AljalalPhys 102 142 Ch 18 page 1 Chapter
- Slides: 61

Aljalal-Phys 102 - 142 -Ch 18 -page 1 Chapter 18 Temperature, Heat, and the First Law of Thermodynamics Objective 18 -1 Temperature 18 -2 The Celsius and Fahrenheit scales 18 -3 Thermal expansion 18 -4 Absorption of heat 18 -5 The First Law of Thermodynamics 18 -6 Heat Transfer Mechanisms

18 -1 Temperature What is thermodynamics? Aljalal-Phys 102 - 142 -Ch 18 -page 2 Thermodynamics is the study of thermal energy (internal energy) of systems. Temperature is one of the central concept of thermodynamics.

18 -1 Temperature Thermal equilibrium and temperature Aljalal-Phys 102 - 142 -Ch 18 -page 3 The zeroth law of thermodynamics Every body has a property called temperature. When two bodies are in thermal equilibrium, their temperatures are equal. And vice versa.

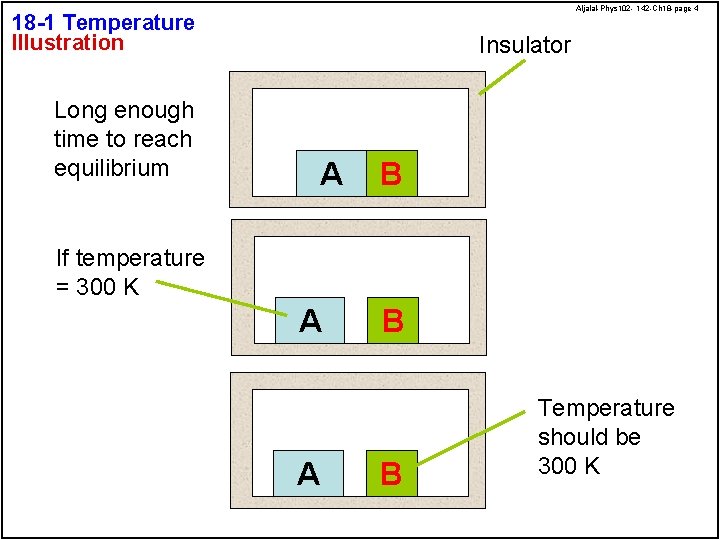
Aljalal-Phys 102 - 142 -Ch 18 -page 4 18 -1 Temperature Illustration Long enough time to reach equilibrium Insulator A B If temperature = 300 K A A B B Temperature should be 300 K

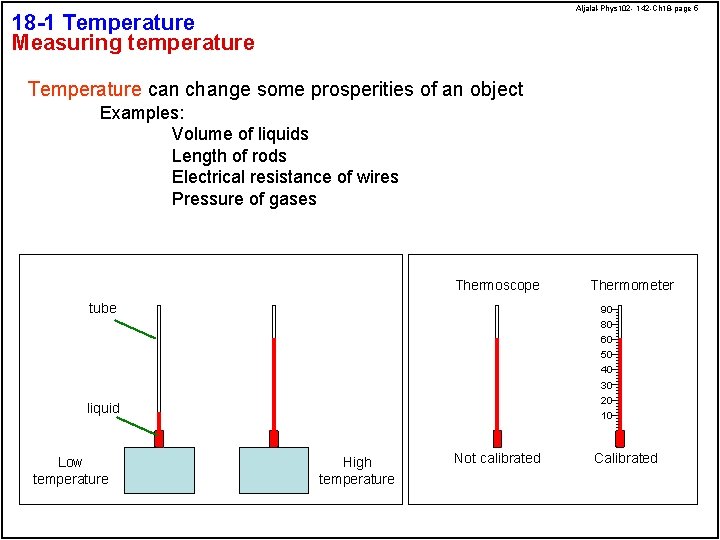
Aljalal-Phys 102 - 142 -Ch 18 -page 5 18 -1 Temperature Measuring temperature Temperature can change some prosperities of an object Examples: Volume of liquids Length of rods Electrical resistance of wires Pressure of gases Thermoscope tube Thermometer 90 80 60 50 40 30 20 liquid Low temperature 10 High temperature Not calibrated Calibrated

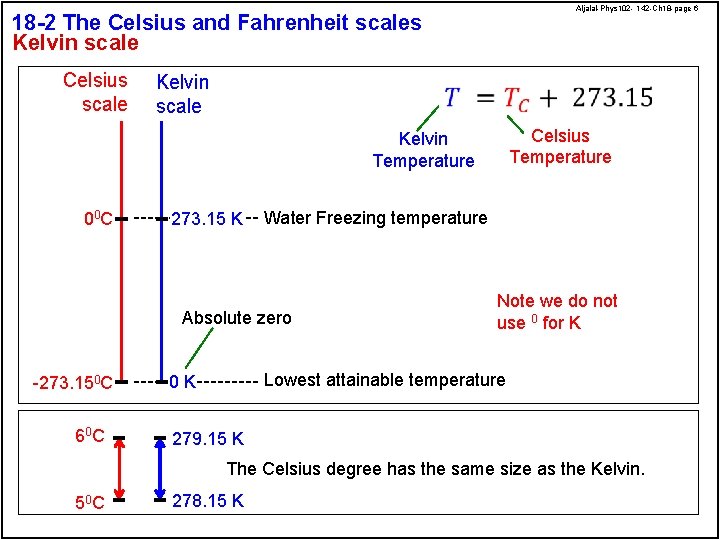
Aljalal-Phys 102 - 142 -Ch 18 -page 6 18 -2 The Celsius and Fahrenheit scales Kelvin scale Celsius scale Kelvin scale Celsius Temperature Kelvin Temperature 00 C 273. 15 K Water Freezing temperature Absolute zero -273. 150 C 60 C Note we do not use 0 for K Lowest attainable temperature 0 K 279. 15 K The Celsius degree has the same size as the Kelvin. 50 C 278. 15 K

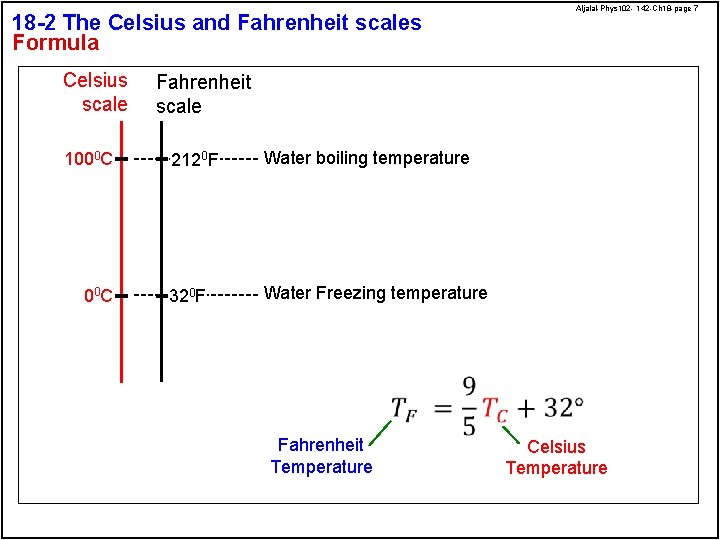
18 -2 The Celsius and Fahrenheit scales Formula Celsius scale 1000 C 00 C Aljalal-Phys 102 - 142 -Ch 18 -page 7 Fahrenheit scale 2120 F Water boiling temperature 320 F Water Freezing temperature Fahrenheit Temperature Celsius Temperature

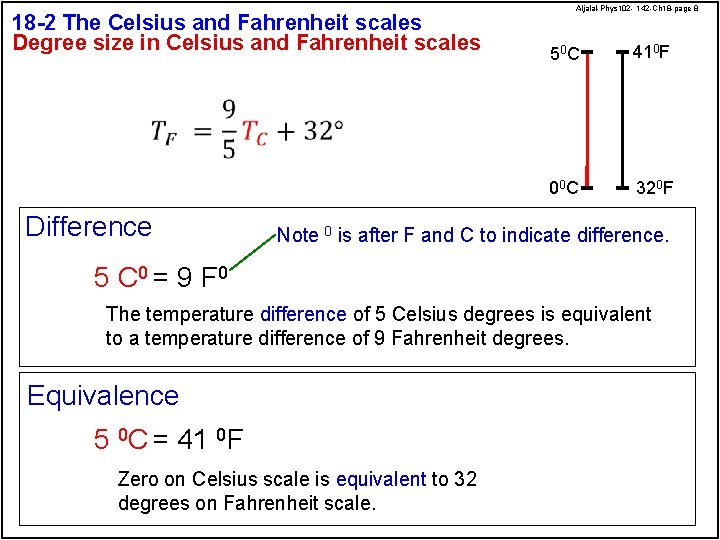
18 -2 The Celsius and Fahrenheit scales Degree size in Celsius and Fahrenheit scales Difference Aljalal-Phys 102 - 142 -Ch 18 -page 8 50 C 410 F 00 C 320 F Note 0 is after F and C to indicate difference. 5 C 0 = 9 F 0 The temperature difference of 5 Celsius degrees is equivalent to a temperature difference of 9 Fahrenheit degrees. Equivalence 5 0 C = 41 0 F Zero on Celsius scale is equivalent to 32 degrees on Fahrenheit scale.

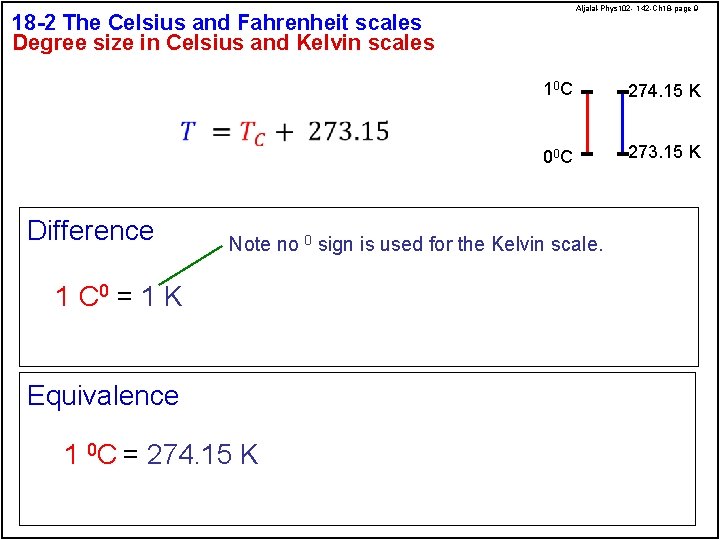
Aljalal-Phys 102 - 142 -Ch 18 -page 9 18 -2 The Celsius and Fahrenheit scales Degree size in Celsius and Kelvin scales Difference 10 C 274. 15 K 00 C 273. 15 K Note no 0 sign is used for the Kelvin scale. 1 C 0 = 1 K Equivalence 1 0 C = 274. 15 K

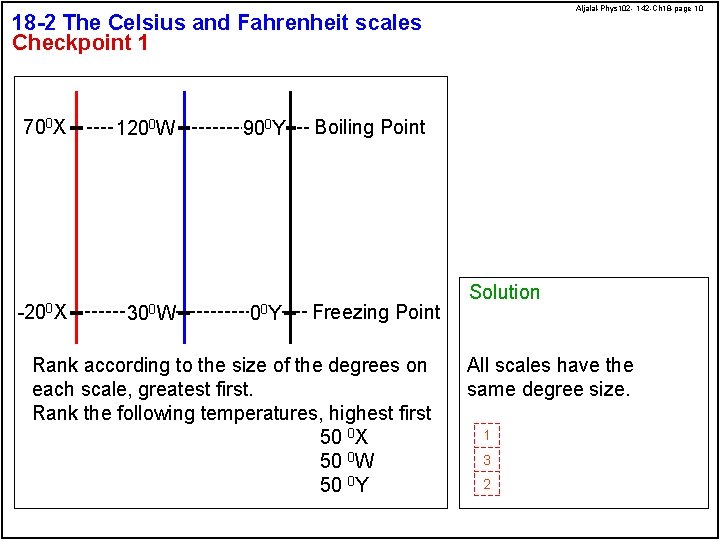
Aljalal-Phys 102 - 142 -Ch 18 -page 10 18 -2 The Celsius and Fahrenheit scales Checkpoint 1 700 X -200 X 1200 W 300 W 900 Y 00 Y Boiling Point Freezing Point Rank according to the size of the degrees on each scale, greatest first. Rank the following temperatures, highest first 50 0 X 50 0 W 50 0 Y Solution All scales have the same degree size. 1 3 2

