Whats coming up Oct 25 Oct 27 Oct




- Slides: 42

What’s coming up? ? ? • • • Oct 25 Oct 27 Oct 29 Nov 1 Nov 3, 5 Nov 8, 10 Nov 12 Nov 15 Nov 17 Nov 19 Nov 22 The atmosphere, part 1 Midterm … No lecture The atmosphere, part 2 Light, blackbodies, Bohr Postulates of QM, p-in-a-box Hydrogen and multi – e atoms Multi-electron atoms Periodic properties Valence-bond; Lewis structures VSEPR Ch. 8 • Nov 24 Hybrid orbitals; VSEPR Ch. 11, 12 • • MO theory bonding wrapup Review for exam Ch. 12 Ch. 11, 12 Nov 26 Nov 29 Dec 1 Dec 2 Ch. 8 Ch. 9, 10 Ch. 11




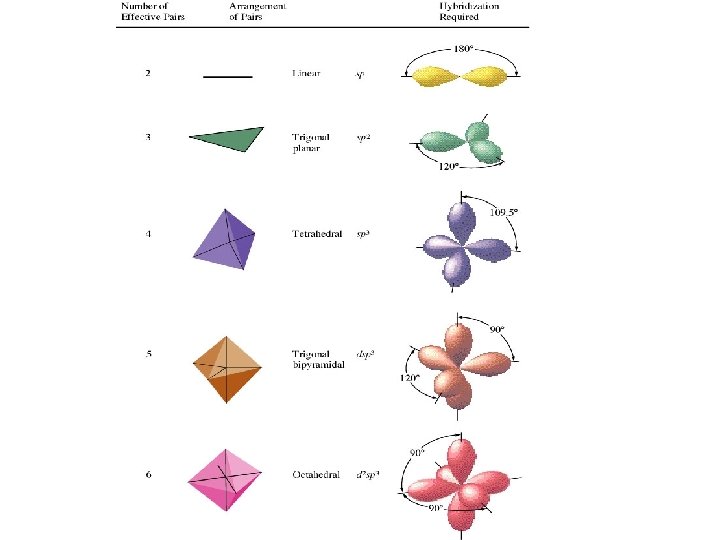
COMBINING ORBITALS TO FORM HYBRIDS HYBRIDIZATION : the combination of two or more “native” atomic orbitals on an atom to produce “hybrid” orbitals RULE: the number of atomic orbitals that are combined must equal the number which are formed All resulting hybrid orbitals are identical.

Note that these are +ive on one side of origin and -ive on the other!

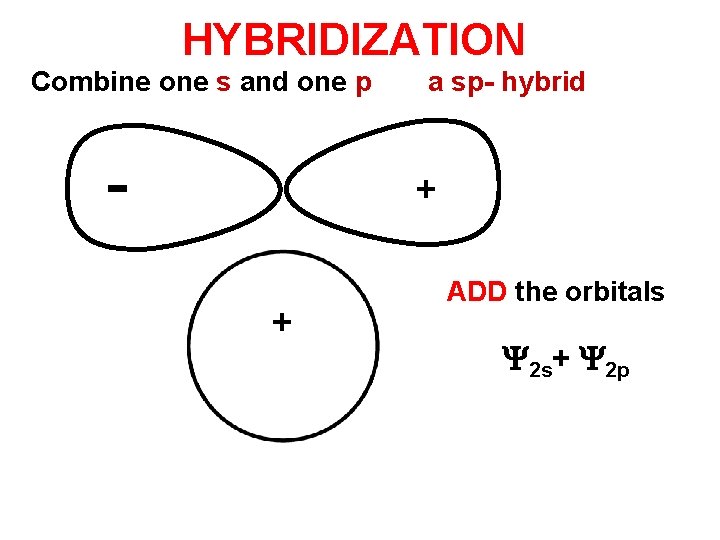
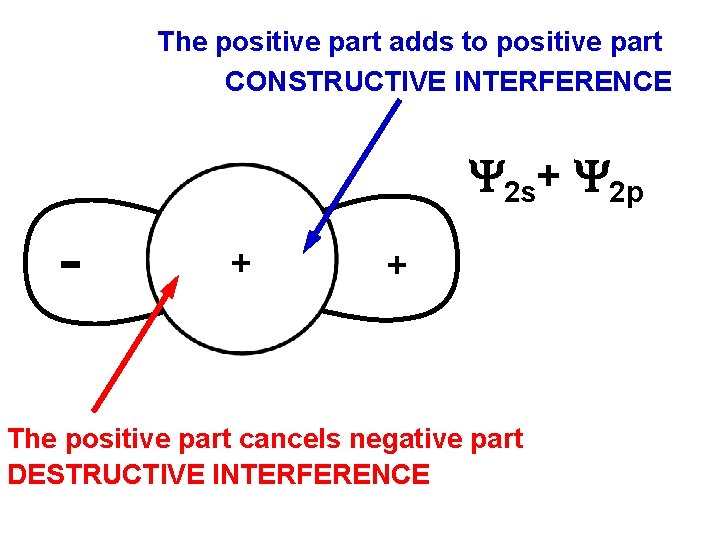
HYBRIDIZATION Combine one s and one p a sp- hybrid + + ADD the orbitals 2 s+ 2 p

The positive part adds to positive part CONSTRUCTIVE INTERFERENCE 2 s+ 2 p + + The positive part cancels negative part DESTRUCTIVE INTERFERENCE

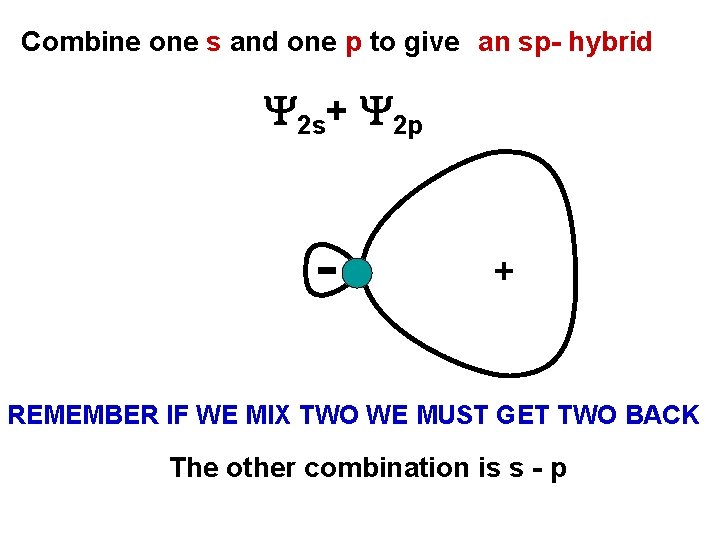
Combine one s and one p to give an sp- hybrid 2 s+ 2 p + REMEMBER IF WE MIX TWO WE MUST GET TWO BACK The other combination is s - p

