AljalalPhys 102 5 March 2006 Ch 19 page

- Slides: 39

Aljalal-Phys. 102 -5 March 2006 -Ch 19 -page 1 Chapter 19 The Kinetic Theory of Gases

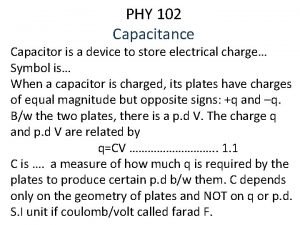
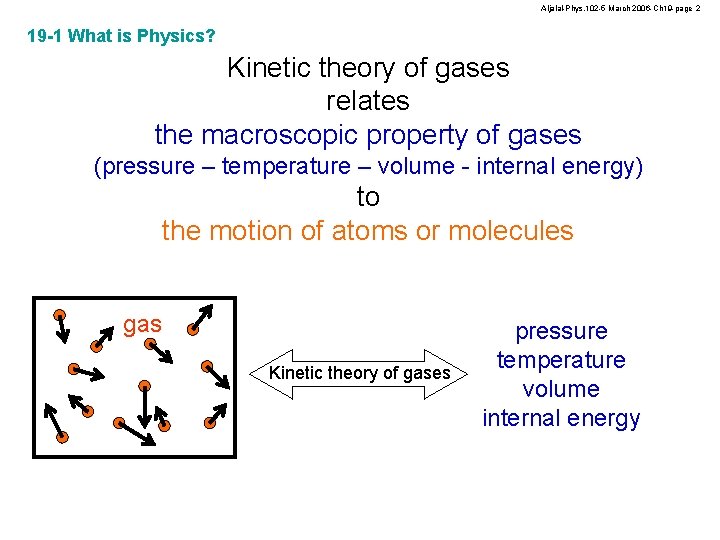
Aljalal-Phys. 102 -5 March 2006 -Ch 19 -page 2 19 -1 What is Physics? Kinetic theory of gases relates the macroscopic property of gases (pressure – temperature – volume - internal energy) to the motion of atoms or molecules gas Kinetic theory of gases pressure temperature volume internal energy

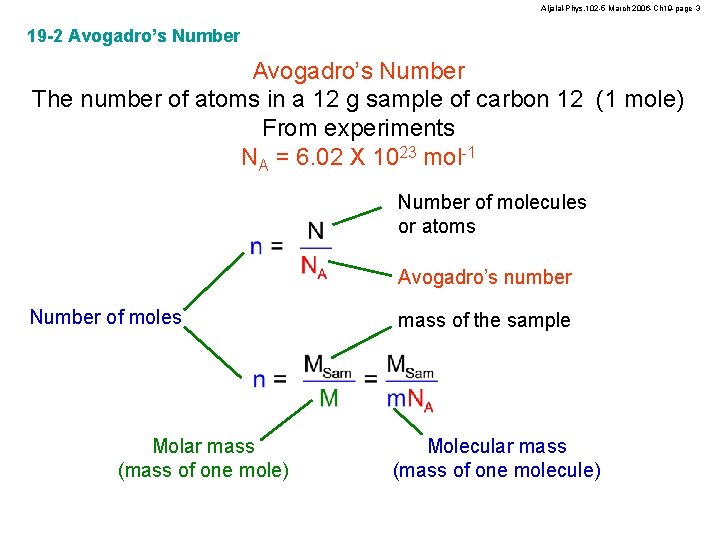
Aljalal-Phys. 102 -5 March 2006 -Ch 19 -page 3 19 -2 Avogadro’s Number The number of atoms in a 12 g sample of carbon 12 (1 mole) From experiments NA = 6. 02 X 1023 mol-1 Number of molecules or atoms Avogadro’s number Number of moles Molar mass (mass of one mole) mass of the sample Molecular mass (mass of one molecule)

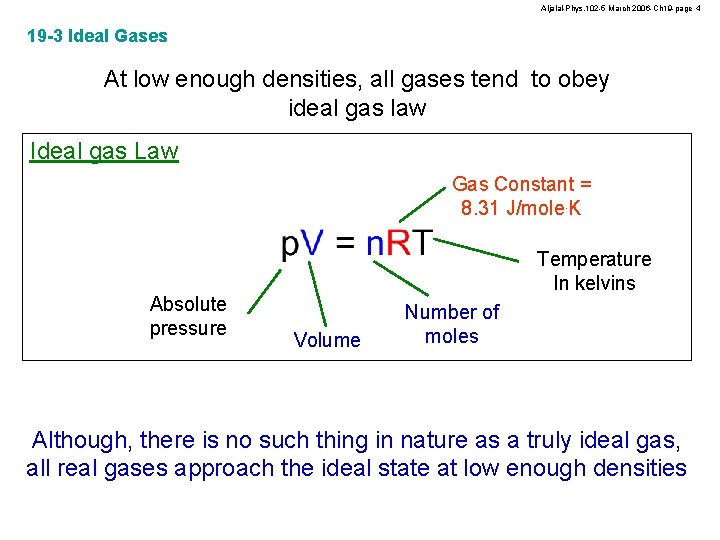
Aljalal-Phys. 102 -5 March 2006 -Ch 19 -page 4 19 -3 Ideal Gases At low enough densities, all gases tend to obey ideal gas law Ideal gas Law Gas Constant = 8. 31 J/mole. K Absolute pressure Temperature In kelvins Volume Number of moles Although, there is no such thing in nature as a truly ideal gas, all real gases approach the ideal state at low enough densities

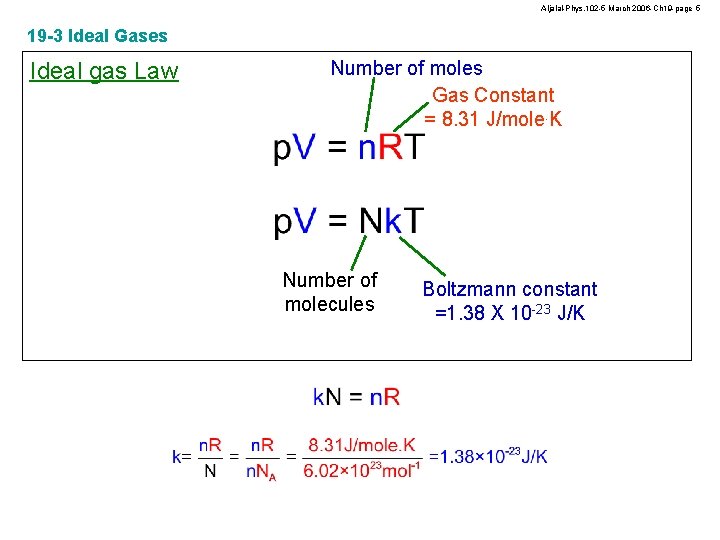
Aljalal-Phys. 102 -5 March 2006 -Ch 19 -page 5 19 -3 Ideal Gases Ideal gas Law Number of moles Gas Constant = 8. 31 J/mole. K Number of molecules Boltzmann constant =1. 38 X 10 -23 J/K

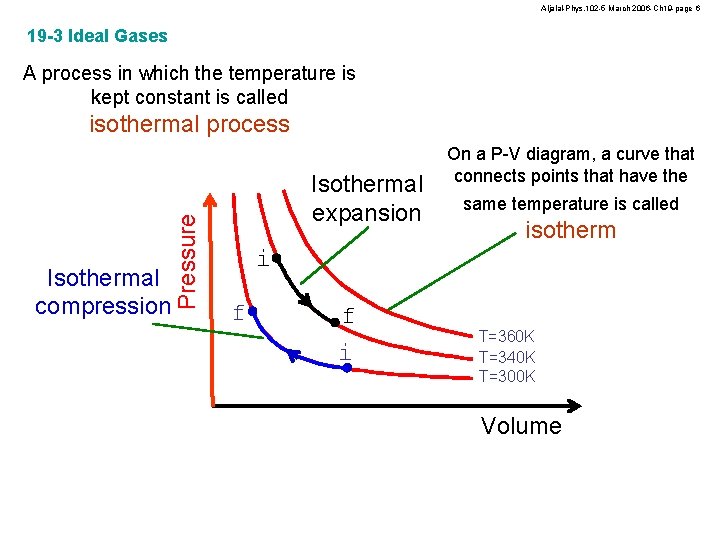
Aljalal-Phys. 102 -5 March 2006 -Ch 19 -page 6 19 -3 Ideal Gases A process in which the temperature is kept constant is called Isothermal compression Pressure isothermal process Isothermal expansion On a P-V diagram, a curve that connects points that have the same temperature is called isotherm i f f i T=360 K T=340 K T=300 K Volume