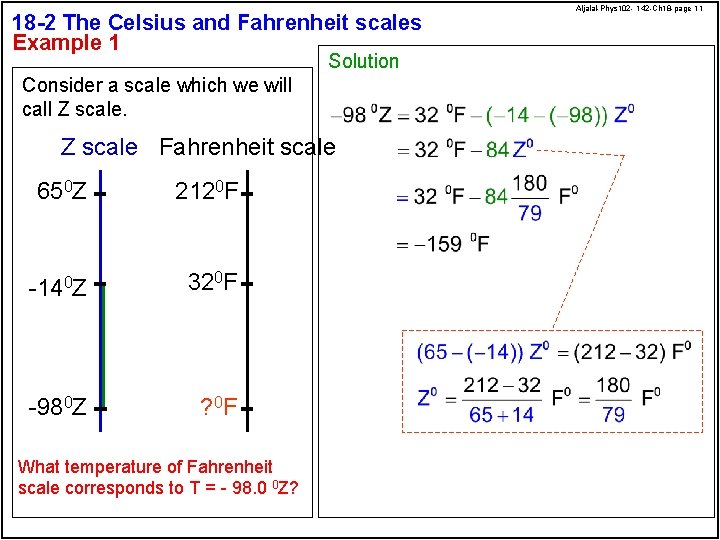
18 -2 The Celsius and Fahrenheit scales Example 1 Solution Consider a scale which we will call Z scale Fahrenheit scale 650 Z 2120 F -140 Z 320 F -980 Z ? 0 F What temperature of Fahrenheit scale corresponds to T = - 98. 0 0 Z? Aljalal-Phys 102 - 142 -Ch 18 -page 11

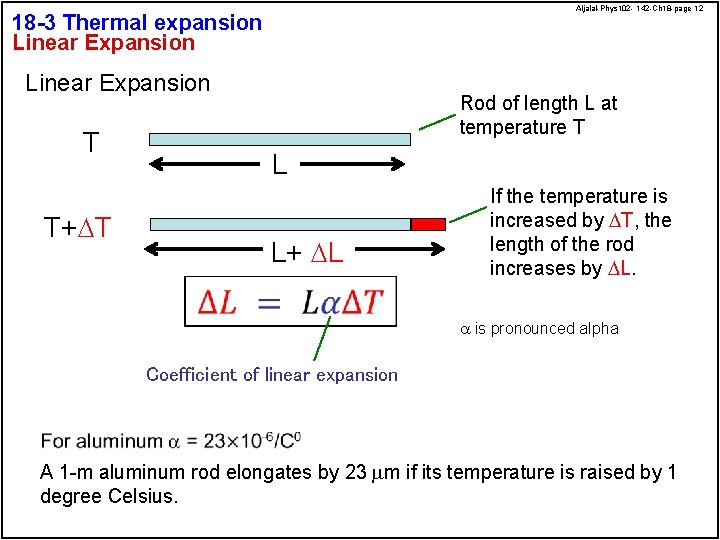
Aljalal-Phys 102 - 142 -Ch 18 -page 12 18 -3 Thermal expansion Linear Expansion T T+DT Rod of length L at temperature T L L+ DL If the temperature is increased by DT, the length of the rod increases by DL. a is pronounced alpha Coefficient of linear expansion A 1 -m aluminum rod elongates by 23 mm if its temperature is raised by 1 degree Celsius.

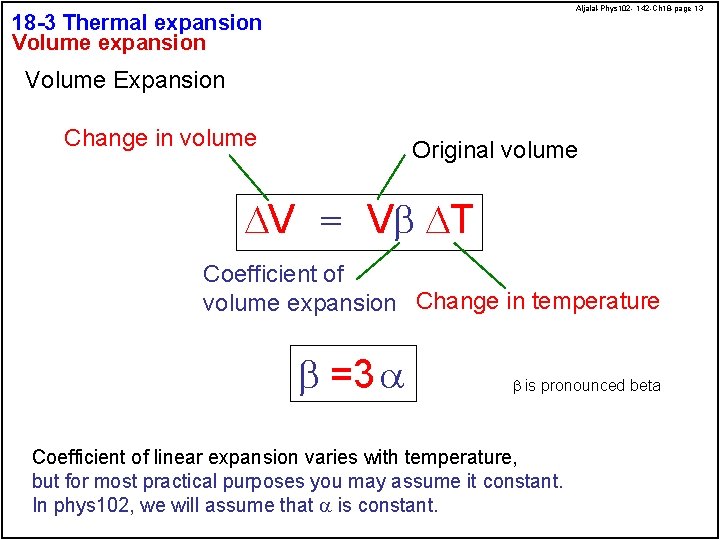
Aljalal-Phys 102 - 142 -Ch 18 -page 13 18 -3 Thermal expansion Volume Expansion Change in volume Original volume DV = Vb DT Coefficient of volume expansion Change in temperature b =3 a b is pronounced beta Coefficient of linear expansion varies with temperature, but for most practical purposes you may assume it constant. In phys 102, we will assume that a is constant.

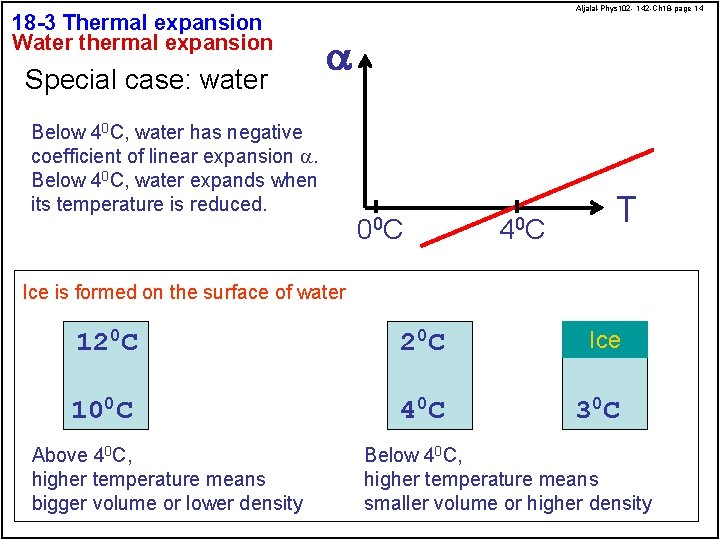
18 -3 Thermal expansion Water thermal expansion Special case: water Aljalal-Phys 102 - 142 -Ch 18 -page 14 a Below 40 C, water has negative coefficient of linear expansion a. Below 40 C, water expands when its temperature is reduced. 0 0 C 4 0 C T Ice is formed on the surface of water 120 C 2 0 C Ice 100 C 4 0 C 3 0 C Above 40 C, higher temperature means bigger volume or lower density Below 40 C, higher temperature means smaller volume or higher density

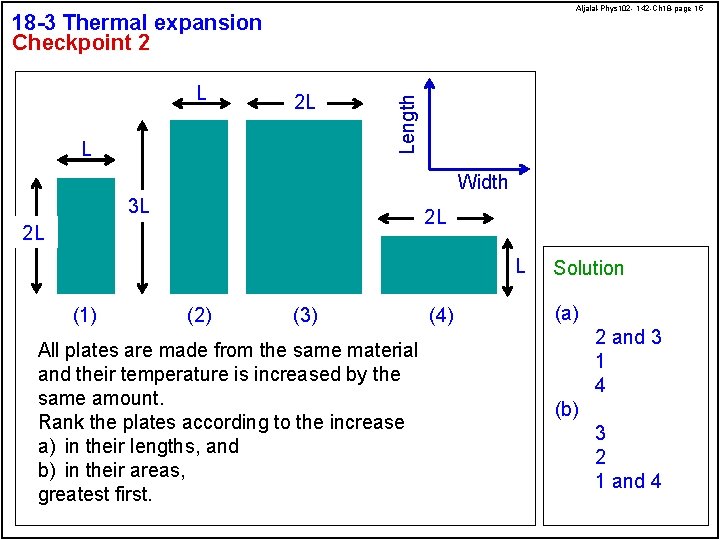
Aljalal-Phys 102 - 142 -Ch 18 -page 15 L 2 L L Length 18 -3 Thermal expansion Checkpoint 2 Width 3 L 2 L 2 L L (1) (2) (3) All plates are made from the same material and their temperature is increased by the same amount. Rank the plates according to the increase a) in their lengths, and b) in their areas, greatest first. (4) Solution (a) 2 and 3 1 4 (b) 3 2 1 and 4

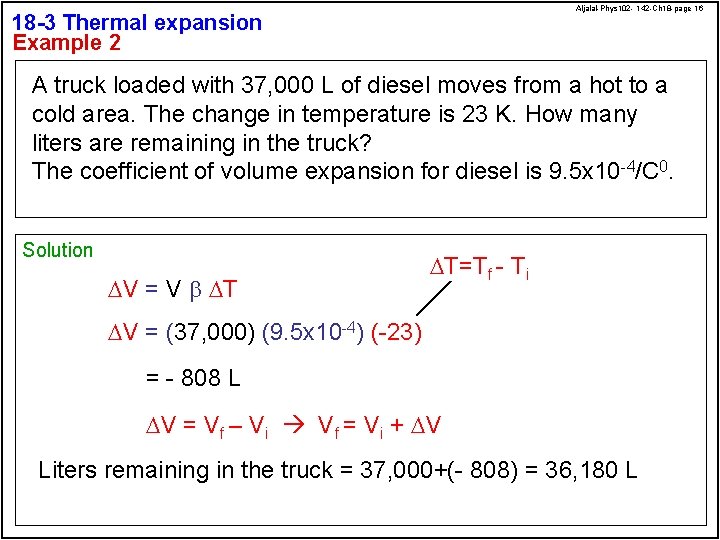
Aljalal-Phys 102 - 142 -Ch 18 -page 16 18 -3 Thermal expansion Example 2 A truck loaded with 37, 000 L of diesel moves from a hot to a cold area. The change in temperature is 23 K. How many liters are remaining in the truck? The coefficient of volume expansion for diesel is 9. 5 x 10 -4/C 0. Solution DV = V b DT DT=Tf - Ti DV = (37, 000) (9. 5 x 10 -4) (-23) = - 808 L DV = Vf – Vi Vf = Vi + DV Liters remaining in the truck = 37, 000+(- 808) = 36, 180 L

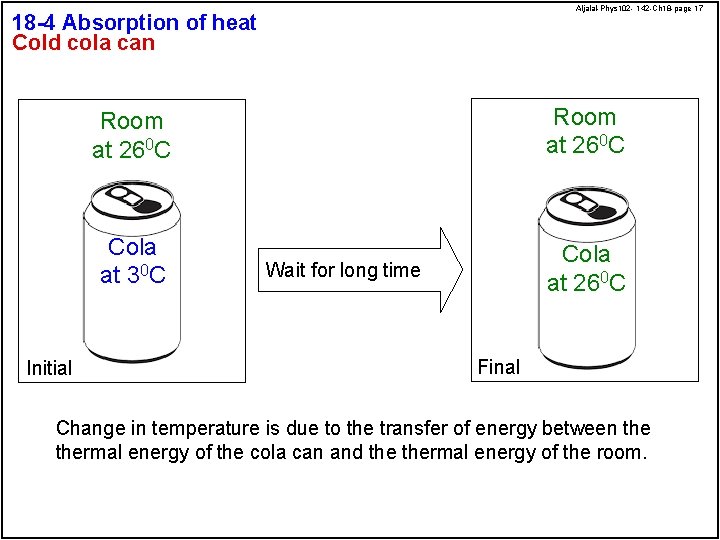
Aljalal-Phys 102 - 142 -Ch 18 -page 17 18 -4 Absorption of heat Cold cola can Initial Room at 260 C Cola at 30 C Cola at 260 C Wait for long time Final Change in temperature is due to the transfer of energy between thermal energy of the cola can and thermal energy of the room.