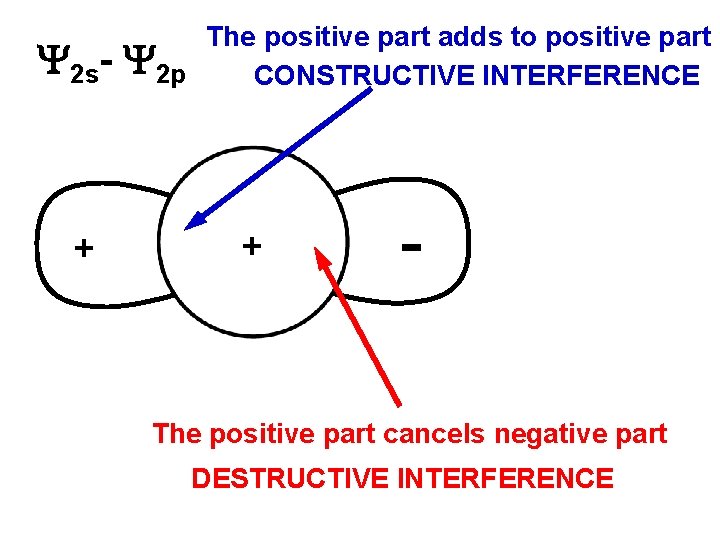
The positive part adds to positive part 2 s- 2 p CONSTRUCTIVE INTERFERENCE + + The positive part cancels negative part DESTRUCTIVE INTERFERENCE

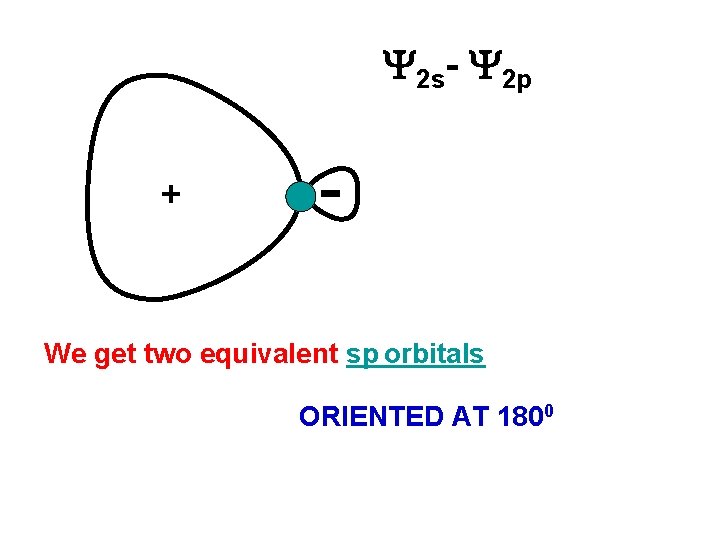
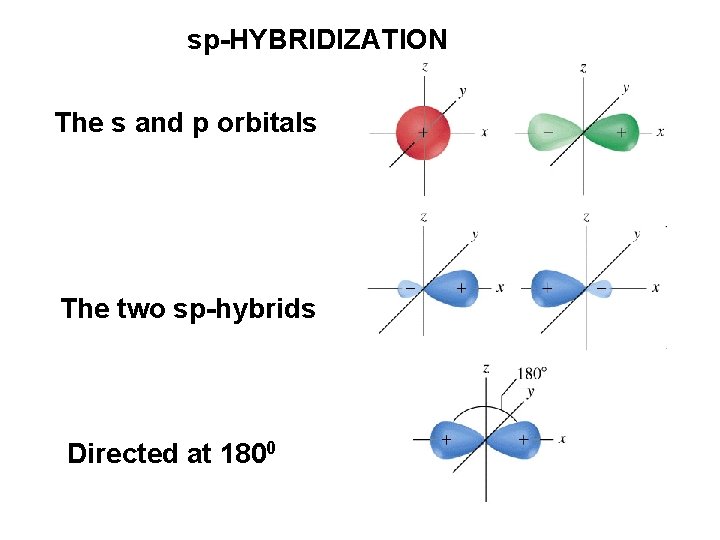
2 s- 2 p + We get two equivalent sp orbitals ORIENTED AT 1800

sp-HYBRIDIZATION The s and p orbitals The two sp-hybrids Directed at 1800

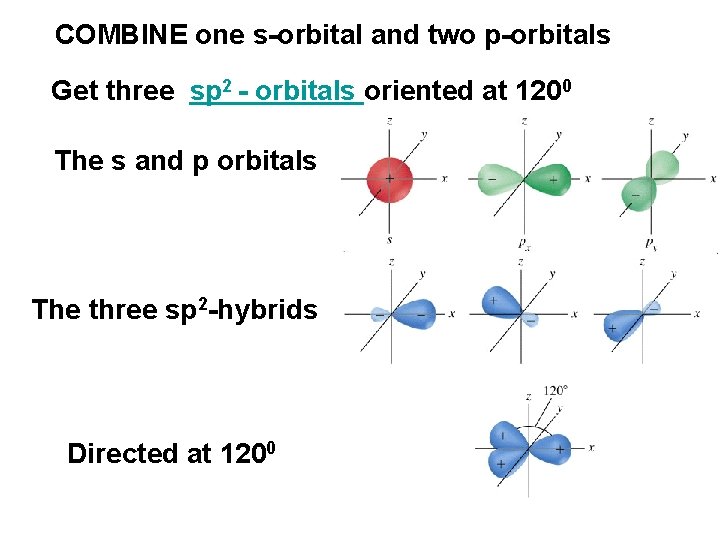
COMBINE one s-orbital and two p-orbitals Get three sp 2 - orbitals oriented at 1200 The s and p orbitals The three sp 2 -hybrids Directed at 1200