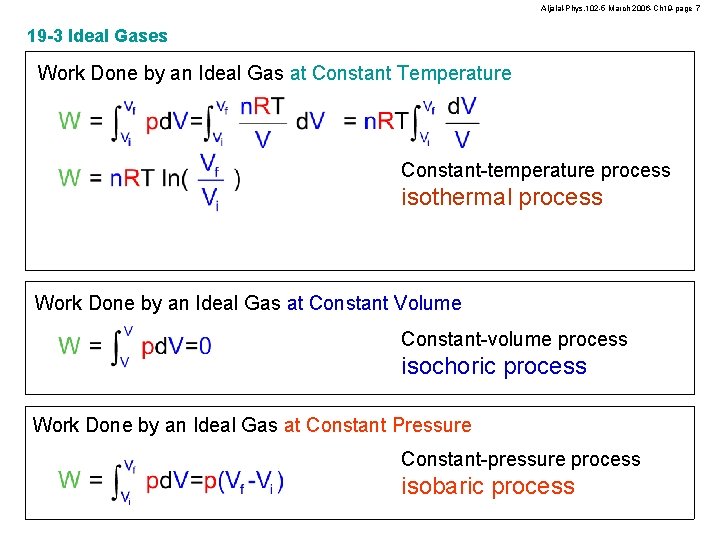
Aljalal-Phys. 102 -5 March 2006 -Ch 19 -page 7 19 -3 Ideal Gases Work Done by an Ideal Gas at Constant Temperature Constant-temperature process isothermal process Work Done by an Ideal Gas at Constant Volume Constant-volume process isochoric process Work Done by an Ideal Gas at Constant Pressure Constant-pressure process isobaric process

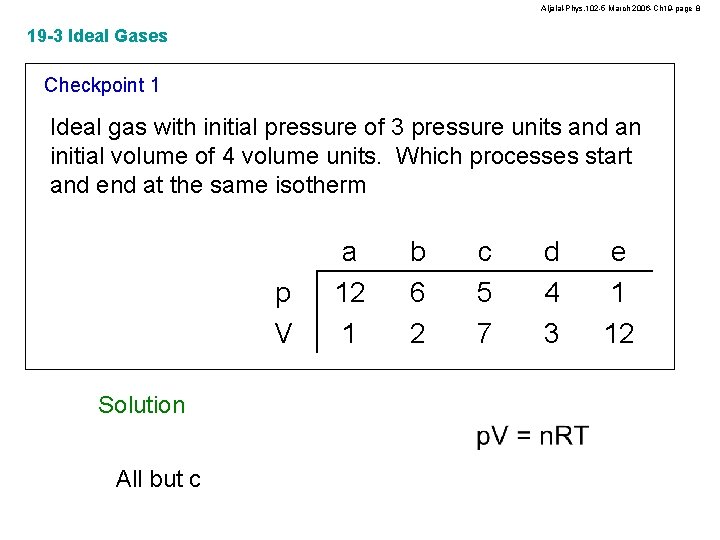
Aljalal-Phys. 102 -5 March 2006 -Ch 19 -page 8 19 -3 Ideal Gases Checkpoint 1 Ideal gas with initial pressure of 3 pressure units and an initial volume of 4 volume units. Which processes start and end at the same isotherm p V Solution All but c a 12 1 b 6 2 c 5 7 d 4 3 e 1 12

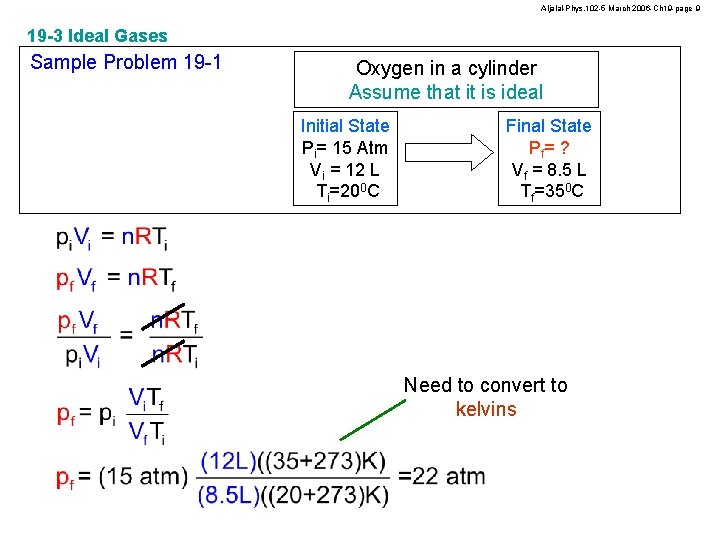
Aljalal-Phys. 102 -5 March 2006 -Ch 19 -page 9 19 -3 Ideal Gases Sample Problem 19 -1 Oxygen in a cylinder Assume that it is ideal Initial State Pi= 15 Atm Vi = 12 L Ti=200 C Final State Pf= ? Vf = 8. 5 L Tf=350 C Need to convert to kelvins

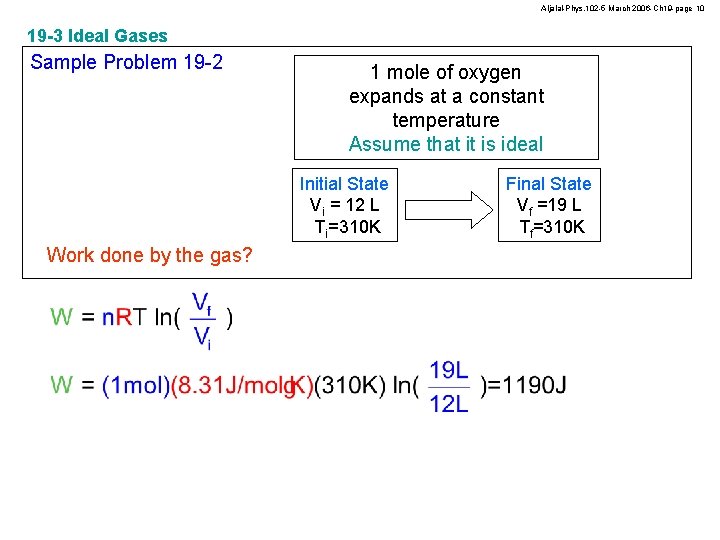
Aljalal-Phys. 102 -5 March 2006 -Ch 19 -page 10 19 -3 Ideal Gases Sample Problem 19 -2 1 mole of oxygen expands at a constant temperature Assume that it is ideal Initial State Vi = 12 L Ti=310 K Work done by the gas? Final State Vf =19 L Tf=310 K

