2015 NHSN Training Teresa Fox CIC Quality Improvement
























































- Slides: 106

2015 NHSN Training Teresa Fox, CIC Quality Improvement Advisor teresa. fox@area-G. hcqis. org Central Line-Associated Bloodstream Infections -CLABSI Surgical Site Infections-SSI Ventilator-Associated Events- VAE

Objectives Define key terms and concepts for primary and secondary bloodstream infections (BSI), surgical site infections (SSI) and ventilator-associated infections (VAE) Identify 2015 surveillance definition and protocol changes Identify 2015 CLABSI and SSI CMS-required reporting for IQR program 2

New CLABSI Reporting Requirements 2015 In addition to ICUs, report CLABSI from all patient care locations which are mapped as adult and pediatric wards: Medical Surgical Medical/surgical http: //www. cdc. gov/nhsn/PDFs/Newsletters/vol 9 -3 -e. NL-Sept-2014. pdf 3

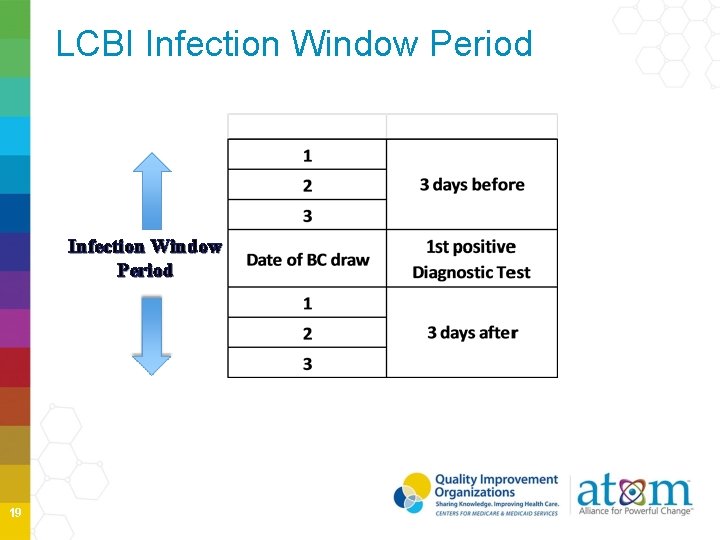
BSI Key Terms Date of Event- the date when the first element used to meet the NHSN site-specific infection criterion occurs for the first time in the Infection Window Period- 7 -day period in which all infection criterion must be met. It includes the date of the first positive diagnostic test. POA- date of event occurs on the day of admission or day after admission. POA period continues to include day of admission, 2 days before and the day after admission HAI- date of event occurs on or after the 3 rd of admission RIT- 14 -day timeframe during which no new infections of the same type are reported 4

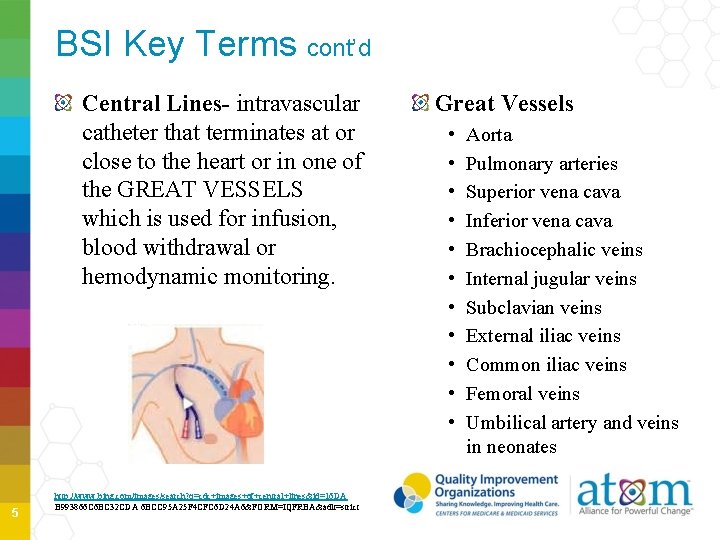
BSI Key Terms cont’d Central Lines- intravascular catheter that terminates at or close to the heart or in one of the GREAT VESSELS which is used for infusion, blood withdrawal or hemodynamic monitoring. 5 http: //www. bing. com/images/search? q=cdc+images+of+central+lines&id=16 DA B 993866 C 6 BC 32 CDA 6 BCC 95 A 25 F 4 CFC 6 D 24 A 6&FORM=IQFRBA&adlt=strict Great Vessels • • • Aorta Pulmonary arteries Superior vena cava Inferior vena cava Brachiocephalic veins Internal jugular veins Subclavian veins External iliac veins Common iliac veins Femoral veins Umbilical artery and veins in neonates