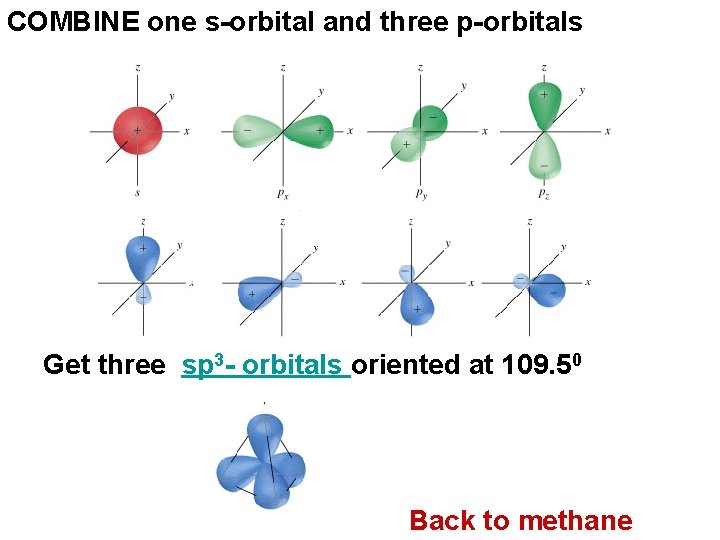
COMBINE one s-orbital and three p-orbitals Get three sp 3 - orbitals oriented at 109. 50 Back to methane

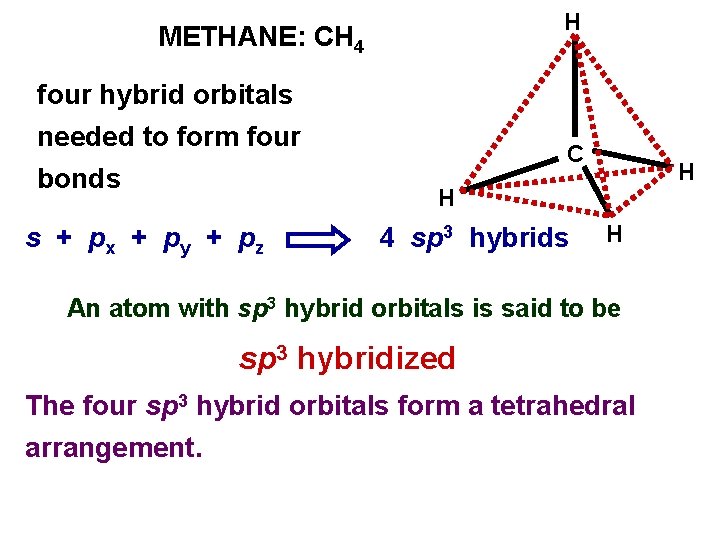
H METHANE: CH 4 four hybrid orbitals needed to form four bonds s + px + py + pz C H H 4 sp 3 hybrids H An atom with sp 3 hybrid orbitals is said to be sp 3 hybridized The four sp 3 hybrid orbitals form a tetrahedral arrangement.

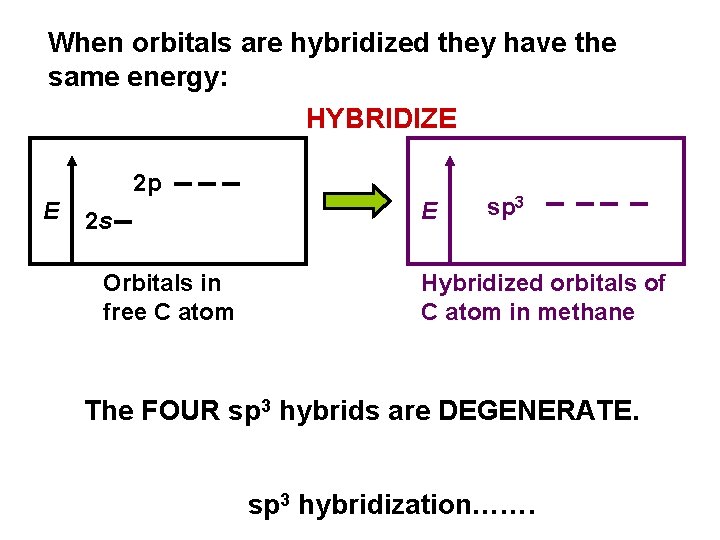
When orbitals are hybridized they have the same energy: HYBRIDIZE 2 p E 2 s Orbitals in free C atom E sp 3 Hybridized orbitals of C atom in methane The FOUR sp 3 hybrids are DEGENERATE. sp 3 hybridization…….

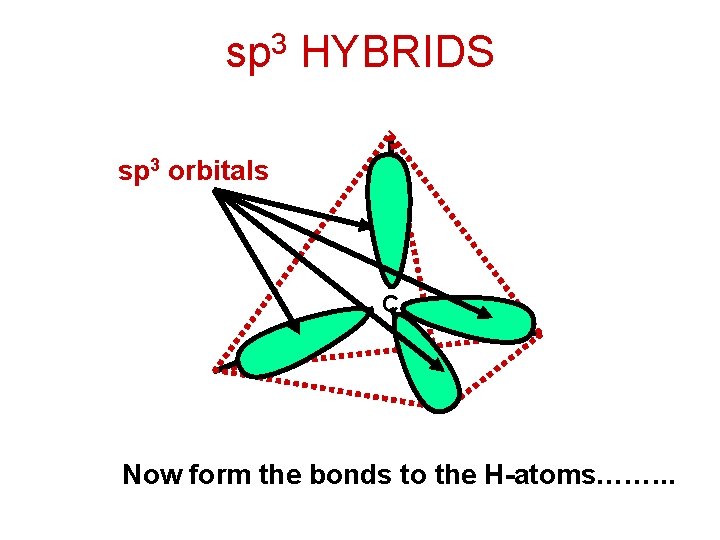
sp 3 HYBRIDS sp 3 orbitals C Now form the bonds to the H-atoms……. . .

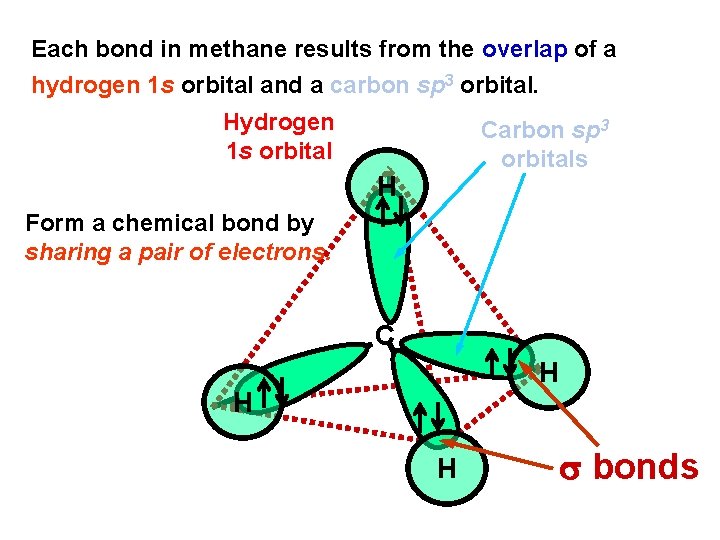
Each bond in methane results from the overlap of a hydrogen 1 s orbital and a carbon sp 3 orbital. Hydrogen 1 s orbital Carbon sp 3 orbitals H Form a chemical bond by sharing a pair of electrons. C H H H bonds