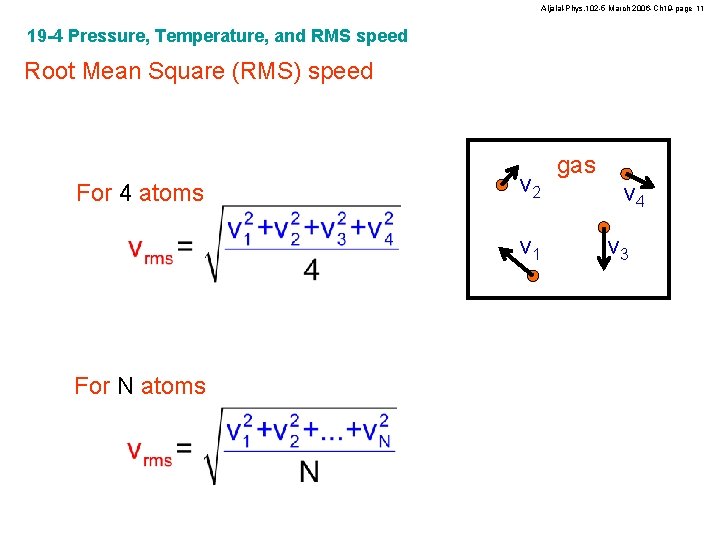
Aljalal-Phys. 102 -5 March 2006 -Ch 19 -page 11 19 -4 Pressure, Temperature, and RMS speed Root Mean Square (RMS) speed For 4 atoms v 2 v 1 For N atoms gas v 4 v 3

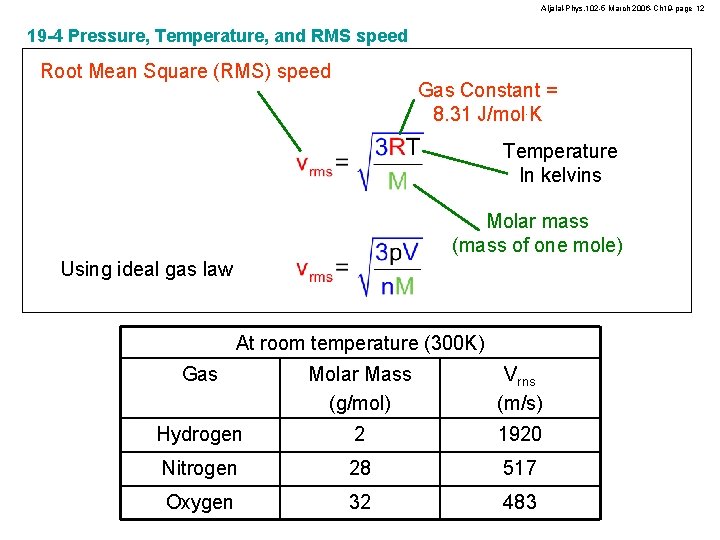
Aljalal-Phys. 102 -5 March 2006 -Ch 19 -page 12 19 -4 Pressure, Temperature, and RMS speed Root Mean Square (RMS) speed Gas Constant = 8. 31 J/mol. K Temperature In kelvins Molar mass (mass of one mole) Using ideal gas law At room temperature (300 K) Gas Molar Mass (g/mol) Vrns (m/s) Hydrogen 2 1920 Nitrogen 28 517 Oxygen 32 483

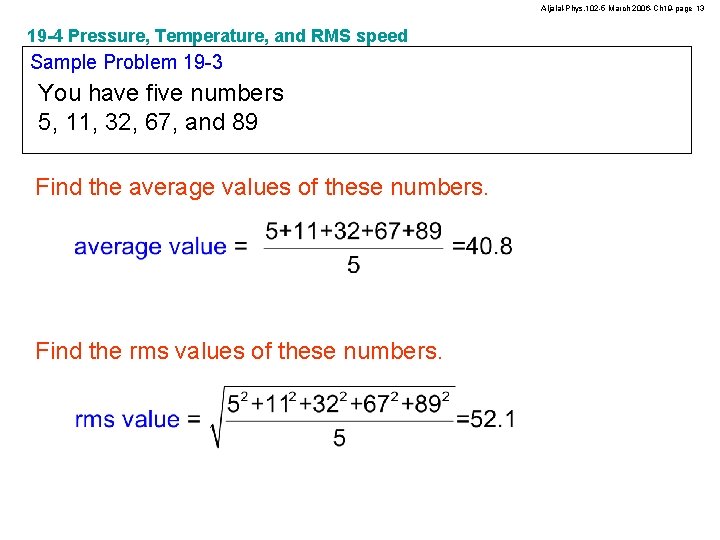
Aljalal-Phys. 102 -5 March 2006 -Ch 19 -page 13 19 -4 Pressure, Temperature, and RMS speed Sample Problem 19 -3 You have five numbers 5, 11, 32, 67, and 89 Find the average values of these numbers. Find the rms values of these numbers.

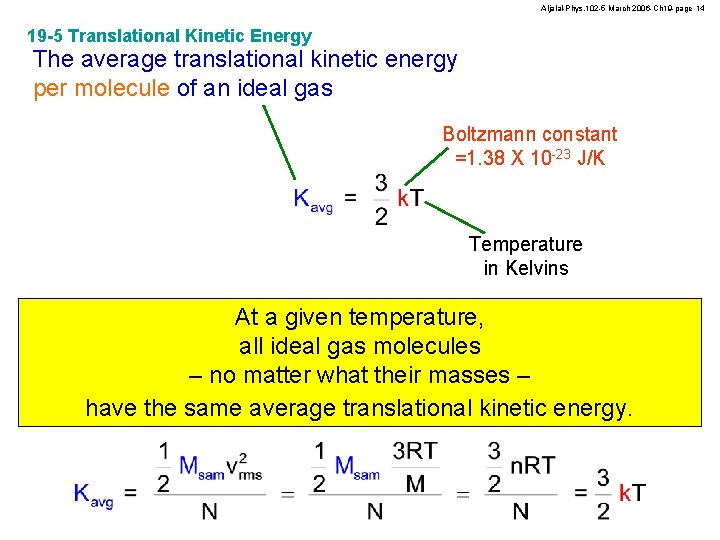
Aljalal-Phys. 102 -5 March 2006 -Ch 19 -page 14 19 -5 Translational Kinetic Energy The average translational kinetic energy per molecule of an ideal gas Boltzmann constant =1. 38 X 10 -23 J/K Temperature in Kelvins At a given temperature, all ideal gas molecules – no matter what their masses – have the same average translational kinetic energy.

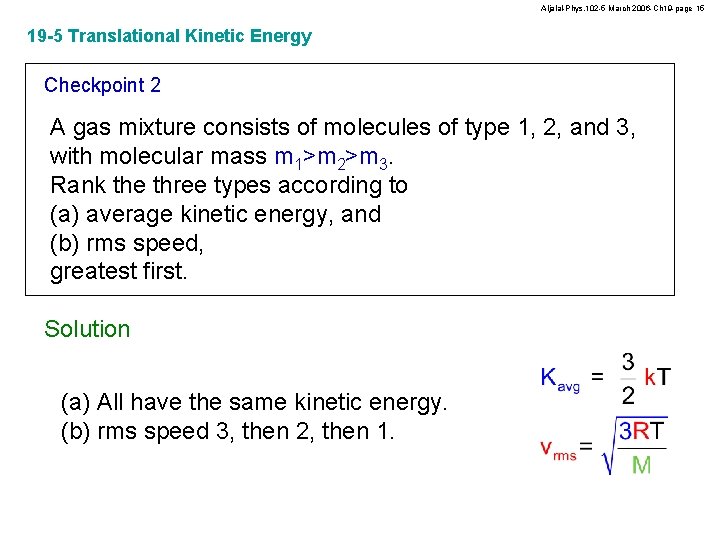
Aljalal-Phys. 102 -5 March 2006 -Ch 19 -page 15 19 -5 Translational Kinetic Energy Checkpoint 2 A gas mixture consists of molecules of type 1, 2, and 3, with molecular mass m 1>m 2>m 3. Rank the three types according to (a) average kinetic energy, and (b) rms speed, greatest first. Solution (a) All have the same kinetic energy. (b) rms speed 3, then 2, then 1.