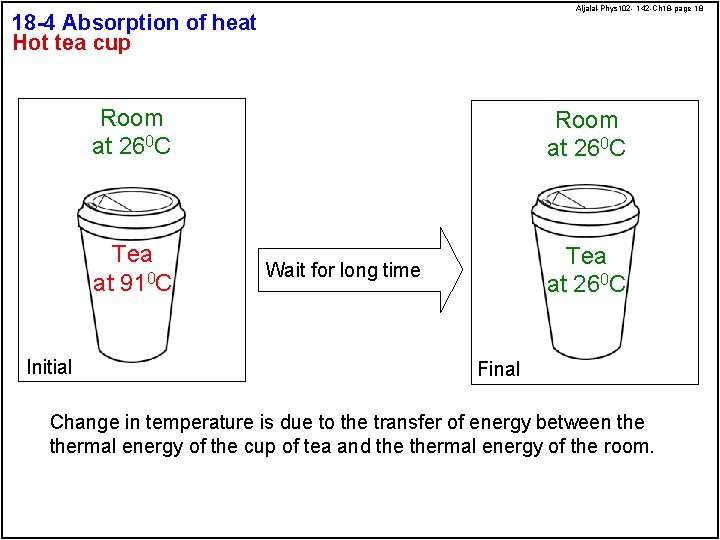
Aljalal-Phys 102 - 142 -Ch 18 -page 18 18 -4 Absorption of heat Hot tea cup Initial Room at 260 C Tea at 910 C Tea at 260 C Wait for long time Final Change in temperature is due to the transfer of energy between thermal energy of the cup of tea and thermal energy of the room.

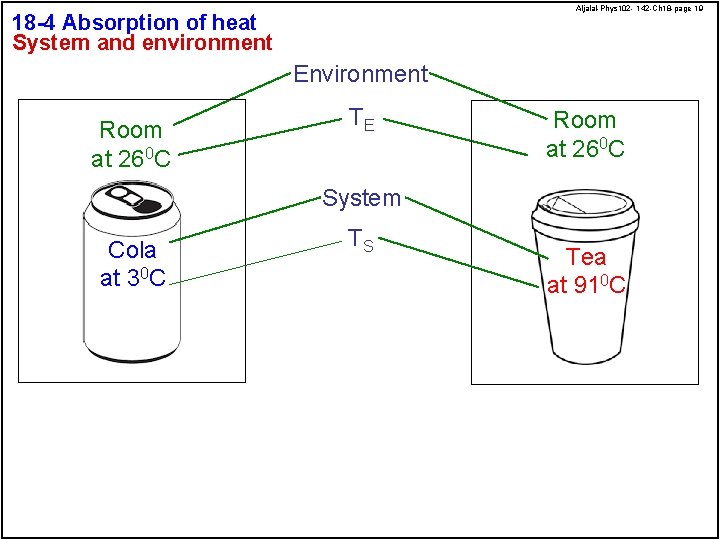
Aljalal-Phys 102 - 142 -Ch 18 -page 19 18 -4 Absorption of heat System and environment Environment Room at 260 C TE Room at 260 C System Cola at 30 C TS Tea at 910 C

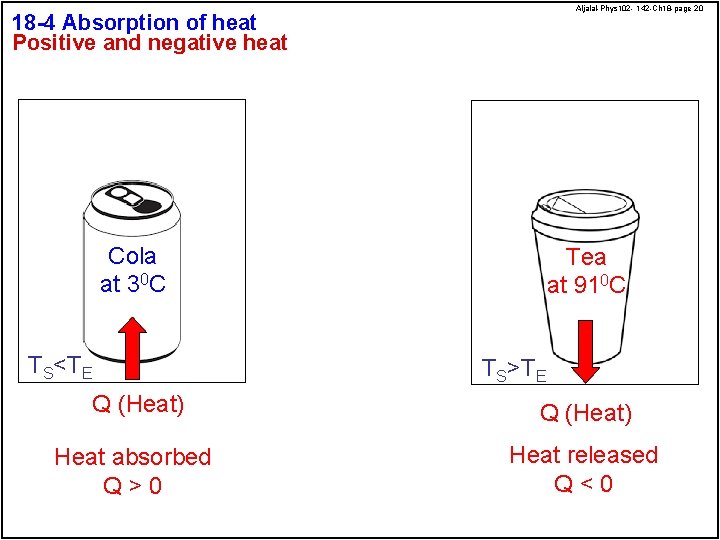
Aljalal-Phys 102 - 142 -Ch 18 -page 20 18 -4 Absorption of heat Positive and negative heat Cola at 30 C TS<TE Tea at 910 C TS>TE Q (Heat) Heat absorbed Q>0 Heat released Q<0

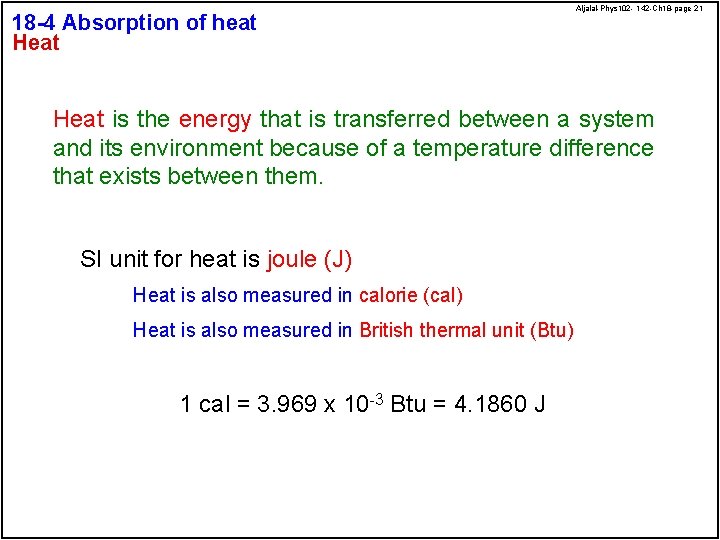
18 -4 Absorption of heat Heat Aljalal-Phys 102 - 142 -Ch 18 -page 21 Heat is the energy that is transferred between a system and its environment because of a temperature difference that exists between them. SI unit for heat is joule (J) Heat is also measured in calorie (cal) Heat is also measured in British thermal unit (Btu) 1 cal = 3. 969 x 10 -3 Btu = 4. 1860 J

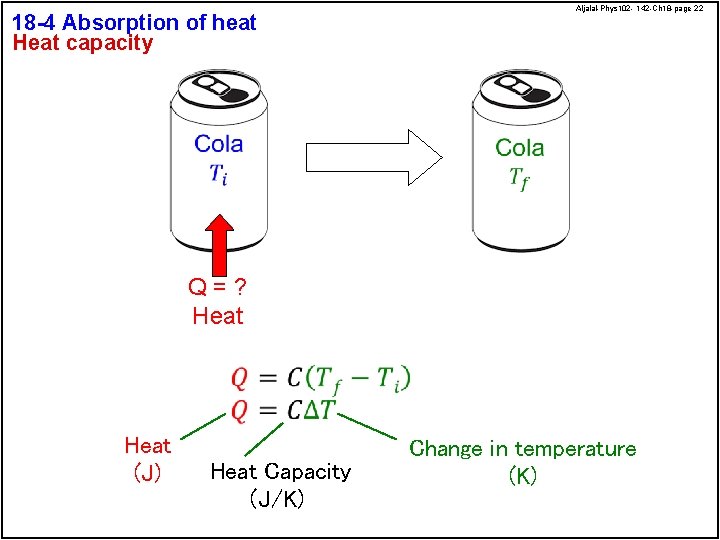
18 -4 Absorption of heat Heat capacity Aljalal-Phys 102 - 142 -Ch 18 -page 22 Q=? Heat (J) Heat Capacity (J/K) Change in temperature (K)

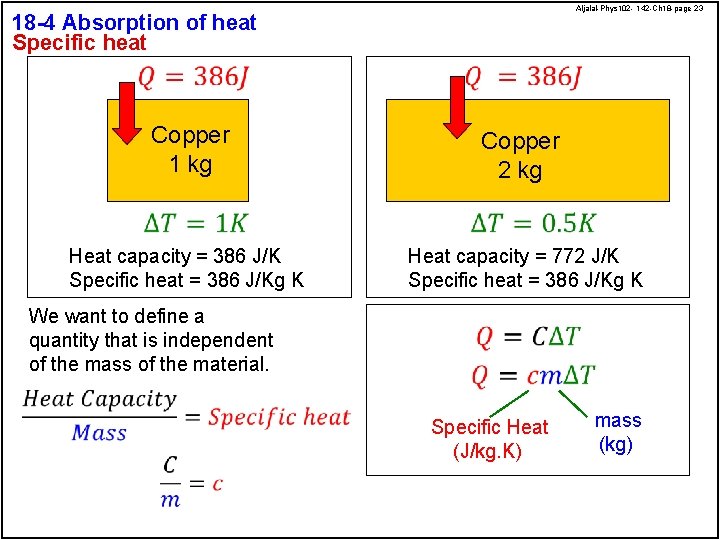
Aljalal-Phys 102 - 142 -Ch 18 -page 23 18 -4 Absorption of heat Specific heat Copper 1 kg Copper 2 kg Heat capacity = 386 J/K Specific heat = 386 J/Kg K Heat capacity = 772 J/K Specific heat = 386 J/Kg K We want to define a quantity that is independent of the mass of the material. Specific Heat (J/kg. K) mass (kg)

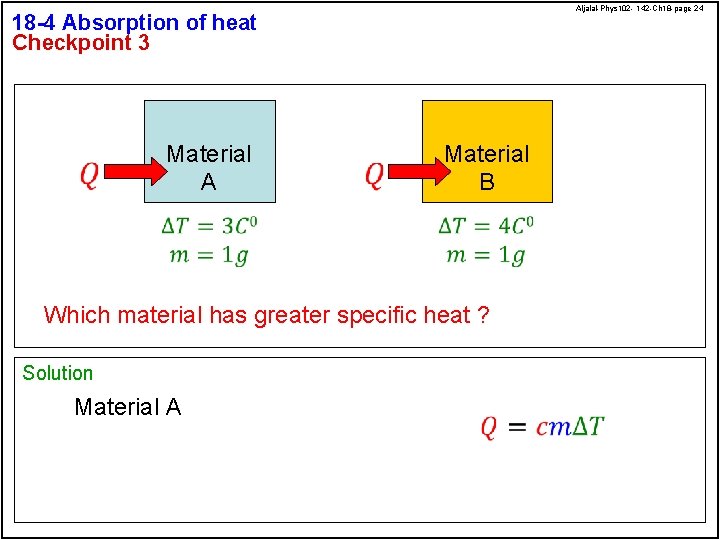
Aljalal-Phys 102 - 142 -Ch 18 -page 24 18 -4 Absorption of heat Checkpoint 3 Material A Material B Which material has greater specific heat ? Solution Material A