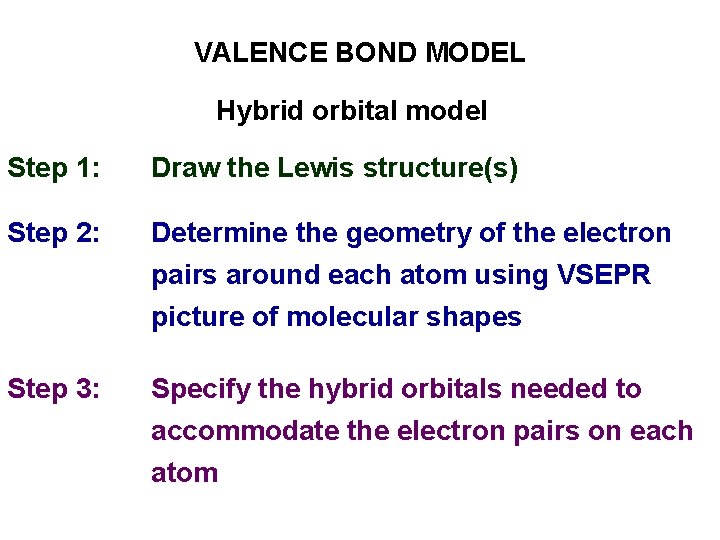
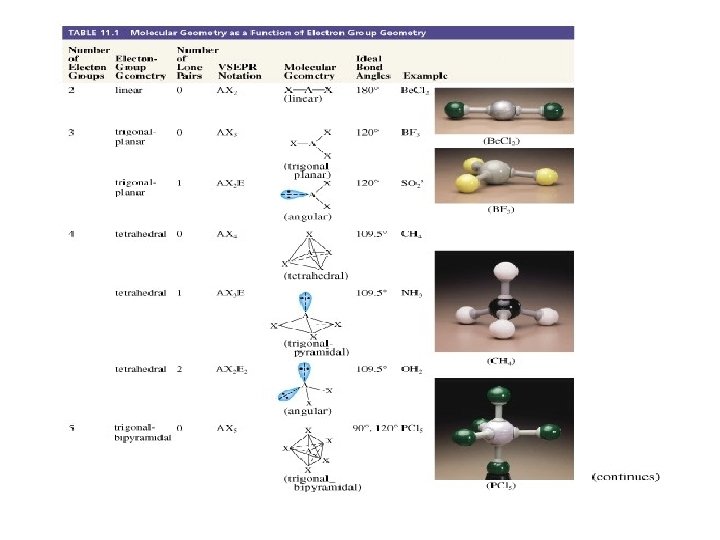
VALENCE BOND MODEL Hybrid orbital model Step 1: Draw the Lewis structure(s) Step 2: Determine the geometry of the electron pairs around each atom using VSEPR picture of molecular shapes Step 3: Specify the hybrid orbitals needed to accommodate the electron pairs on each atom

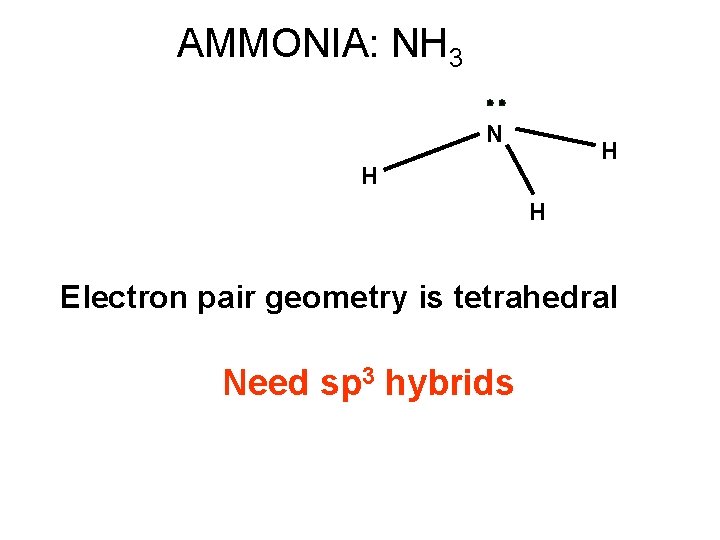
AMMONIA: NH 3 N H H H Electron pair geometry is tetrahedral Need sp 3 hybrids

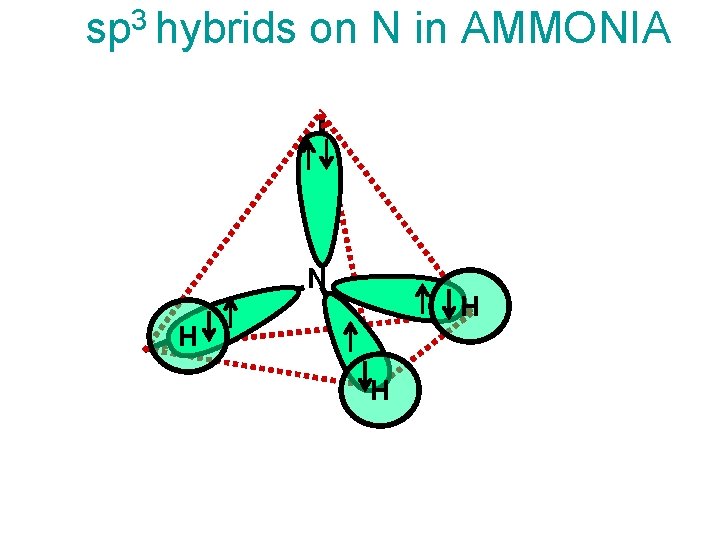
sp 3 hybrids on N in AMMONIA N H H H

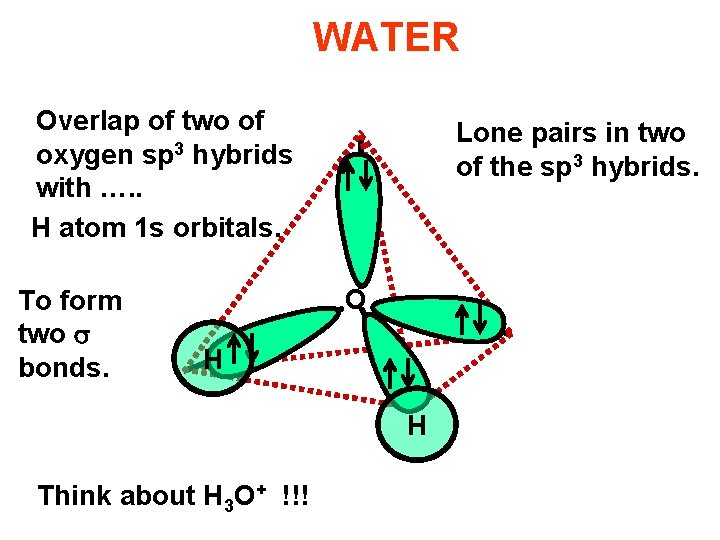
WATER Overlap of two of oxygen sp 3 hybrids with …. . H atom 1 s orbitals. To form two bonds. Lone pairs in two of the sp 3 hybrids. O H H Think about H 3 O+ !!!