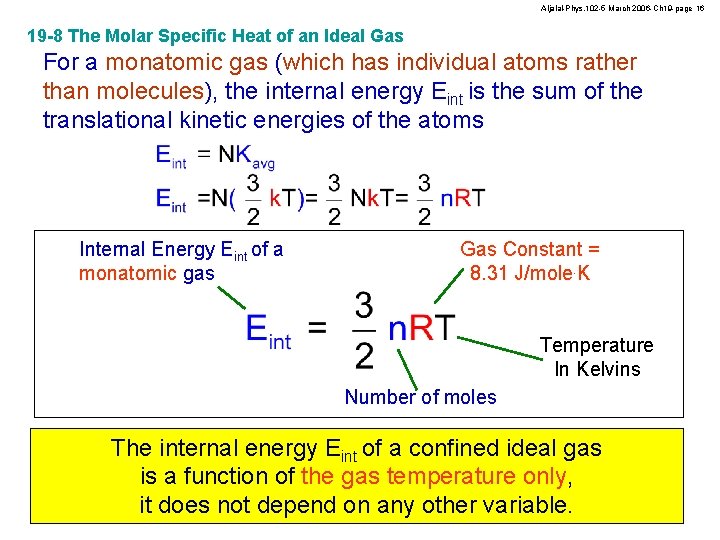
Aljalal-Phys. 102 -5 March 2006 -Ch 19 -page 16 19 -8 The Molar Specific Heat of an Ideal Gas For a monatomic gas (which has individual atoms rather than molecules), the internal energy Eint is the sum of the translational kinetic energies of the atoms Internal Energy Eint of a monatomic gas Gas Constant = 8. 31 J/mole. K Temperature In Kelvins Number of moles The internal energy Eint of a confined ideal gas is a function of the gas temperature only, it does not depend on any other variable.

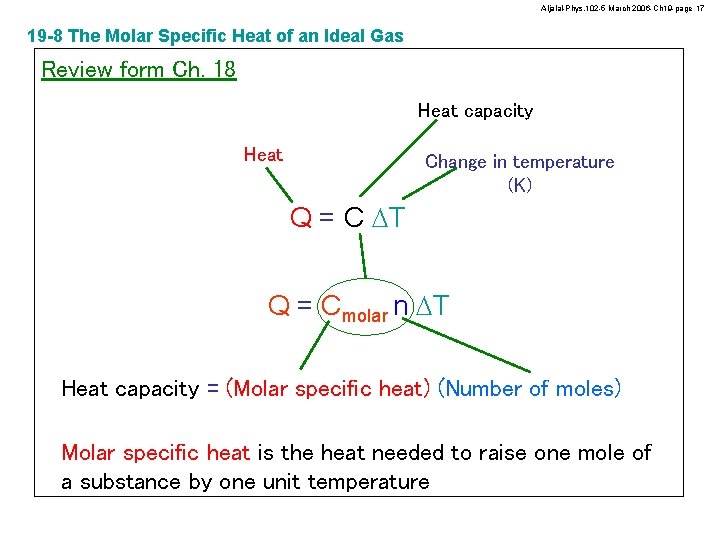
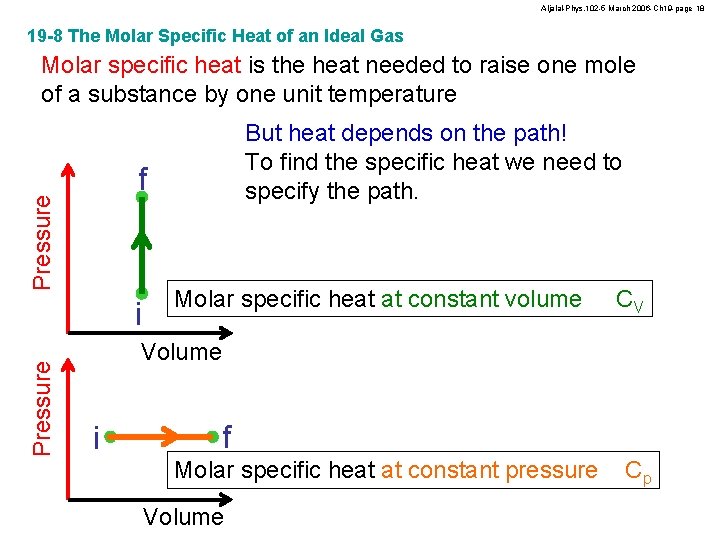
Aljalal-Phys. 102 -5 March 2006 -Ch 19 -page 17 19 -8 The Molar Specific Heat of an Ideal Gas Review form Ch. 18 Heat capacity Heat Change in temperature (K) Q = C DT Q = Cmolar n DT Heat capacity = (Molar specific heat) (Number of moles) Molar specific heat is the heat needed to raise one mole of a substance by one unit temperature

Aljalal-Phys. 102 -5 March 2006 -Ch 19 -page 18 19 -8 The Molar Specific Heat of an Ideal Gas Molar specific heat is the heat needed to raise one mole of a substance by one unit temperature Pressure f i Pressure But heat depends on the path! To find the specific heat we need to specify the path. Molar specific heat at constant volume CV Volume i f Molar specific heat at constant pressure Volume Cp

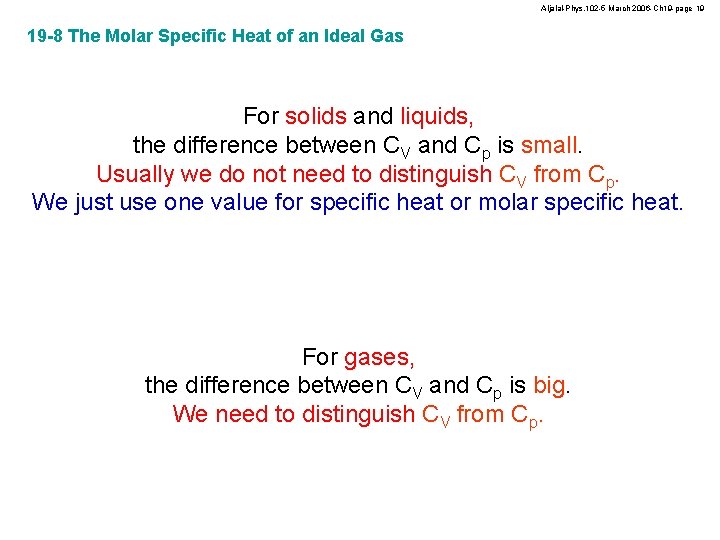
Aljalal-Phys. 102 -5 March 2006 -Ch 19 -page 19 19 -8 The Molar Specific Heat of an Ideal Gas For solids and liquids, the difference between CV and Cp is small. Usually we do not need to distinguish CV from Cp. We just use one value for specific heat or molar specific heat. For gases, the difference between CV and Cp is big. We need to distinguish CV from Cp.

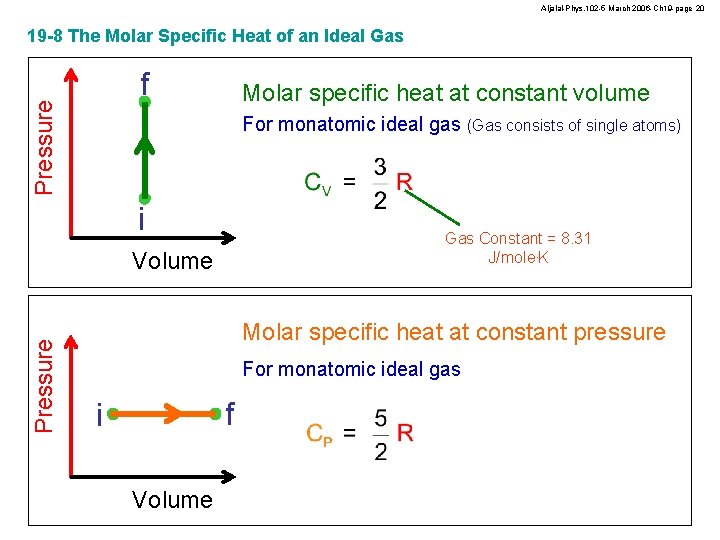
Aljalal-Phys. 102 -5 March 2006 -Ch 19 -page 20 19 -8 The Molar Specific Heat of an Ideal Gas Pressure f Molar specific heat at constant volume For monatomic ideal gas (Gas consists of single atoms) i Gas Constant = 8. 31 J/mole. K Pressure Volume Molar specific heat at constant pressure For monatomic ideal gas f i Volume