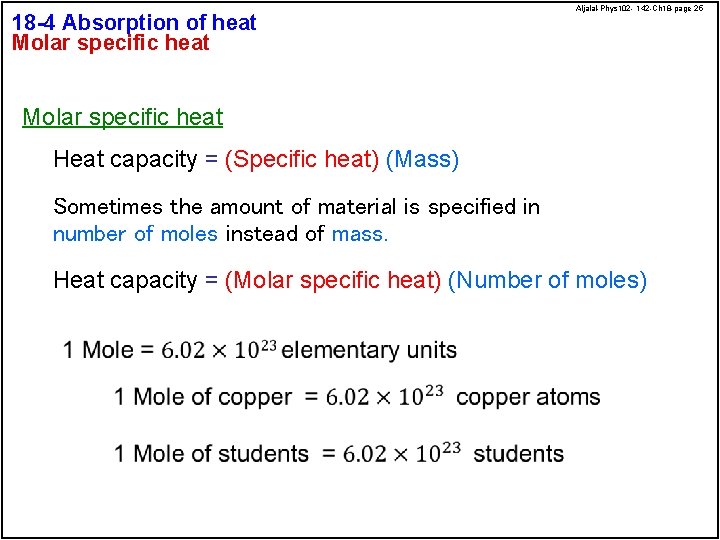
18 -4 Absorption of heat Molar specific heat Aljalal-Phys 102 - 142 -Ch 18 -page 25 Molar specific heat Heat capacity = (Specific heat) (Mass) Sometimes the amount of material is specified in number of moles instead of mass. Heat capacity = (Molar specific heat) (Number of moles)

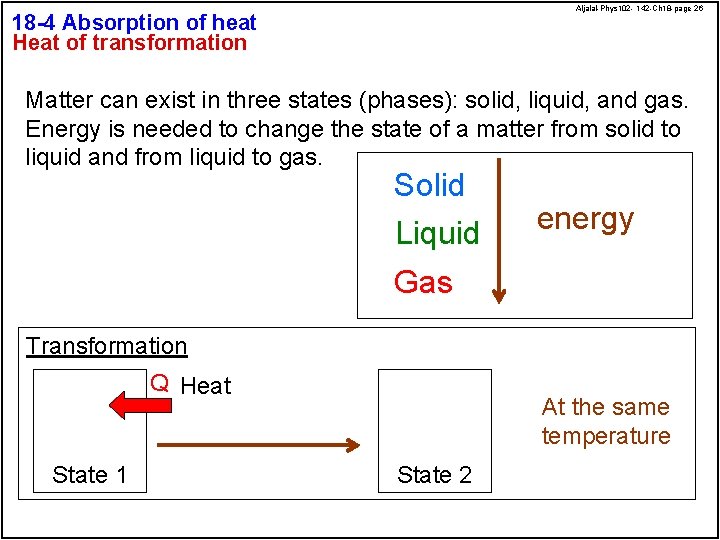
Aljalal-Phys 102 - 142 -Ch 18 -page 26 18 -4 Absorption of heat Heat of transformation Matter can exist in three states (phases): solid, liquid, and gas. Energy is needed to change the state of a matter from solid to liquid and from liquid to gas. Solid Liquid energy Gas Transformation Q Heat State 1 At the same temperature State 2

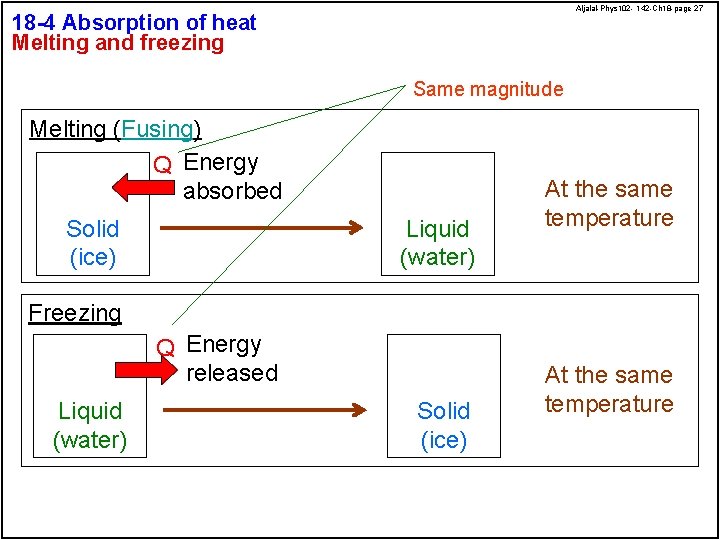
Aljalal-Phys 102 - 142 -Ch 18 -page 27 18 -4 Absorption of heat Melting and freezing Same magnitude Melting (Fusing) Q Energy absorbed Solid (ice) Liquid (water) At the same temperature Freezing Q Energy released Liquid (water) Solid (ice) At the same temperature

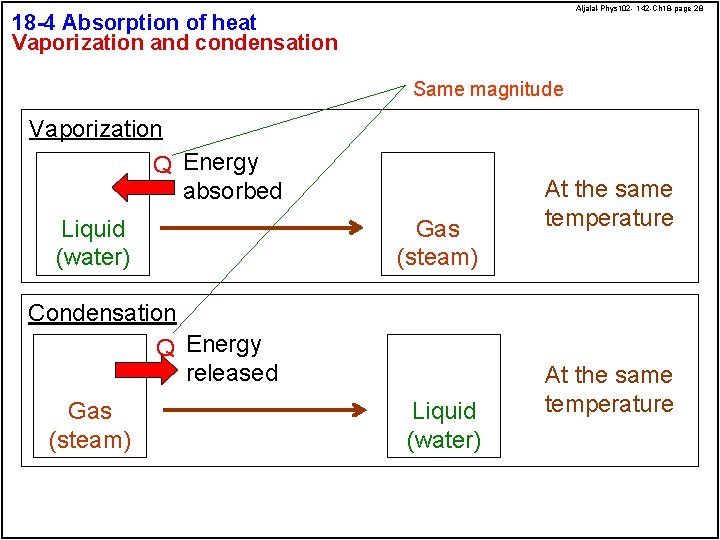
Aljalal-Phys 102 - 142 -Ch 18 -page 28 18 -4 Absorption of heat Vaporization and condensation Same magnitude Vaporization Q Energy absorbed Liquid (water) Gas (steam) Condensation Q Energy released Gas (steam) Liquid (water) At the same temperature

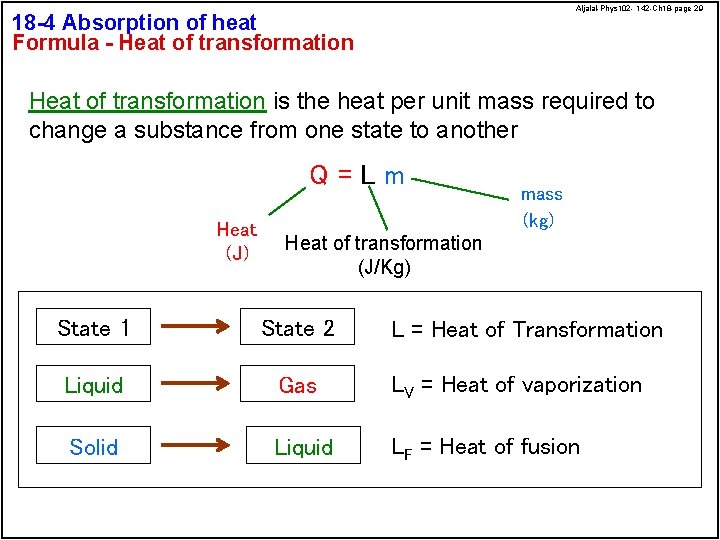
Aljalal-Phys 102 - 142 -Ch 18 -page 29 18 -4 Absorption of heat Formula - Heat of transformation is the heat per unit mass required to change a substance from one state to another Q=Lm Heat (J) mass (kg) Heat of transformation (J/Kg) State 1 State 2 Liquid Gas LV = Heat of vaporization Solid Liquid LF = Heat of fusion L = Heat of Transformation

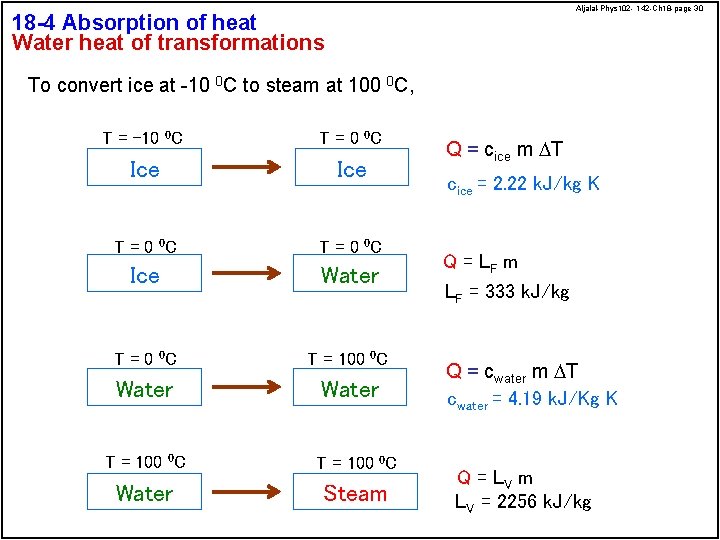
Aljalal-Phys 102 - 142 -Ch 18 -page 30 18 -4 Absorption of heat Water heat of transformations To convert ice at -10 0 C to steam at 100 0 C, T = -10 0 C T = 0 0 C Ice Water T = 0 0 C T = 100 0 C Water Steam Q = cice m DT cice = 2. 22 k. J/kg K Q = LF m LF = 333 k. J/kg Q = cwater m DT cwater = 4. 19 k. J/Kg K Q = LV m LV = 2256 k. J/kg

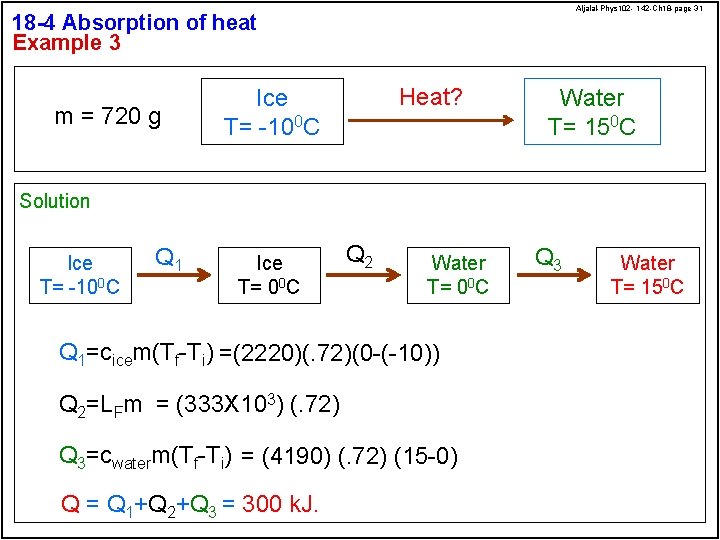
Aljalal-Phys 102 - 142 -Ch 18 -page 31 18 -4 Absorption of heat Example 3 m = 720 g Heat? Ice T= -100 C Water T= 150 C Solution Ice T= -100 C Q 1 Ice T= 00 C Q 2 Water T= 00 C Q 1=cicem(Tf-Ti) =(2220)(. 72)(0 -(-10)) Q 2=LFm = (333 X 103) (. 72) Q 3=cwaterm(Tf-Ti) = (4190) (. 72) (15 -0) Q = Q 1+Q 2+Q 3 = 300 k. J. Q 3 Water T= 150 C