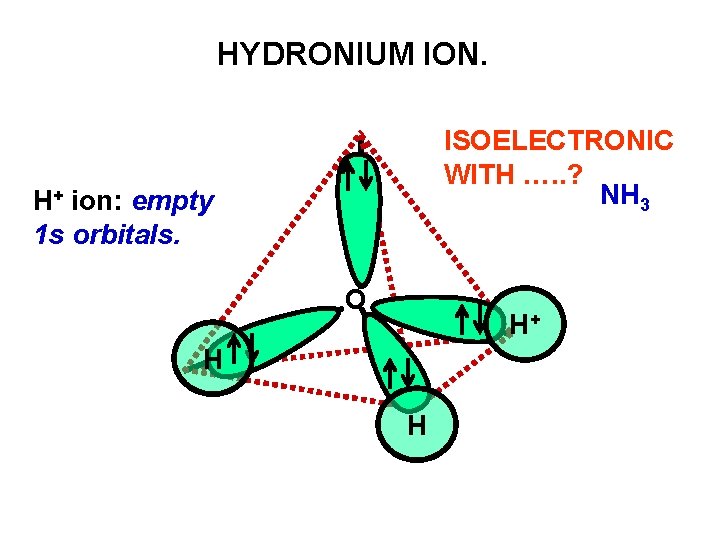
HYDRONIUM ION. ISOELECTRONIC WITH …. . ? NH 3 H+ ion: empty 1 s orbitals. O H+ H H

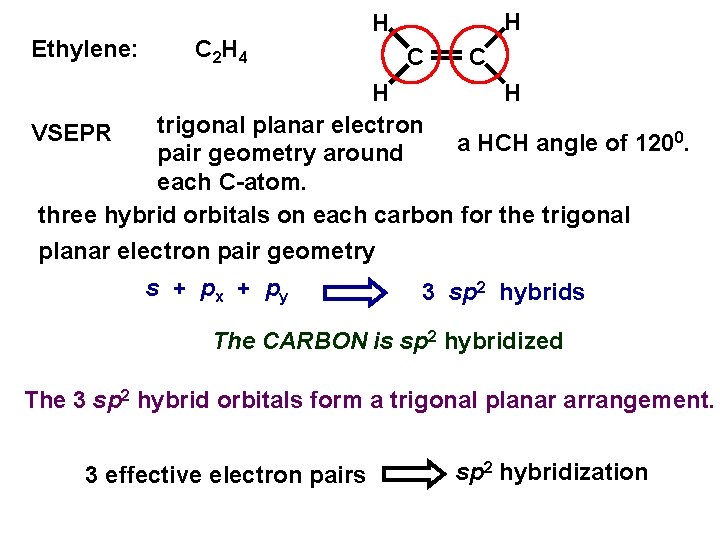
Ethylene: C 2 H 4 H H C C H H trigonal planar electron VSEPR 0. a HCH angle of 120 pair geometry around each C-atom. three hybrid orbitals on each carbon for the trigonal planar electron pair geometry s + px + py 3 sp 2 hybrids The CARBON is sp 2 hybridized The 3 sp 2 hybrid orbitals form a trigonal planar arrangement. 3 effective electron pairs sp 2 hybridization

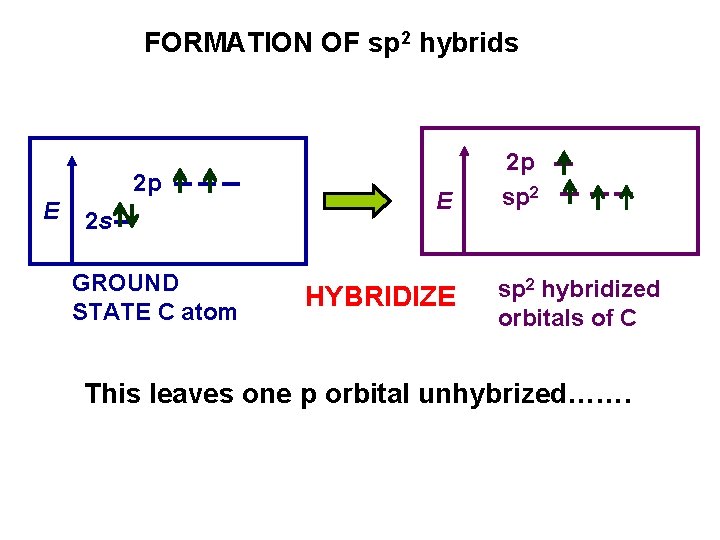
FORMATION OF sp 2 hybrids 2 p E 2 s GROUND STATE C atom E HYBRIDIZE 2 p sp 2 hybridized orbitals of C This leaves one p orbital unhybrized…….

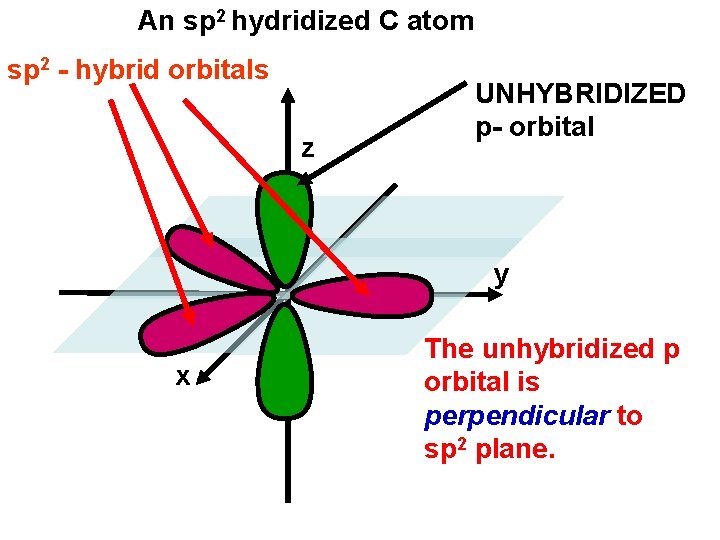
An sp 2 hydridized C atom sp 2 - hybrid orbitals z UNHYBRIDIZED p- orbital y x The unhybridized p orbital is perpendicular to sp 2 plane.