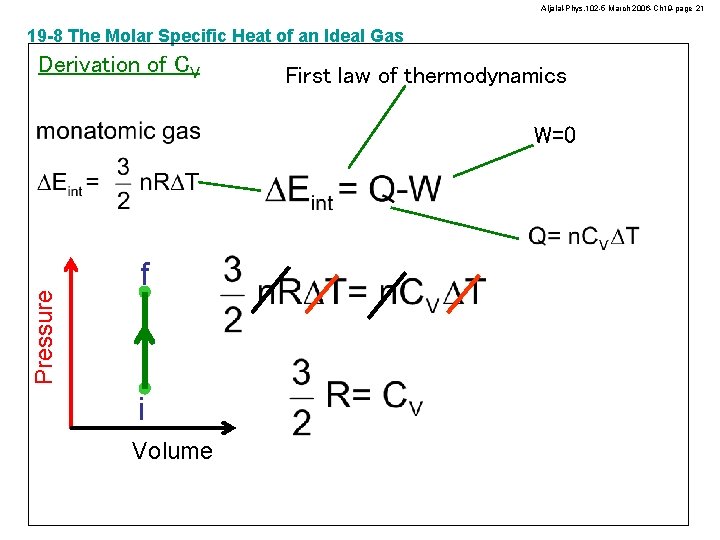
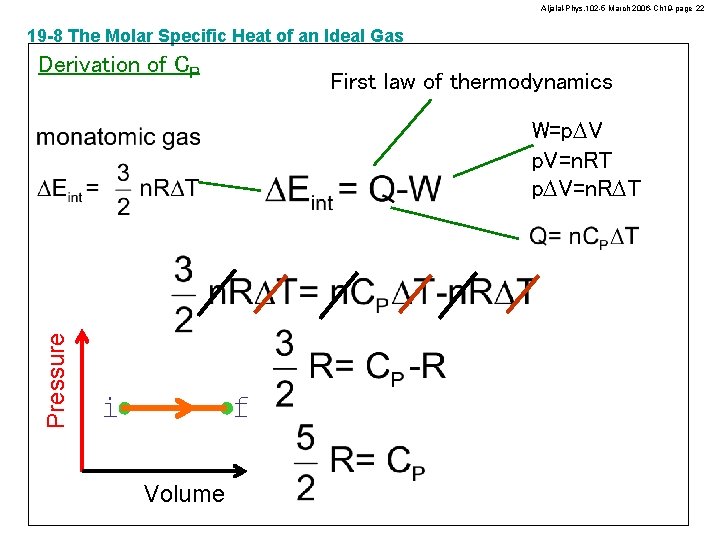
Aljalal-Phys. 102 -5 March 2006 -Ch 19 -page 21 19 -8 The Molar Specific Heat of an Ideal Gas Derivation of CV First law of thermodynamics Pressure W=0 f i Volume

Aljalal-Phys. 102 -5 March 2006 -Ch 19 -page 22 19 -8 The Molar Specific Heat of an Ideal Gas Derivation of CP First law of thermodynamics Pressure W=p. DV p. V=n. RT p. DV=n. RDT f i Volume

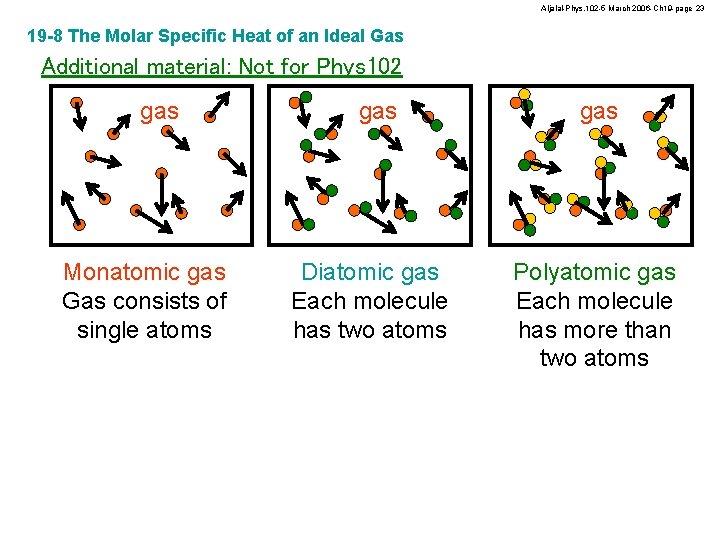
Aljalal-Phys. 102 -5 March 2006 -Ch 19 -page 23 19 -8 The Molar Specific Heat of an Ideal Gas Additional material: Not for Phys 102 gas Monatomic gas Gas consists of single atoms gas Diatomic gas Each molecule has two atoms gas Polyatomic gas Each molecule has more than two atoms

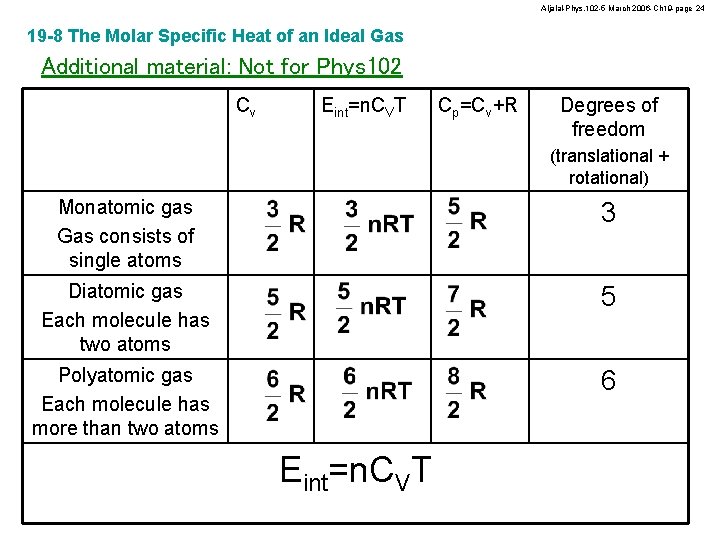
Aljalal-Phys. 102 -5 March 2006 -Ch 19 -page 24 19 -8 The Molar Specific Heat of an Ideal Gas Additional material: Not for Phys 102 Cv Eint=n. CVT Cp=Cv+R Degrees of freedom (translational + rotational) Monatomic gas Gas consists of single atoms 3 Diatomic gas Each molecule has two atoms 5 Polyatomic gas Each molecule has more than two atoms 6 Eint=n. CVT

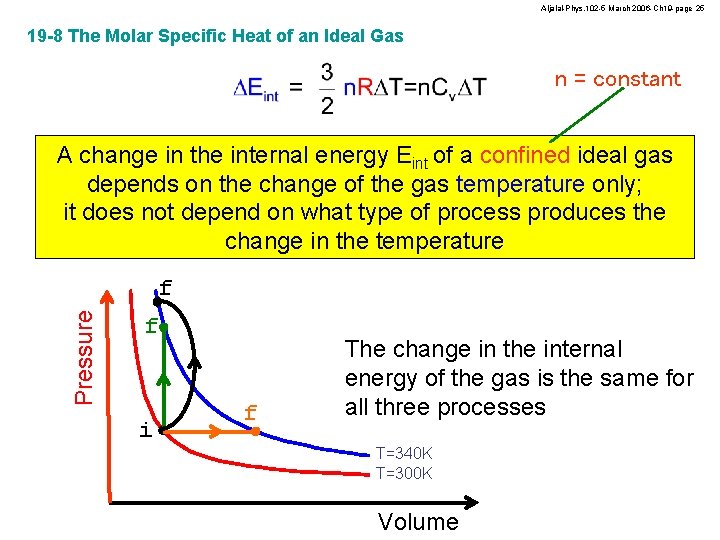
Aljalal-Phys. 102 -5 March 2006 -Ch 19 -page 25 19 -8 The Molar Specific Heat of an Ideal Gas n = constant A change in the internal energy Eint of a confined ideal gas depends on the change of the gas temperature only; it does not depend on what type of process produces the change in the temperature Pressure f f i f The change in the internal energy of the gas is the same for all three processes T=340 K T=300 K Volume