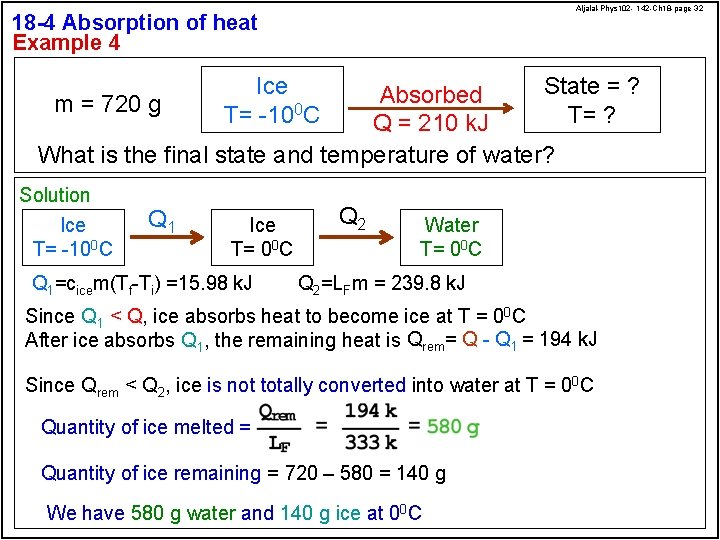
Aljalal-Phys 102 - 142 -Ch 18 -page 32 18 -4 Absorption of heat Example 4 Ice T= -100 C State = ? Absorbed m = 720 g T= ? Q = 210 k. J What is the final state and temperature of water? Solution Ice T= -100 C Q 1 Ice T= 00 C Q 1=cicem(Tf-Ti) =15. 98 k. J Q 2 Water T= 00 C Q 2=LFm = 239. 8 k. J Since Q 1 < Q, ice absorbs heat to become ice at T = 00 C After ice absorbs Q 1, the remaining heat is Qrem= Q - Q 1 = 194 k. J Since Qrem < Q 2, ice is not totally converted into water at T = 00 C Quantity of ice melted = Quantity of ice remaining = 720 – 580 = 140 g We have 580 g water and 140 g ice at 00 C

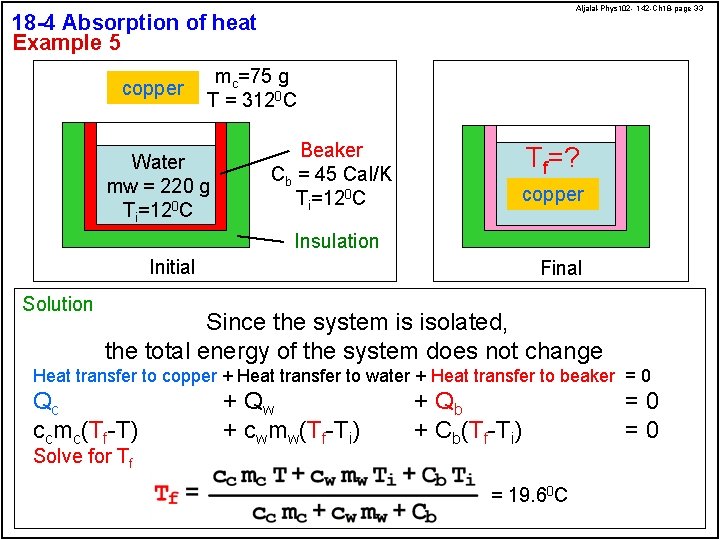
Aljalal-Phys 102 - 142 -Ch 18 -page 33 18 -4 Absorption of heat Example 5 copper mc=75 g T = 3120 C Water mw = 220 g Ti=120 C Beaker Cb = 45 Cal/K Ti=120 C Tf=? copper Insulation Initial Solution Final Since the system is isolated, the total energy of the system does not change Heat transfer to copper + Heat transfer to water + Heat transfer to beaker = 0 Qc ccmc(Tf-T) Solve for Tf + Qw + cwmw(Tf-Ti) + Qb + Cb(Tf-Ti) = 19. 60 C =0 =0

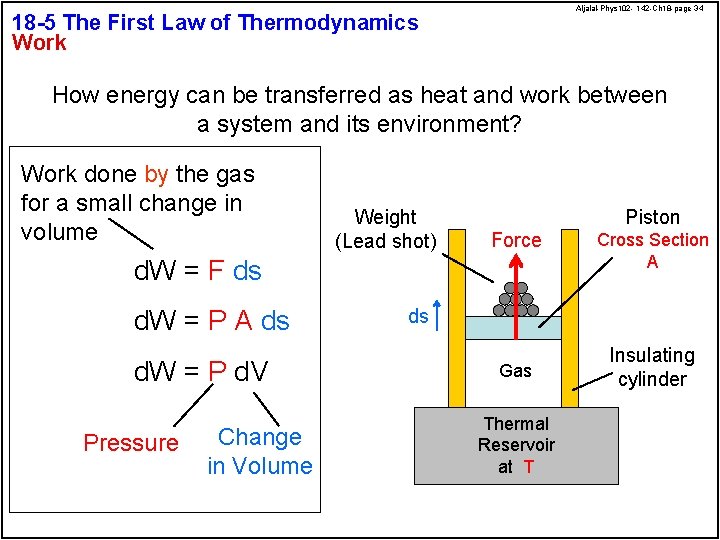
Aljalal-Phys 102 - 142 -Ch 18 -page 34 18 -5 The First Law of Thermodynamics Work How energy can be transferred as heat and work between a system and its environment? Work done by the gas for a small change in volume Weight (Lead shot) Piston Force d. W = F ds d. W = P A ds d. W = P d. V Pressure Change in Volume Cross Section A ds Gas Thermal Reservoir at T Insulating cylinder

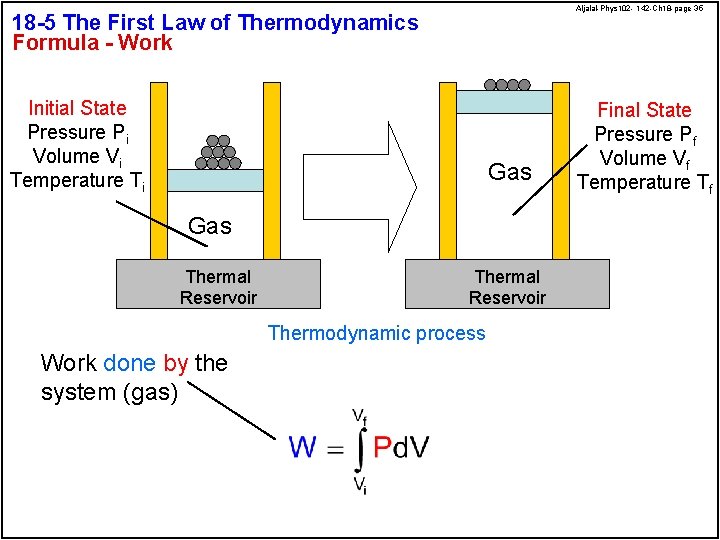
Aljalal-Phys 102 - 142 -Ch 18 -page 35 18 -5 The First Law of Thermodynamics Formula - Work Initial State Pressure Pi Volume Vi Temperature Ti Gas Thermal Reservoir Thermodynamic process Work done by the system (gas) Final State Pressure Pf Volume Vf Temperature Tf

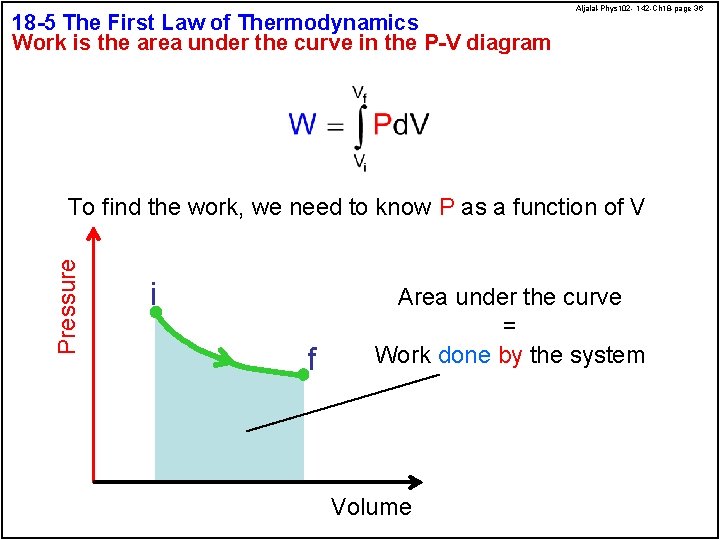
18 -5 The First Law of Thermodynamics Work is the area under the curve in the P-V diagram Aljalal-Phys 102 - 142 -Ch 18 -page 36 Pressure To find the work, we need to know P as a function of V i f Area under the curve = Work done by the system Volume

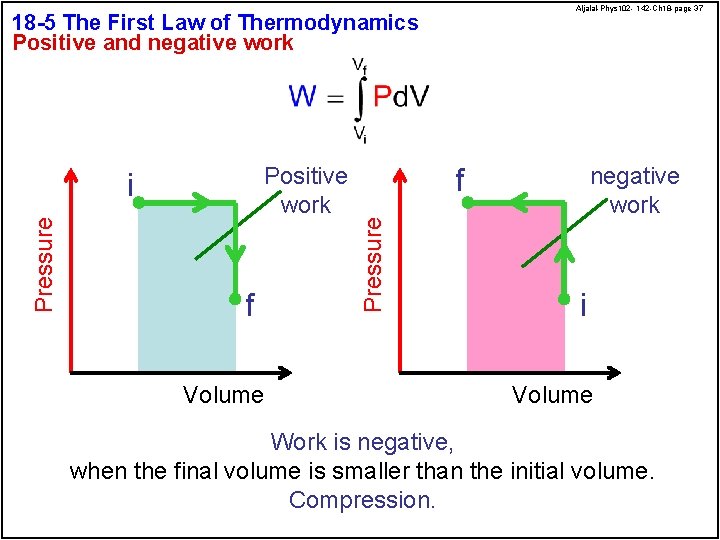
Aljalal-Phys 102 - 142 -Ch 18 -page 37 18 -5 The First Law of Thermodynamics Positive and negative work Pressure f Volume f Pressure Positive work i negative work i Volume Work is negative, when the final volume is smaller than the initial volume. Compression.