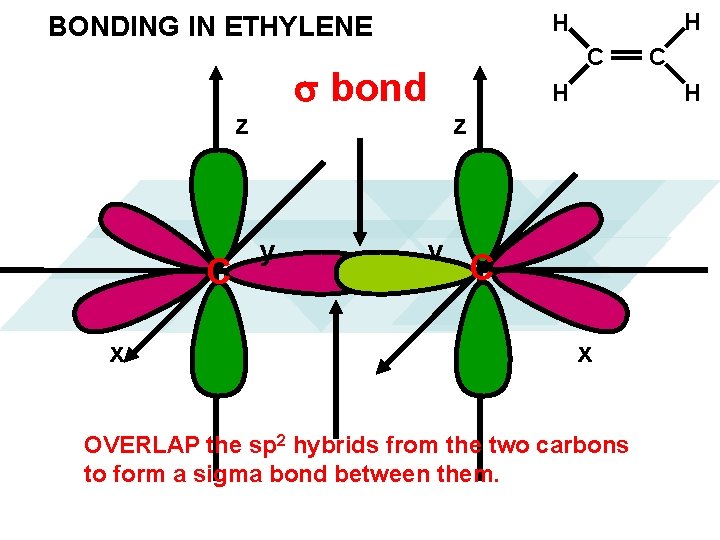
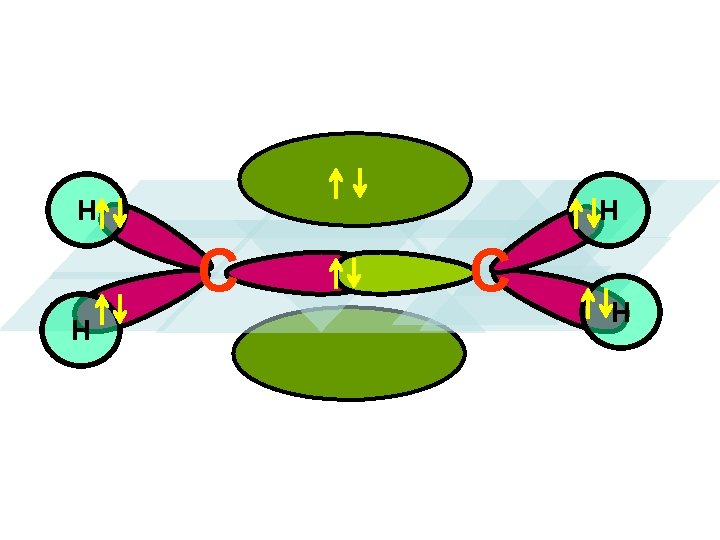
bond z C x y H H BONDING IN ETHYLENE y C C H H z C x OVERLAP the sp 2 hybrids from the two carbons to form a sigma bond between them.

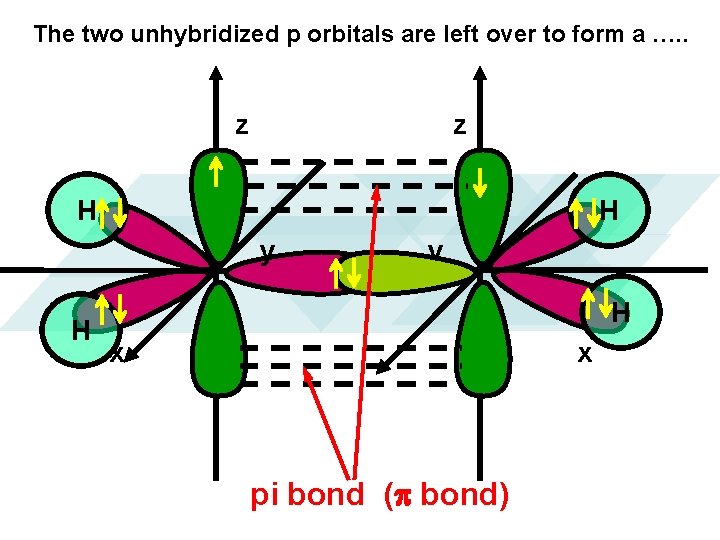
The two unhybridized p orbitals are left over to form a …. . z z H H y H x x pi bond (p bond)

H H C H

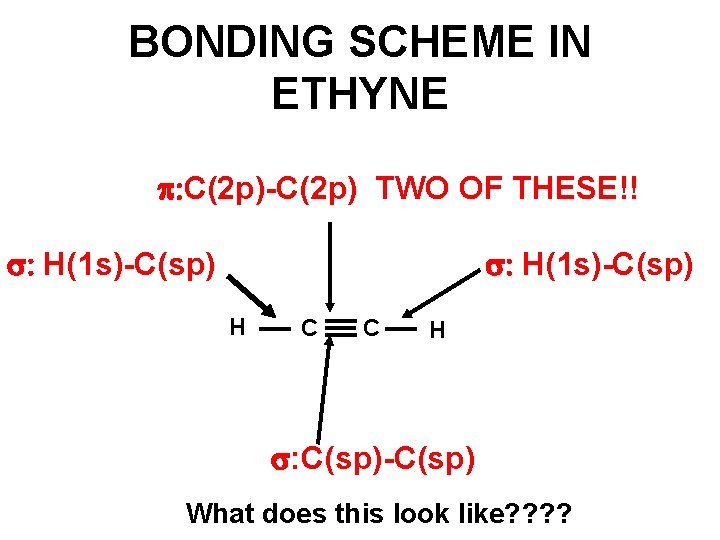
BONDING SCHEME IN ETHYNE p: C(2 p)-C(2 p) TWO OF THESE!! : H(1 s)-C(sp) H C C H : C(sp)-C(sp) What does this look like? ?