Central Line Notes Introducer is considered an intravascular catheter and depending on the location of the tip it may be a central line Pacemaker wires and other non-lumened devices into great vessels or heart are not considered central lines Extracoporeal membrane oxygenation, femoral arterial catheters and intraaortic balloon pumps are not considered central lines Assistance with determining if a device is a central line, email to NHSN@cdc. gov 6

Infusions Introduction of a solution through a catheter lumen into a blood vessel Includes: • Continuous infusions such as nutritious fluids or medications • Intermittent infusions such as flushed or IV antimicrobial administration • Administration of blood or blood products in the case of transfusion or hemodialysis 7

Types of Central Lines Temporary Central Lines • A non-tunneled, nonimplanted catheter 8 Permanent Central Lines • Tunneled catheters, including certain dialysis catheters • Implanted catheters (including ports)

Central Line-associated Bloodstream Infection (CLABSI) CLABSI surveillance utilizes the Major Event Type: BSI Specific Event Type: Laboratory Confirmed Bloodstream Infection (LCBI) 9

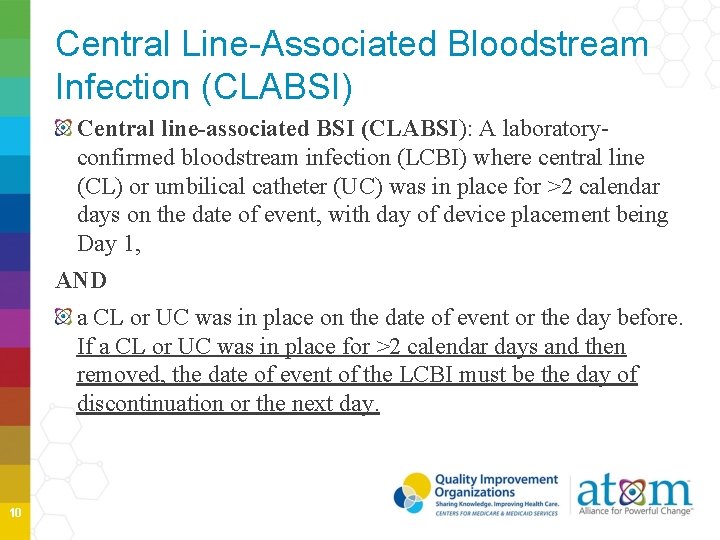
Central Line-Associated Bloodstream Infection (CLABSI) Central line-associated BSI (CLABSI): A laboratoryconfirmed bloodstream infection (LCBI) where central line (CL) or umbilical catheter (UC) was in place for >2 calendar days on the date of event, with day of device placement being Day 1, AND a CL or UC was in place on the date of event or the day before. If a CL or UC was in place for >2 calendar days and then removed, the date of event of the LCBI must be the day of discontinuation or the next day. 10

Central Line-Associated Bloodstream Infection (CLABSI) cont’d If patient is admitted or transferred into a facility with an implanted central line (port) in place, and that is the patient’s only central line, the day of first access in an inpatient location is considered Day 1. Access is defined as line placement, infusion or withdrawal through the line. Such lines continue to eligible for CLABSI once they are accessed until patient is discharged or line is discontinued. 11

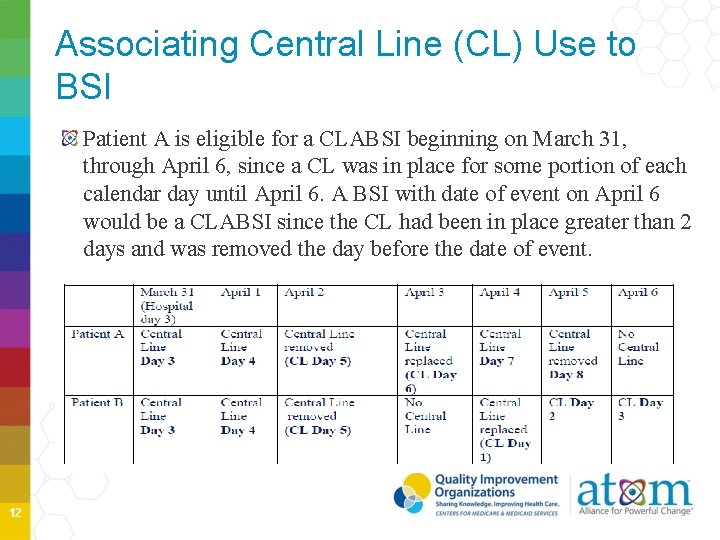
Associating Central Line (CL) Use to BSI Patient A is eligible for a CLABSI beginning on March 31, through April 6, since a CL was in place for some portion of each calendar day until April 6. A BSI with date of event on April 6 would be a CLABSI since the CL had been in place greater than 2 days and was removed the day before the date of event. 12