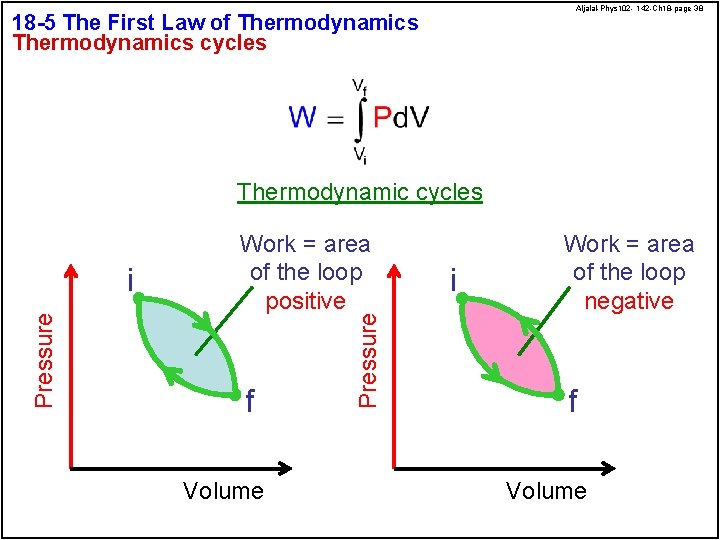
Aljalal-Phys 102 - 142 -Ch 18 -page 38 18 -5 The First Law of Thermodynamics cycles Thermodynamic cycles f Volume Pressure i Work = area of the loop positive i Work = area of the loop negative f Volume

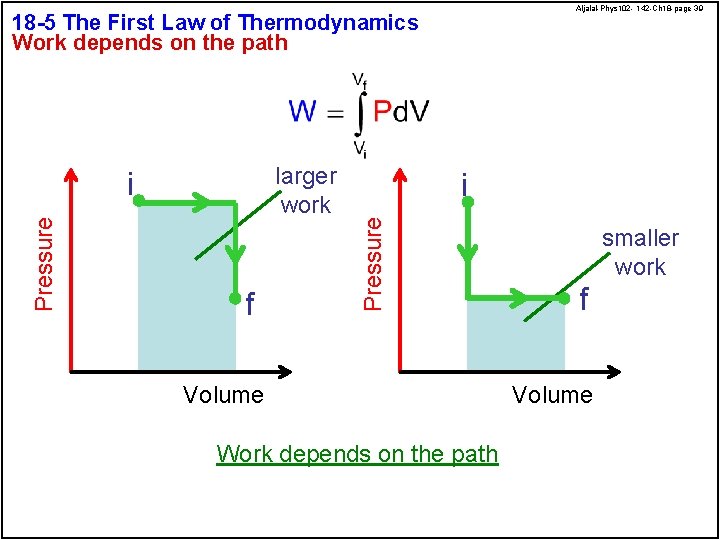
Aljalal-Phys 102 - 142 -Ch 18 -page 39 18 -5 The First Law of Thermodynamics Work depends on the path Pressure f i Pressure larger work i Volume Work depends on the path smaller work f Volume

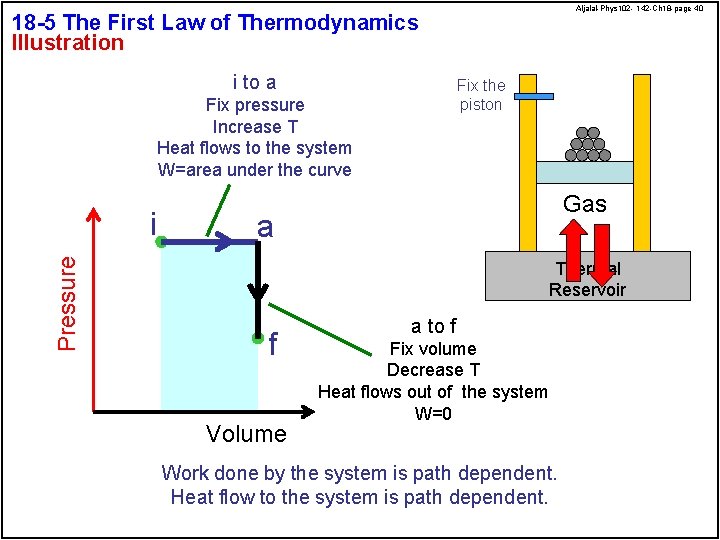
Aljalal-Phys 102 - 142 -Ch 18 -page 40 18 -5 The First Law of Thermodynamics Illustration i to a Fix the piston Fix pressure Increase T Heat flows to the system W=area under the curve Pressure i Gas a Thermal Reservoir f Volume a to f Fix volume Decrease T Heat flows out of the system W=0 Work done by the system is path dependent. Heat flow to the system is path dependent.

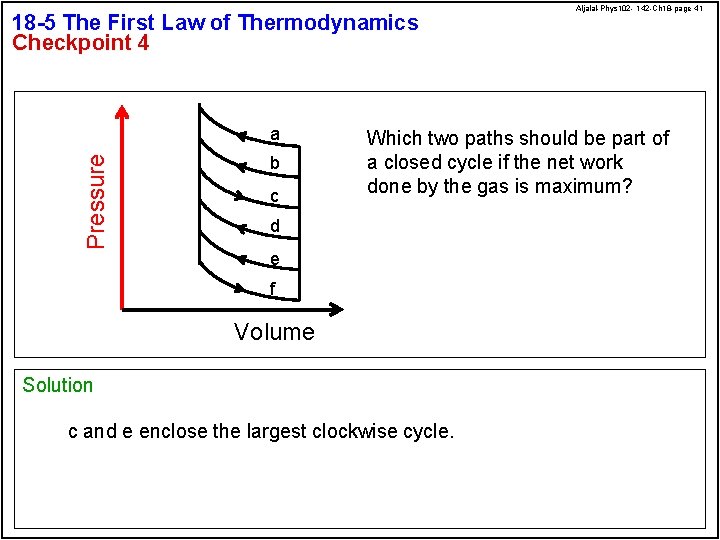
18 -5 The First Law of Thermodynamics Checkpoint 4 Pressure a b c Aljalal-Phys 102 - 142 -Ch 18 -page 41 Which two paths should be part of a closed cycle if the net work done by the gas is maximum? d e f Volume Solution c and e enclose the largest clockwise cycle.

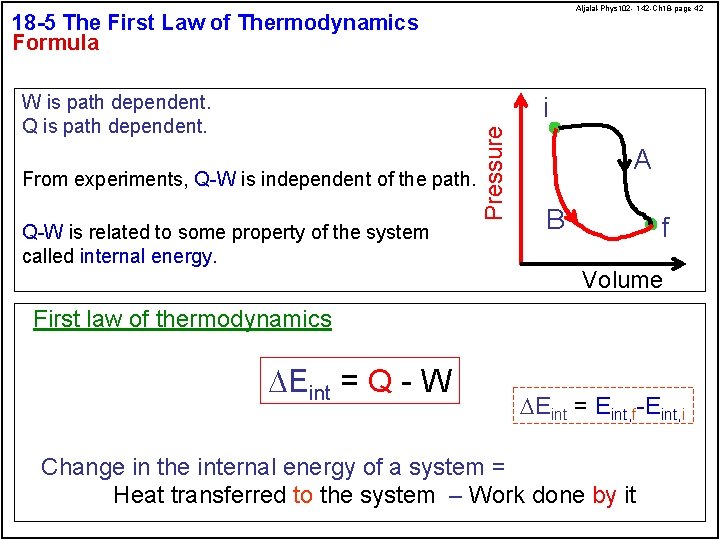
Aljalal-Phys 102 - 142 -Ch 18 -page 42 18 -5 The First Law of Thermodynamics Formula W is path dependent. Q is path dependent. From experiments, Q-W is independent of the path. Q-W is related to some property of the system called internal energy. Pressure i A B f Volume First law of thermodynamics DEint = Q - W DEint = Eint, f-Eint, i Change in the internal energy of a system = Heat transferred to the system – Work done by it

18 -5 The First Law of Thermodynamics Conservation of energy Aljalal-Phys 102 - 142 -Ch 18 -page 43 First law of thermodynamics DEint = Q - W The internal energy of a system tends to increase if energy is added as heat Q to the system and tends to decrease if energy is lost as work W done by the system The first law of thermodynamics is the same as the principle of conservation of energy. In the above form of the first law of thermodynamics, we assume that the kinetic and potential energies of the system do not change.

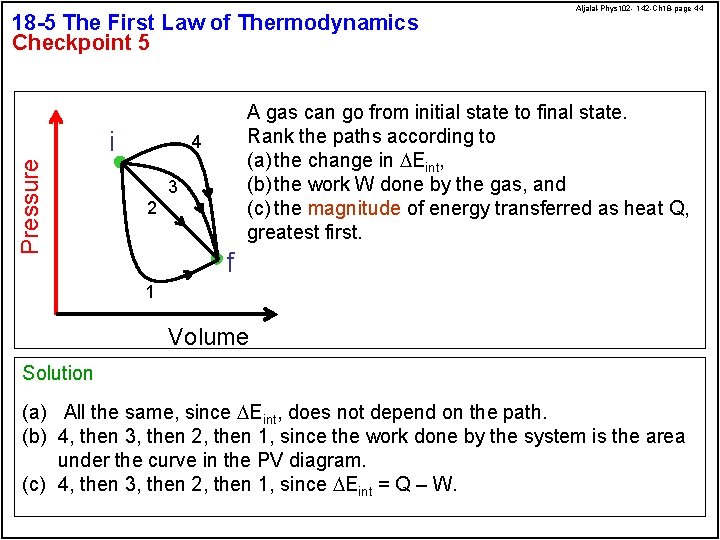
18 -5 The First Law of Thermodynamics Checkpoint 5 Pressure i Aljalal-Phys 102 - 142 -Ch 18 -page 44 A gas can go from initial state to final state. Rank the paths according to (a) the change in DEint, (b) the work W done by the gas, and (c) the magnitude of energy transferred as heat Q, greatest first. 4 3 2 f 1 Volume Solution (a) All the same, since DEint, does not depend on the path. (b) 4, then 3, then 2, then 1, since the work done by the system is the area under the curve in the PV diagram. (c) 4, then 3, then 2, then 1, since DEint = Q – W.

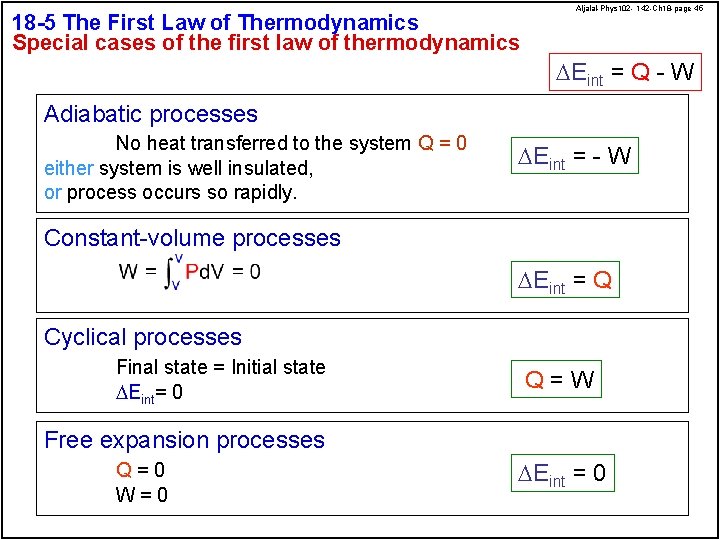
18 -5 The First Law of Thermodynamics Special cases of the first law of thermodynamics Aljalal-Phys 102 - 142 -Ch 18 -page 45 DEint = Q - W Adiabatic processes No heat transferred to the system Q = 0 either system is well insulated, or process occurs so rapidly. DEint = - W Constant-volume processes DEint = Q Cyclical processes Final state = Initial state DEint= 0 Q=W Free expansion processes Q=0 W=0 DEint = 0

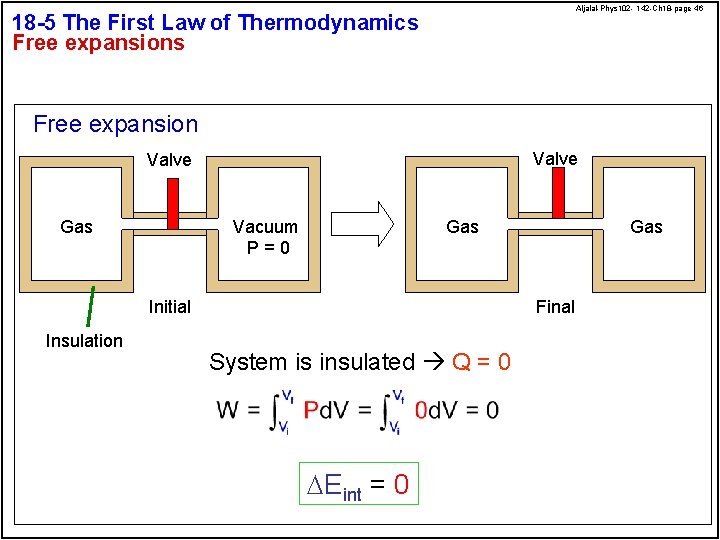
Aljalal-Phys 102 - 142 -Ch 18 -page 46 18 -5 The First Law of Thermodynamics Free expansion Valve Gas Vacuum P=0 Gas Final Initial Insulation Gas System is insulated Q = 0 DEint = 0