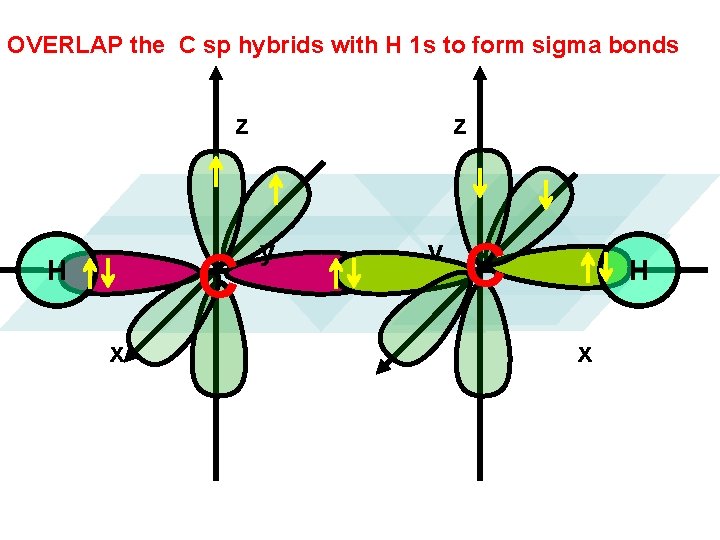
OVERLAP the C sp hybrids with H 1 s to form sigma bonds z C H x z y y C H x

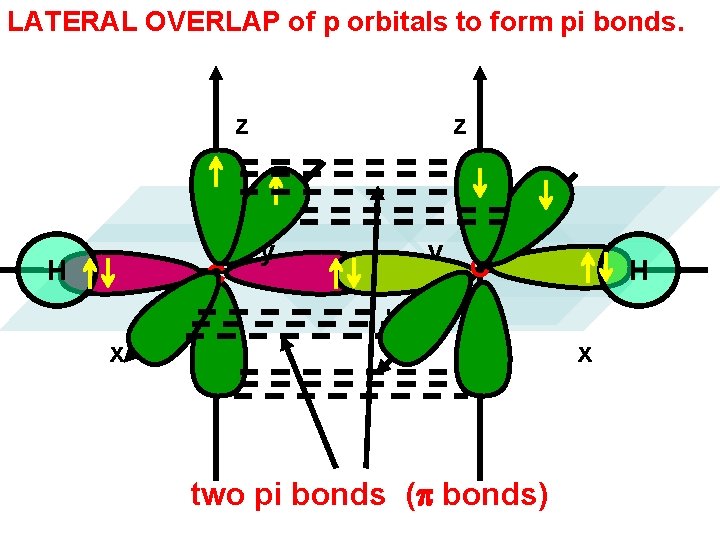
LATERAL OVERLAP of p orbitals to form pi bonds. z H C z y y C x H x two pi bonds (p bonds)

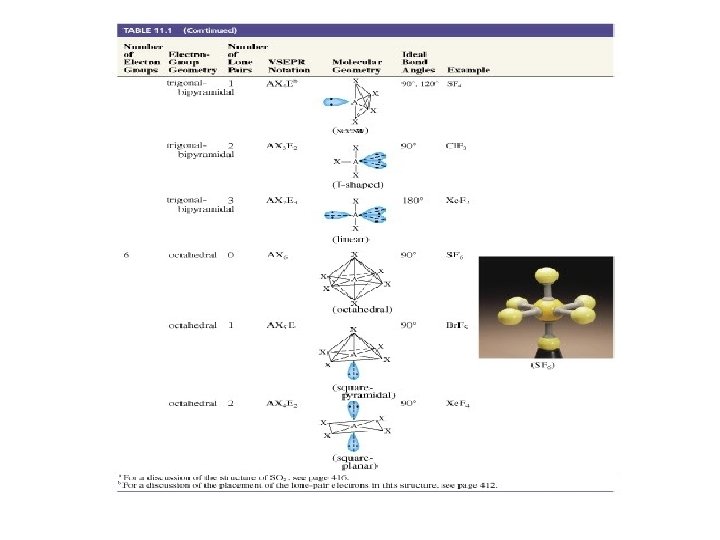
What about molecules with more than an octet around the central atom? Examples: PCl 5, or SF 4 or Si. F 62 - etc…. . Four pairs needs Four orbitals Five pairs needs Five orbitals six pairs needs six orbitals So? ? ?

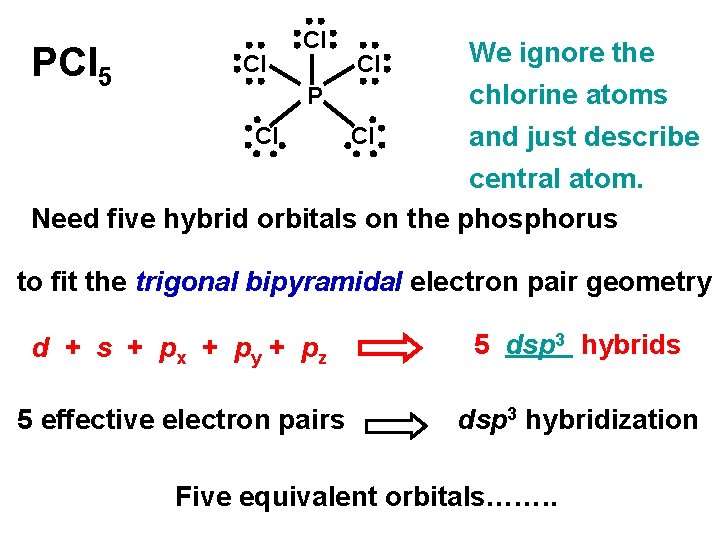
PCl 5 Cl Cl Cl We ignore the chlorine atoms Cl Cl and just describe central atom. Need five hybrid orbitals on the phosphorus P to fit the trigonal bipyramidal electron pair geometry d + s + px + py + pz 5 dsp 3 hybrids 5 effective electron pairs dsp 3 hybridization Five equivalent orbitals……. .

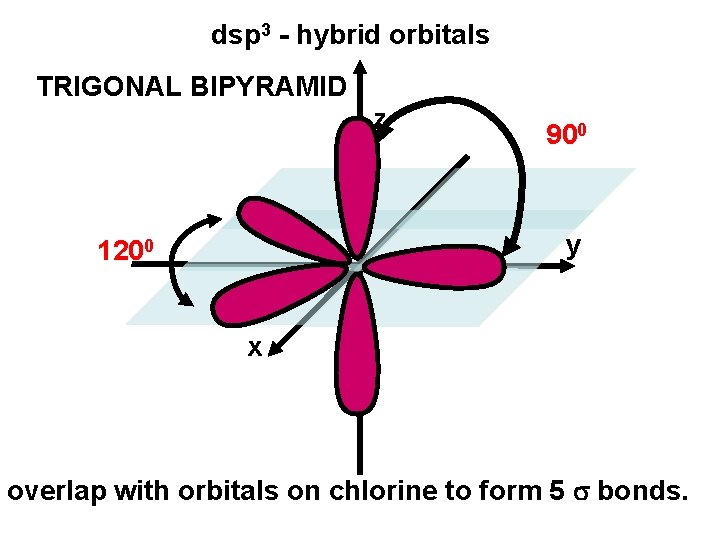
dsp 3 - hybrid orbitals TRIGONAL BIPYRAMID z 900 y 1200 x overlap with orbitals on chlorine to form 5 bonds.