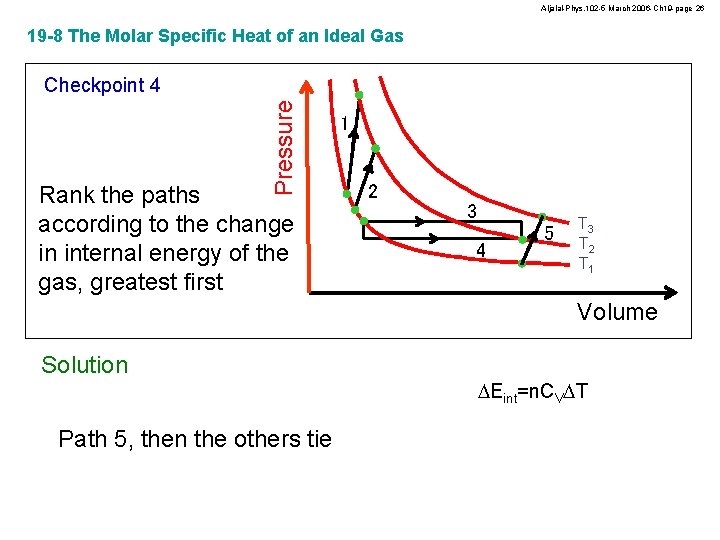
Aljalal-Phys. 102 -5 March 2006 -Ch 19 -page 26 19 -8 The Molar Specific Heat of an Ideal Gas Pressure Checkpoint 4 Rank the paths according to the change in internal energy of the gas, greatest first 1 2 3 4 5 T 3 T 2 T 1 Volume Solution Path 5, then the others tie DEint=n. CVDT

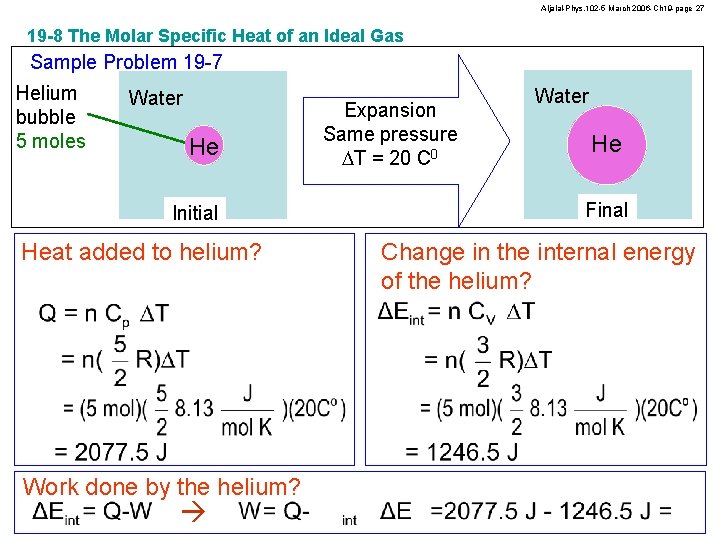
Aljalal-Phys. 102 -5 March 2006 -Ch 19 -page 27 19 -8 The Molar Specific Heat of an Ideal Gas Sample Problem 19 -7 Helium bubble 5 moles Water He Initial Heat added to helium? Work done by the helium? Expansion Same pressure DT = 20 C 0 Water He Final Change in the internal energy of the helium?

Aljalal-Phys. 102 -5 March 2006 -Ch 19 -page 28 19 -11 Adiabatic Expansion Review form Ch. 18 Adiabatic processes No heat transferred to the system Q=0 Either system is well insulated, or process occurs so rapidly. Adiabatic Expansion DEint = - W

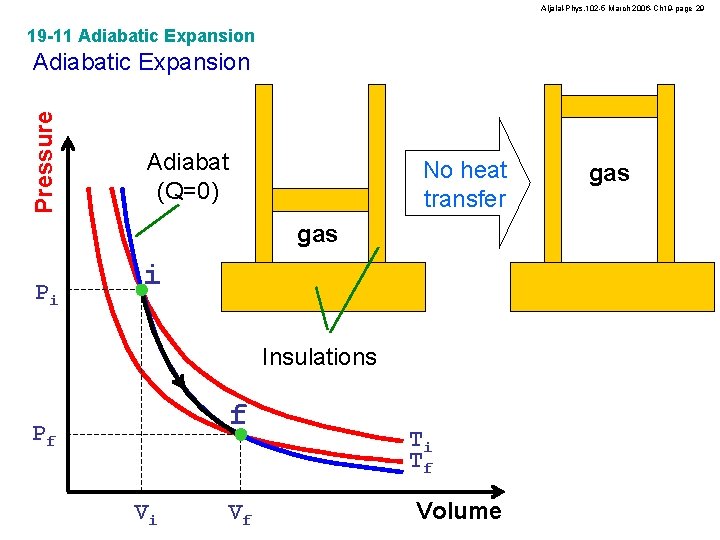
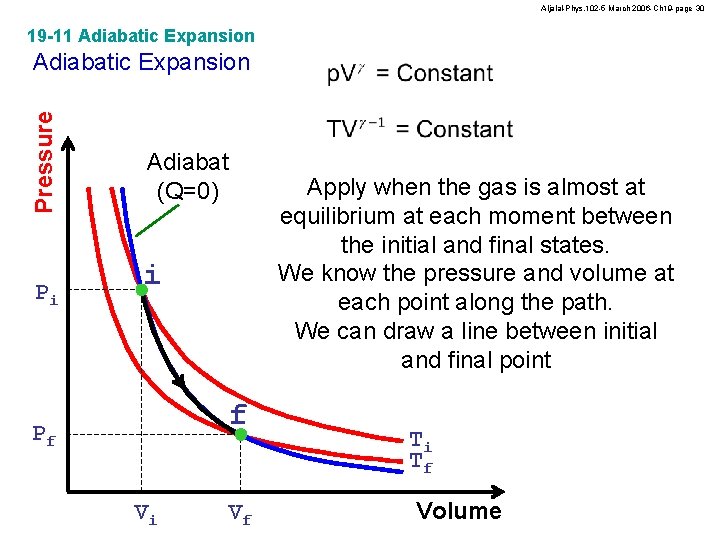
Aljalal-Phys. 102 -5 March 2006 -Ch 19 -page 29 19 -11 Adiabatic Expansion Pressure Adiabatic Expansion Adiabat (Q=0) No heat transfer gas Pi i Insulations f Pf Vi Vf Ti Tf Volume gas

Aljalal-Phys. 102 -5 March 2006 -Ch 19 -page 30 19 -11 Adiabatic Expansion Pressure Adiabatic Expansion Pi Adiabat (Q=0) Apply when the gas is almost at equilibrium at each moment between the initial and final states. We know the pressure and volume at each point along the path. We can draw a line between initial and final point i f Pf Vi Vf Ti Tf Volume