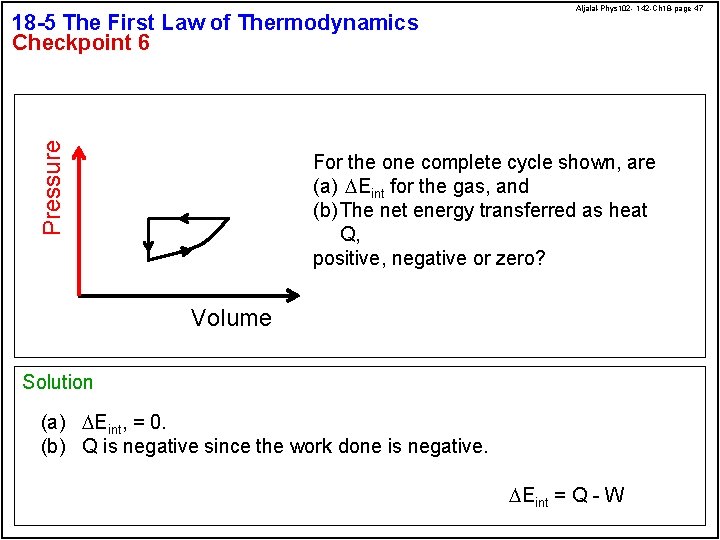
Pressure 18 -5 The First Law of Thermodynamics Checkpoint 6 Aljalal-Phys 102 - 142 -Ch 18 -page 47 For the one complete cycle shown, are (a) DEint for the gas, and (b) The net energy transferred as heat Q, positive, negative or zero? Volume Solution (a) DEint, = 0. (b) Q is negative since the work done is negative. DEint = Q - W

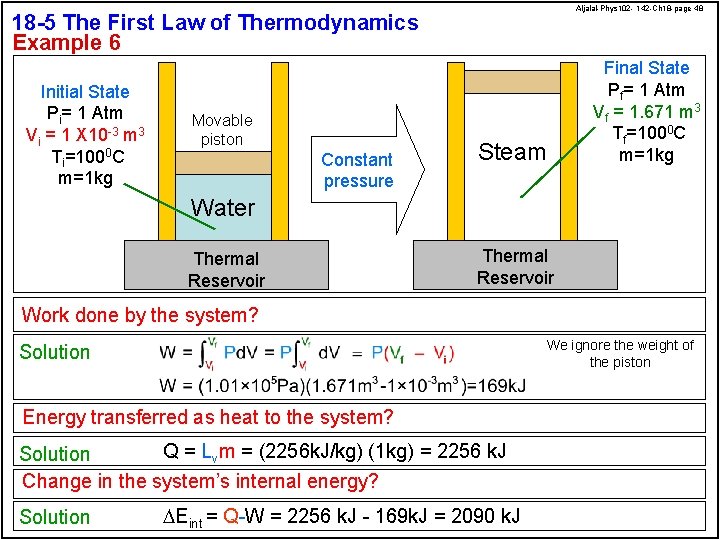
Aljalal-Phys 102 - 142 -Ch 18 -page 48 18 -5 The First Law of Thermodynamics Example 6 Initial State Pi= 1 Atm Vi = 1 X 10 -3 m 3 Ti=1000 C m=1 kg Movable piston Constant pressure Steam Final State Pf= 1 Atm Vf = 1. 671 m 3 Tf=1000 C m=1 kg Water Thermal Reservoir Work done by the system? We ignore the weight of the piston Solution Energy transferred as heat to the system? Q = Lvm = (2256 k. J/kg) (1 kg) = 2256 k. J Solution Change in the system’s internal energy? Solution DEint = Q-W = 2256 k. J - 169 k. J = 2090 k. J

18 -10 Heat Transfer Mechanisms Aljalal-Phys 102 - 142 -Ch 18 -page 49 How does heat transfer take place? There are three transfer mechanisms 1 - Conduction 2 - Convection 3 - Radiation

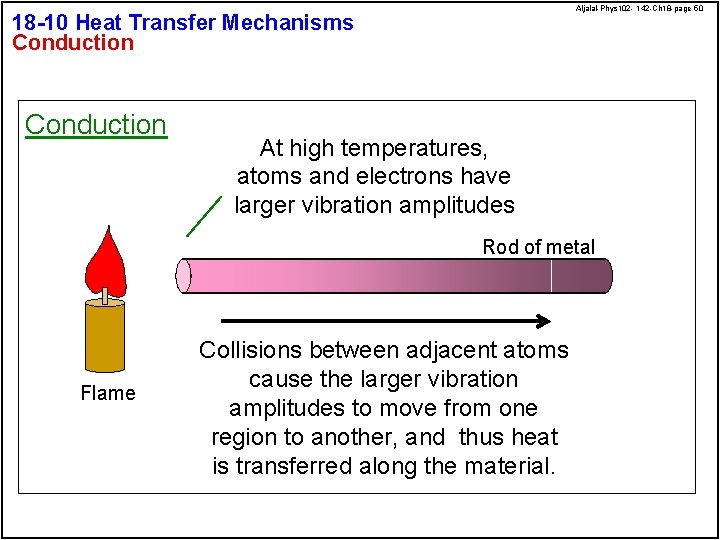
Aljalal-Phys 102 - 142 -Ch 18 -page 50 18 -10 Heat Transfer Mechanisms Conduction At high temperatures, atoms and electrons have larger vibration amplitudes Rod of metal Flame Collisions between adjacent atoms cause the larger vibration amplitudes to move from one region to another, and thus heat is transferred along the material.

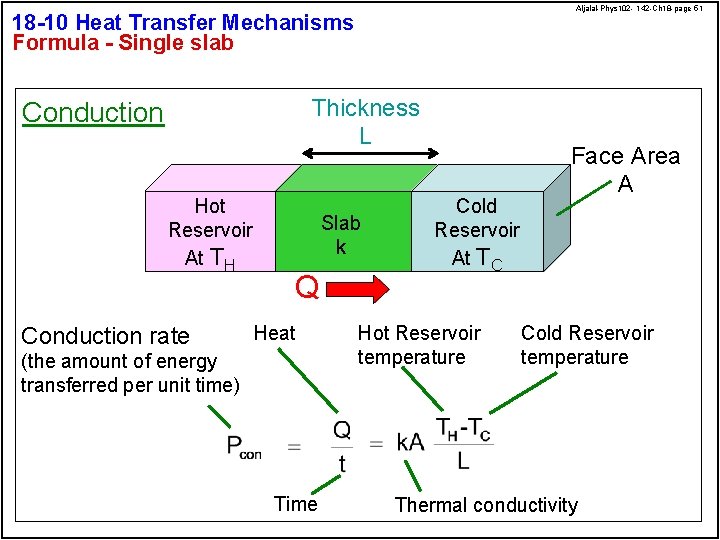
Aljalal-Phys 102 - 142 -Ch 18 -page 51 18 -10 Heat Transfer Mechanisms Formula - Single slab Thickness L Conduction Hot Reservoir At TH Conduction rate Slab k Q Heat (the amount of energy transferred per unit time) Time Cold Reservoir At TC Hot Reservoir temperature Face Area A Cold Reservoir temperature Thermal conductivity

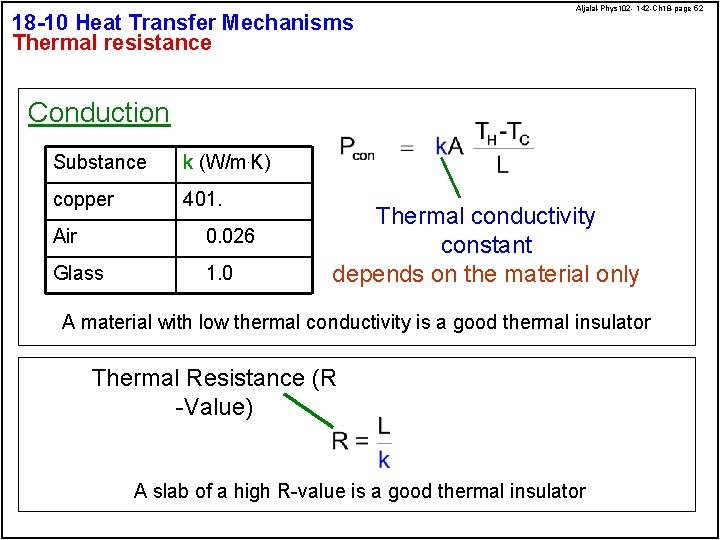
18 -10 Heat Transfer Mechanisms Thermal resistance Aljalal-Phys 102 - 142 -Ch 18 -page 52 Conduction Substance k (W/m. K) copper 401. Air 0. 026 Glass 1. 0 Thermal conductivity constant depends on the material only A material with low thermal conductivity is a good thermal insulator Thermal Resistance (R -Value) A slab of a high R-value is a good thermal insulator

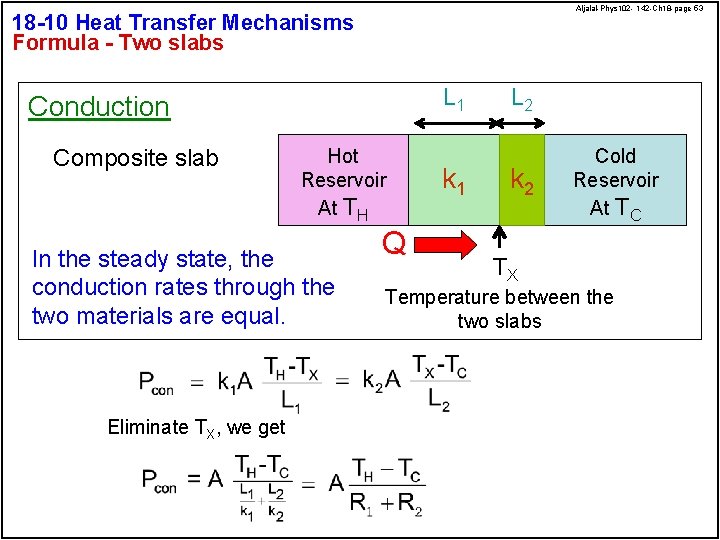
Aljalal-Phys 102 - 142 -Ch 18 -page 53 18 -10 Heat Transfer Mechanisms Formula - Two slabs L 1 Conduction Composite slab Hot Reservoir At TH In the steady state, the conduction rates through the two materials are equal. Eliminate TX, we get Q k 1 L 2 k 2 Cold Reservoir At TC TX Temperature between the two slabs

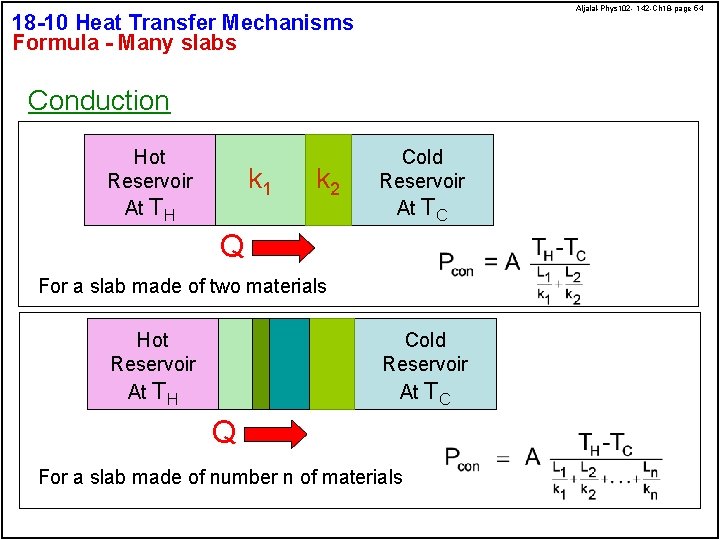
Aljalal-Phys 102 - 142 -Ch 18 -page 54 18 -10 Heat Transfer Mechanisms Formula - Many slabs Conduction Hot Reservoir At TH k 1 k 2 Cold Reservoir At TC Q For a slab made of two materials Hot Reservoir At TH Cold Reservoir At TC Q For a slab made of number n of materials