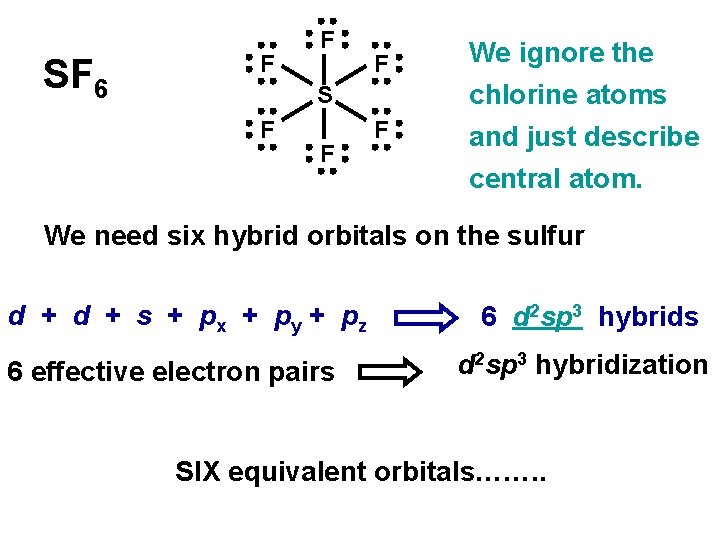
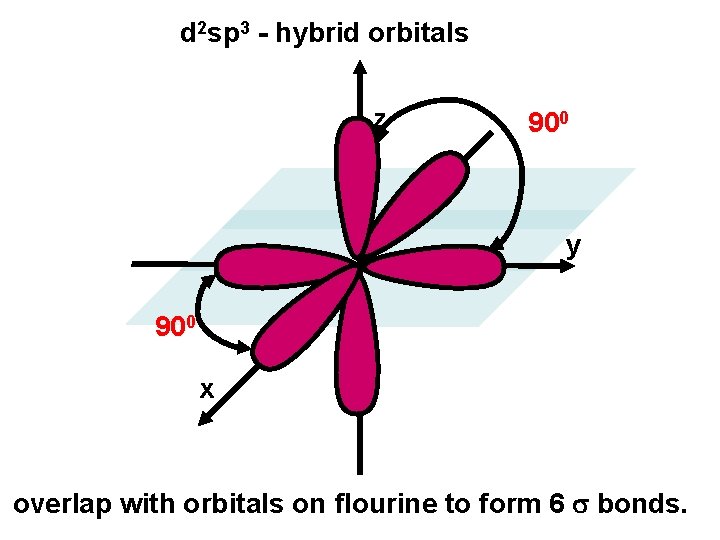
SF 6 F F F S F F F We ignore the chlorine atoms and just describe central atom. We need six hybrid orbitals on the sulfur d + s + px + py + pz 6 effective electron pairs 6 d 2 sp 3 hybrids d 2 sp 3 hybridization SIX equivalent orbitals……. .

d 2 sp 3 - hybrid orbitals z 900 y 900 x overlap with orbitals on flourine to form 6 bonds.




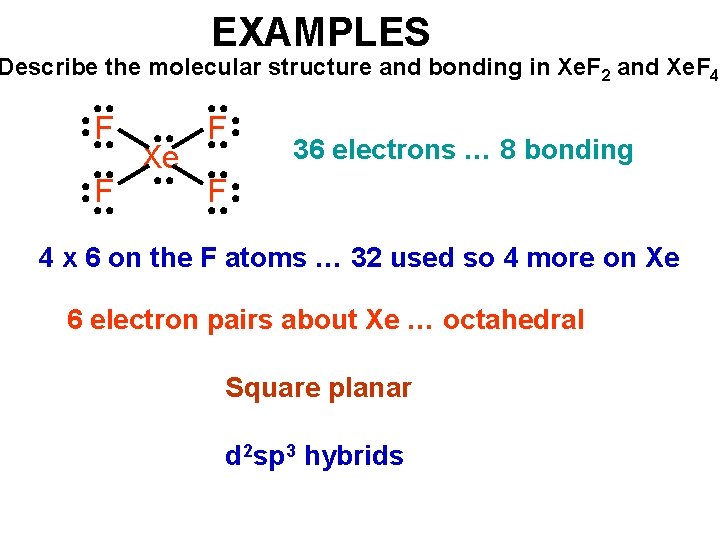
EXAMPLES Describe the molecular structure and bonding in Xe. F 2 and Xe. F 4 F F Xe F 36 electrons … 8 bonding F 4 x 6 on the F atoms … 32 used so 4 more on Xe 6 electron pairs about Xe … octahedral Square planar d 2 sp 3 hybrids

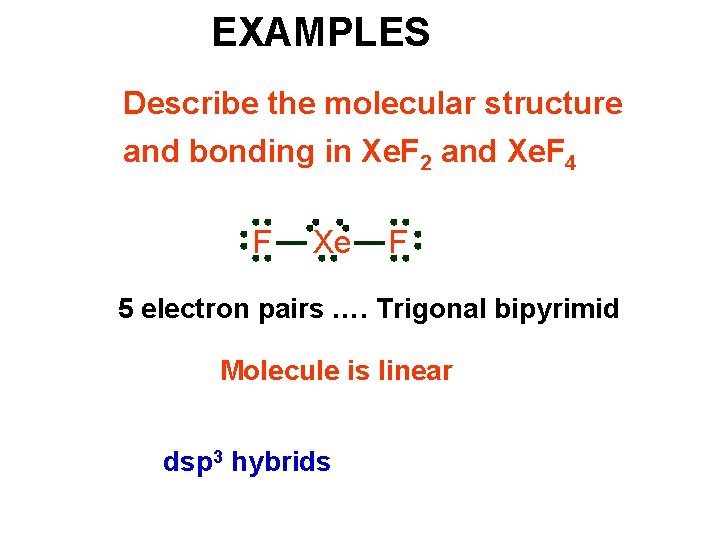
EXAMPLES Describe the molecular structure and bonding in Xe. F 2 and Xe. F 4 F Xe F 5 electron pairs …. Trigonal bipyrimid Molecule is linear dsp 3 hybrids