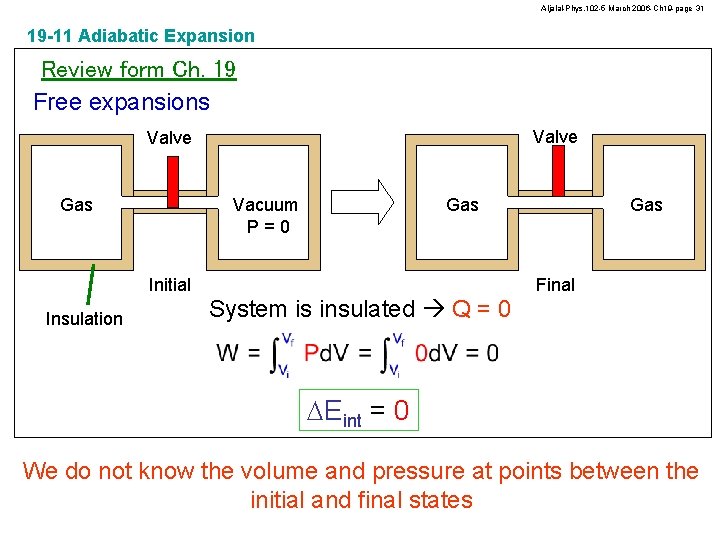
Aljalal-Phys. 102 -5 March 2006 -Ch 19 -page 31 19 -11 Adiabatic Expansion Review form Ch. 19 Free expansions Valve Gas Vacuum P=0 Initial Insulation Gas Final System is insulated Q = 0 DEint = 0 We do not know the volume and pressure at points between the initial and final states

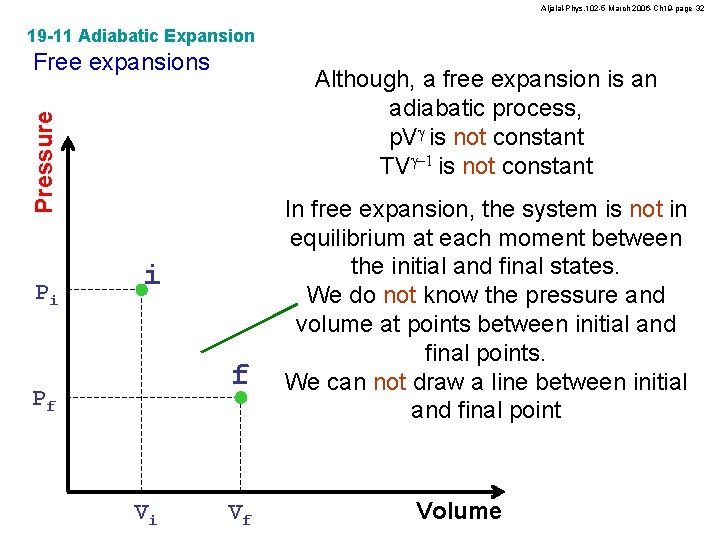
Aljalal-Phys. 102 -5 March 2006 -Ch 19 -page 32 19 -11 Adiabatic Expansion Free expansions Pressure Although, a free expansion is an adiabatic process, p. Vg is not constant TVg-1 is not constant Pi i f Pf Vi Vf In free expansion, the system is not in equilibrium at each moment between the initial and final states. We do not know the pressure and volume at points between initial and final points. We can not draw a line between initial and final point Volume

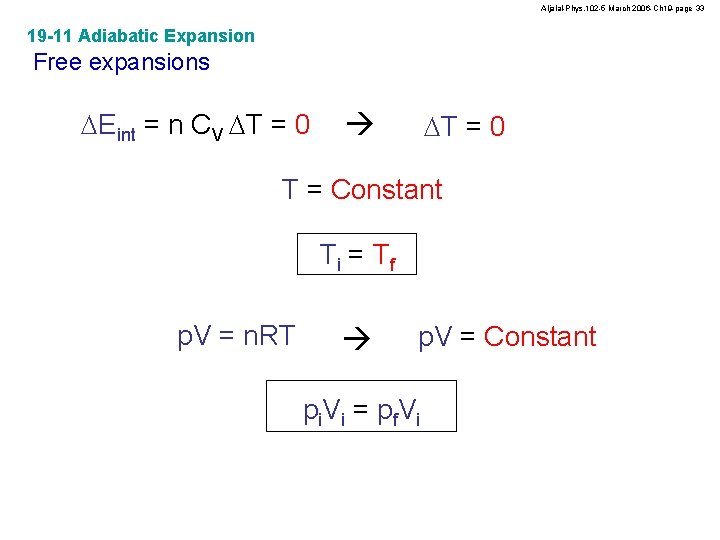
Aljalal-Phys. 102 -5 March 2006 -Ch 19 -page 33 19 -11 Adiabatic Expansion Free expansions DEint = n CV DT = 0 T = Constant Ti = Tf p. V = n. RT p. V = Constant p i. V i = p f V i

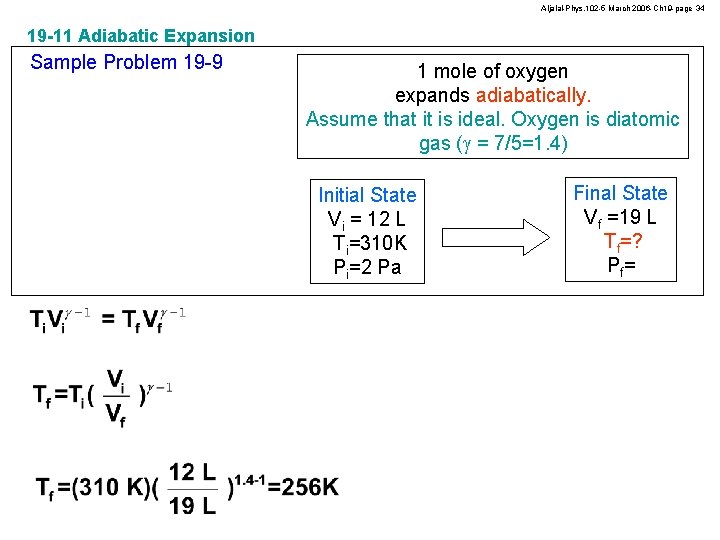
Aljalal-Phys. 102 -5 March 2006 -Ch 19 -page 34 19 -11 Adiabatic Expansion Sample Problem 19 -9 1 mole of oxygen expands adiabatically. Assume that it is ideal. Oxygen is diatomic gas (g = 7/5=1. 4) Initial State Vi = 12 L Ti=310 K Pi=2 Pa Final State Vf =19 L Tf=? Pf=

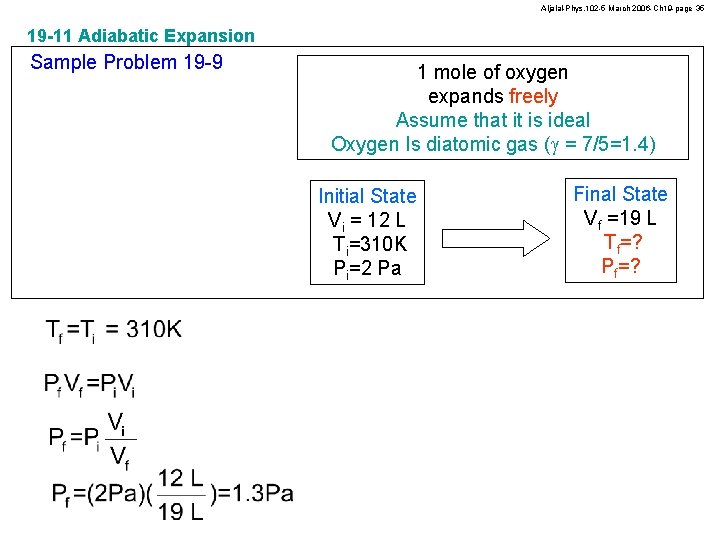
Aljalal-Phys. 102 -5 March 2006 -Ch 19 -page 35 19 -11 Adiabatic Expansion Sample Problem 19 -9 1 mole of oxygen expands freely Assume that it is ideal Oxygen Is diatomic gas (g = 7/5=1. 4) Initial State Vi = 12 L Ti=310 K Pi=2 Pa Final State Vf =19 L Tf=? Pf=?