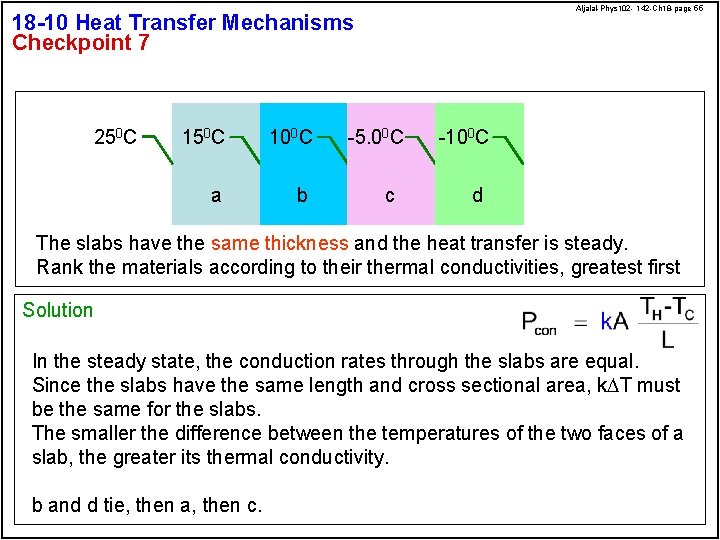
Aljalal-Phys 102 - 142 -Ch 18 -page 55 18 -10 Heat Transfer Mechanisms Checkpoint 7 250 C 100 C a b -5. 00 C c -100 C d The slabs have the same thickness and the heat transfer is steady. Rank the materials according to their thermal conductivities, greatest first Solution In the steady state, the conduction rates through the slabs are equal. Since the slabs have the same length and cross sectional area, k. DT must be the same for the slabs. The smaller the difference between the temperatures of the two faces of a slab, the greater its thermal conductivity. b and d tie, then a, then c.

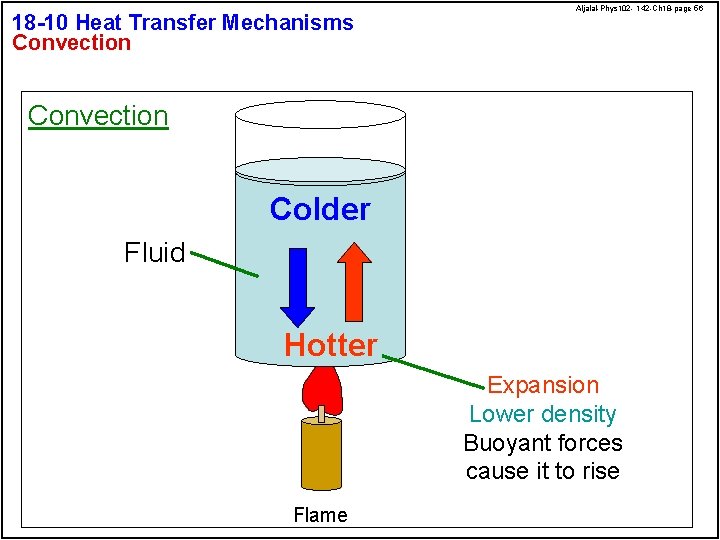
18 -10 Heat Transfer Mechanisms Convection Aljalal-Phys 102 - 142 -Ch 18 -page 56 Convection Colder Fluid Hotter Expansion Lower density Buoyant forces cause it to rise Flame

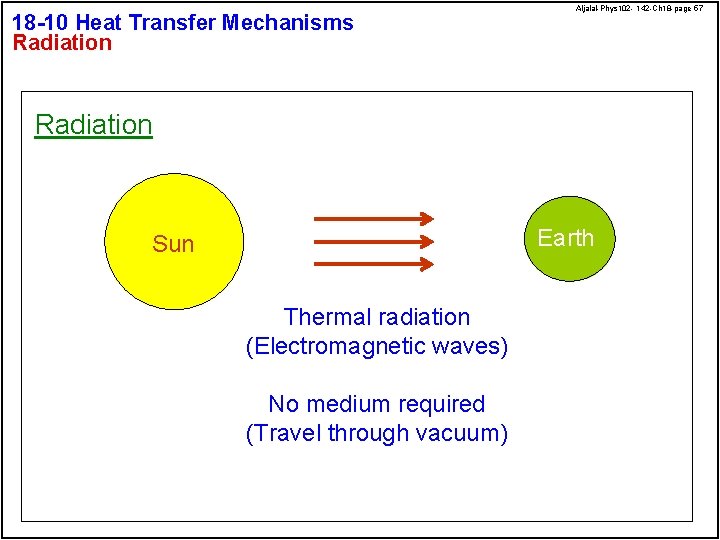
18 -10 Heat Transfer Mechanisms Radiation Aljalal-Phys 102 - 142 -Ch 18 -page 57 Radiation Earth Sun Thermal radiation (Electromagnetic waves) No medium required (Travel through vacuum)

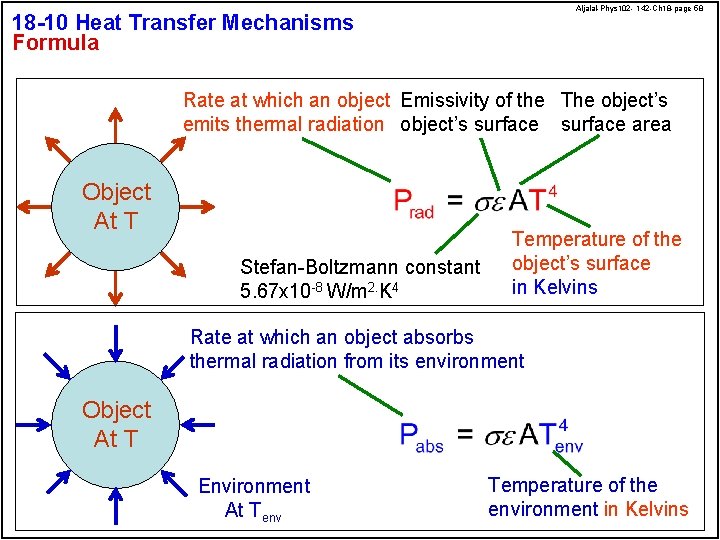
Aljalal-Phys 102 - 142 -Ch 18 -page 58 18 -10 Heat Transfer Mechanisms Formula Rate at which an object Emissivity of the The object’s emits thermal radiation object’s surface area Object At T Stefan-Boltzmann constant 5. 67 x 10 -8 W/m 2. K 4 Temperature of the object’s surface in Kelvins Rate at which an object absorbs thermal radiation from its environment Object At T Environment At Tenv Temperature of the environment in Kelvins

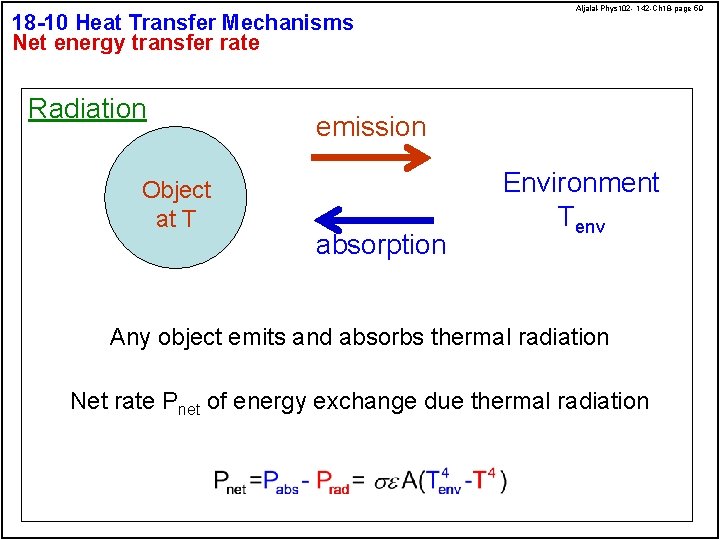
18 -10 Heat Transfer Mechanisms Net energy transfer rate Radiation Object at T Aljalal-Phys 102 - 142 -Ch 18 -page 59 emission absorption Environment Tenv Any object emits and absorbs thermal radiation Net rate Pnet of energy exchange due thermal radiation

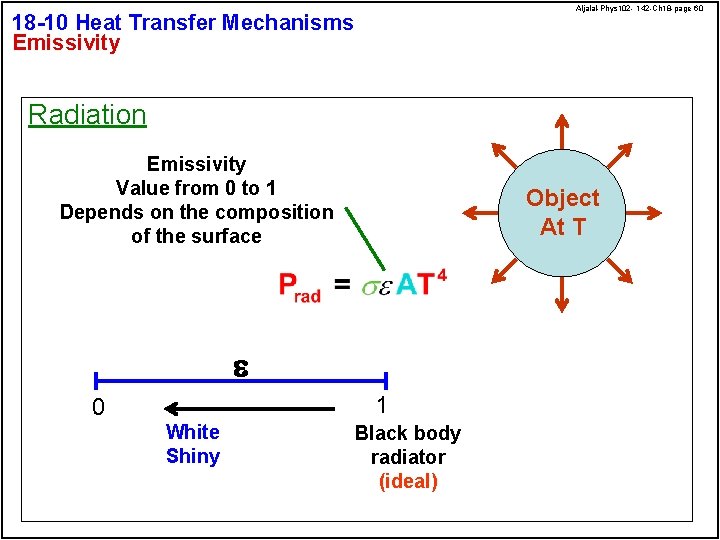
Aljalal-Phys 102 - 142 -Ch 18 -page 60 18 -10 Heat Transfer Mechanisms Emissivity Radiation Emissivity Value from 0 to 1 Depends on the composition of the surface Object At T e 1 0 White Shiny Black body radiator (ideal)

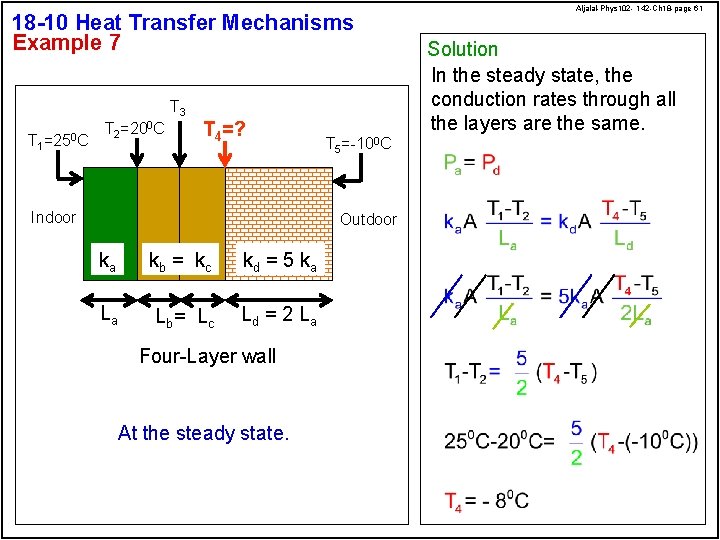
18 -10 Heat Transfer Mechanisms Example 7 T 3 T 1 =250 C T 2=200 C T 4=? Indoor T 5=-100 C Outdoor ka kb = kc kd = 5 ka La Lb = L c Ld = 2 L a Four-Layer wall At the steady state. Aljalal-Phys 102 - 142 -Ch 18 -page 61 Solution In the steady state, the conduction rates through all the layers are the same.