)2

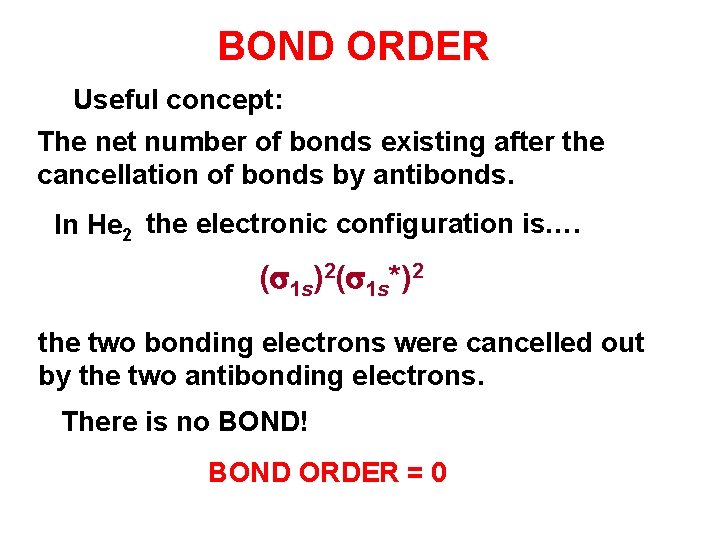
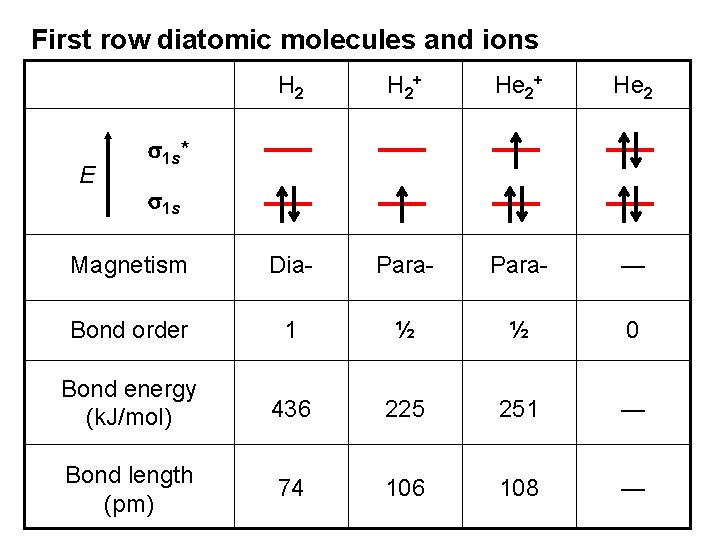
BOND ORDER Useful concept: The net number of bonds existing after the cancellation of bonds by antibonds. In He 2 the electronic configuration is…. (s 1 s)2(s 1 s*)2 the two bonding electrons were cancelled out by the two antibonding electrons. There is no BOND! BOND ORDER = 0

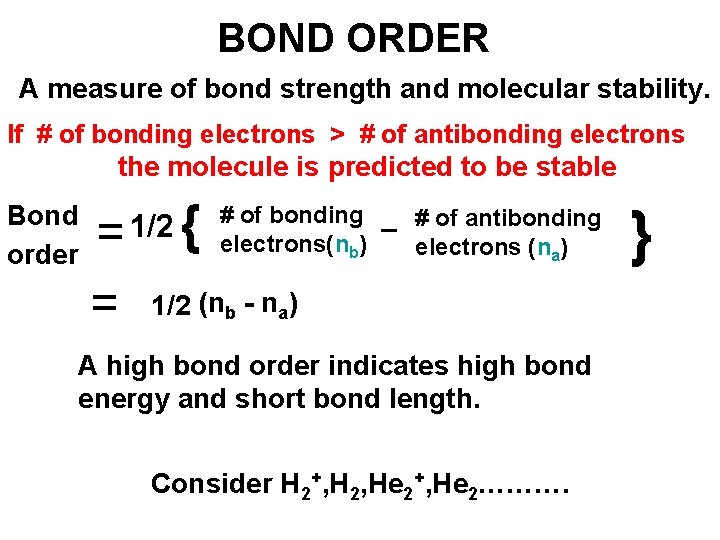
BOND ORDER A measure of bond strength and molecular stability. If # of bonding electrons > # of antibonding electrons the molecule is predicted to be stable Bond order = 1/2 { = 1/2 (n # of bonding # of antibonding – electrons(nb) electrons (na) b - n a) A high bond order indicates high bond energy and short bond length. Consider H 2+, H 2, He 2+, He 2………. }

First row diatomic molecules and ions H 2+ He 2 Magnetism Dia- Para- — Bond order 1 ½ ½ 0 Bond energy (k. J/mol) 436 225 251 — Bond length (pm) 74 106 108 — E s 1 s* s 1 s