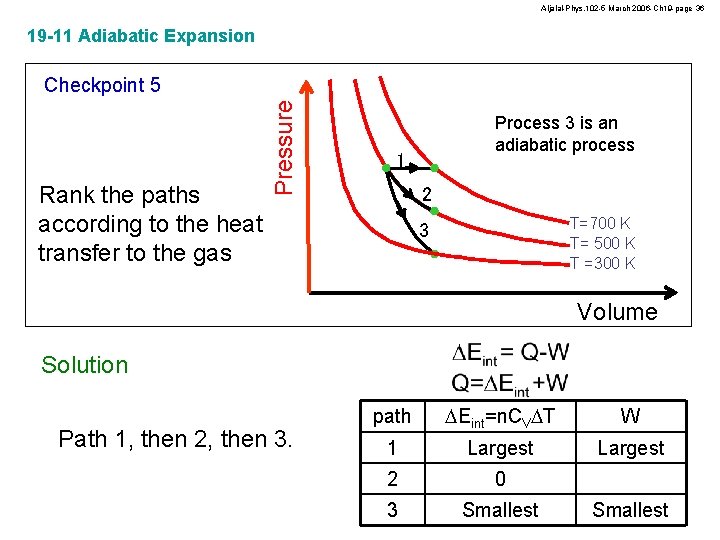
Aljalal-Phys. 102 -5 March 2006 -Ch 19 -page 36 19 -11 Adiabatic Expansion Rank the paths according to the heat transfer to the gas Pressure Checkpoint 5 Process 3 is an adiabatic process 1 2 T=700 K T= 500 K T =300 K 3 Volume Solution Path 1, then 2, then 3. path DEint=n. CVDT W 1 Largest 2 0 3 Smallest

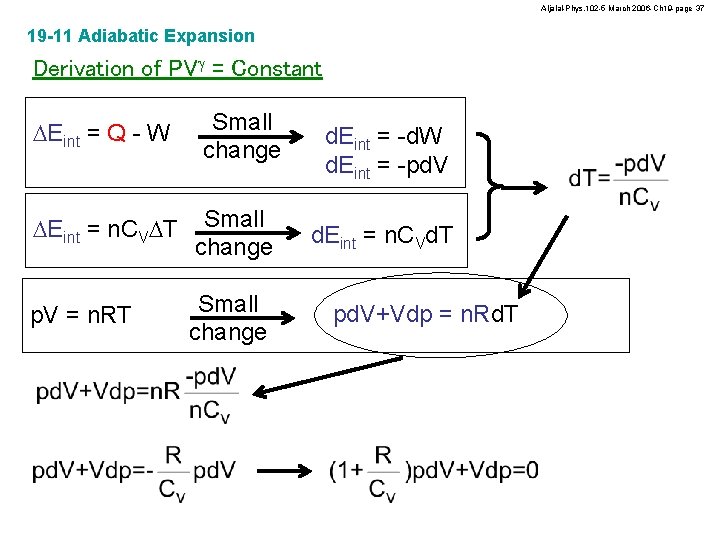
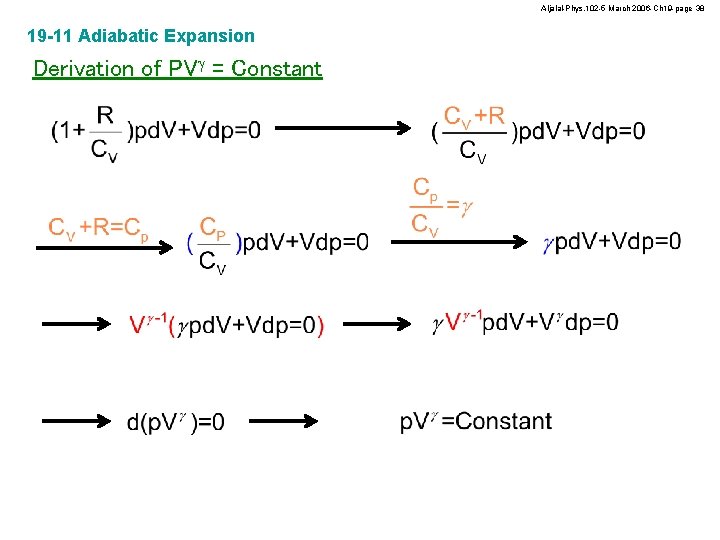
Aljalal-Phys. 102 -5 March 2006 -Ch 19 -page 37 19 -11 Adiabatic Expansion Derivation of PVg = Constant DEint = Q - W Small change DEint = n. CVDT Small change p. V = n. RT Small change d. Eint = -d. W d. Eint = -pd. V d. Eint = n. CVd. T pd. V+Vdp = n. Rd. T

Aljalal-Phys. 102 -5 March 2006 -Ch 19 -page 38 19 -11 Adiabatic Expansion Derivation of PVg = Constant

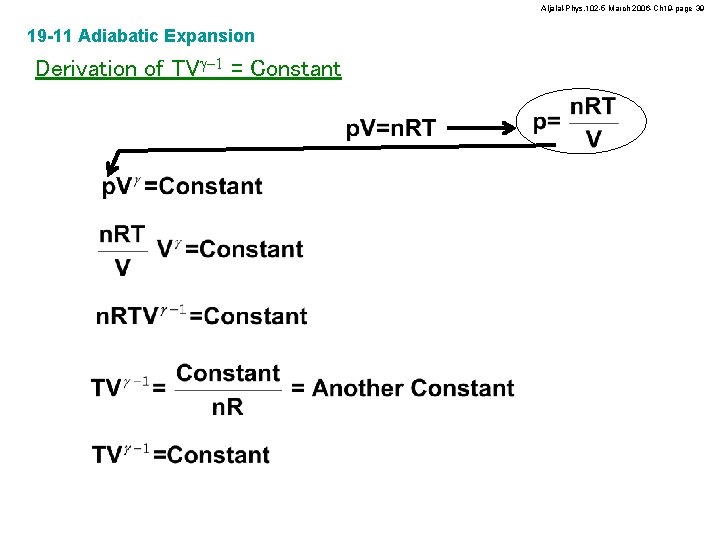
Aljalal-Phys. 102 -5 March 2006 -Ch 19 -page 39 19 -11 Adiabatic Expansion Derivation of TVg-1 = Constant
 Anthem of poland
Anthem of poland March 9, 2006
March 9, 2006 Astronomy picture of the day march 29 2006
Astronomy picture of the day march 29 2006 Starts new page numbered sequentially
Starts new page numbered sequentially Apa heading format
Apa heading format Iat 334
Iat 334 Art.102 tfue
Art.102 tfue Ess 102
Ess 102 102
102 Electric potential lecture
Electric potential lecture Mc 338
Mc 338 Area 102
Area 102 Psychology 102 practice test
Psychology 102 practice test Faisal fairag kfupm
Faisal fairag kfupm 102 capacitor
102 capacitor 102 lgx
102 lgx 102 graphic
102 graphic Nur 102
Nur 102 Phys 102 uiuc
Phys 102 uiuc Convenio 102 oit
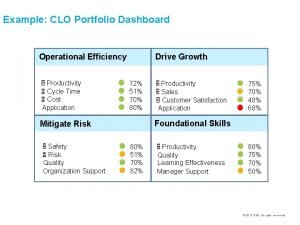
Convenio 102 oit Clo-102
Clo-102 Legea nr. 102/2020
Legea nr. 102/2020 I 102 104
I 102 104 Swiss international style
Swiss international style Physics 102
Physics 102 Parallel lines cut by a transversal vocab practice
Parallel lines cut by a transversal vocab practice Fitness chapter 102
Fitness chapter 102 Comp102 ecs
Comp102 ecs Nur 102
Nur 102 Nur 102
Nur 102 Kmvss 102
Kmvss 102 Clo-102
Clo-102 Bisp 196 ucsd
Bisp 196 ucsd Psalm 28 niv
Psalm 28 niv Aljalal phys 102
Aljalal phys 102 Phép trừ bù
Phép trừ bù Janviers tabell
Janviers tabell Egr 102
Egr 102 Devlet memurları kanunu 102.madde
Devlet memurları kanunu 102.madde Physics 102
Physics 102