Positive Blood Cultures One or more blood cultures means that at least one bottle from a blood draw is reported as having at least one organism Recognized pathogen does not include organisms considered as common commensals by NHSN • Bacillus, Corynebacterium, Staph epi or hominis, etc 13

Blood Culture Specimens All blood culture specimens must be included in surveillance if participating in NHSN CLABSI surveillance • Venipuncture • Vascular catheter • Cannot be considered a contaminant unless a single unmatched common commensal is reported. 14

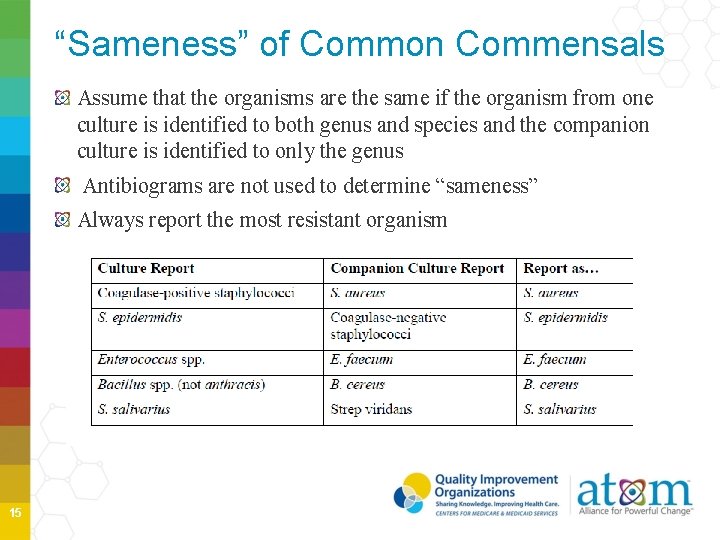
“Sameness” of Common Commensals Assume that the organisms are the same if the organism from one culture is identified to both genus and species and the companion culture is identified to only the genus Antibiograms are not used to determine “sameness” Always report the most resistant organism 15

CLABSI Examples Patient has a central line inserted on June 1. On June 3, the central line is removed and on June 4 the patient has a positive blood culture with S. aureus. This is a CLABSI because the central line was in place for >2 calendar days (June 1, 2, and 3), and was in place the day before the date of event (June 4). A central line is placed in the facility on May 30 th. On June 3, the central line is removed and on June 5 patient spikes a fever of 38. 3°C. Two blood culture sets collected on June 6 are positive for S. epidermidis. May be a healthcare-associated bloodstream infection but it is not a CLABSI because the Date of Event (June 5) did not occur on the day the central line was discontinued (June 3) or the next day (June 4). 16

Inpatient Dialysis Inpatients receiving dialysis are included in any CLABSI surveillance in the location in which they are housed, regardless of whether or not the central line is the only central line and only accessed for dialysis. This also applies to patients in Long-Term Acute Care (LTAC) facilities within Acute Care Facilities when dialysis is received from the Acute Care Facility staff. • • Example: Patient on Unit A receives onsite dialysis by contracted dialysis staff Dialysis staff travels to Unit A to provide dialysis to Unit A patient Patient resides on Unit A for inpatient care, but is transported to dialysis unit within the facility for dialysis. • Since CLABSIs cannot be attributed to non-bedded locations, such an event must be attributed to the inpatient location housing the patient 17

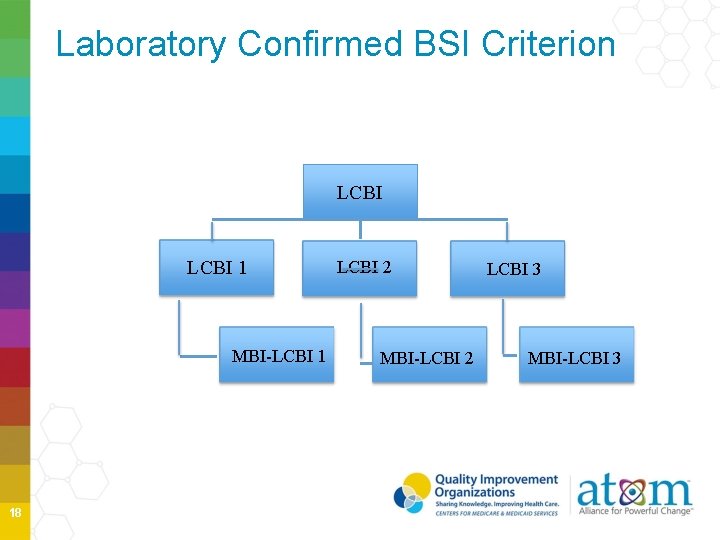
Laboratory Confirmed BSI Criterion LCBI 1 MBI-LCBI 1 18 LCBI 2 MBI-LCBI 2 LCBI 3 MBI-LCBI 3

LCBI Infection Window Period 19

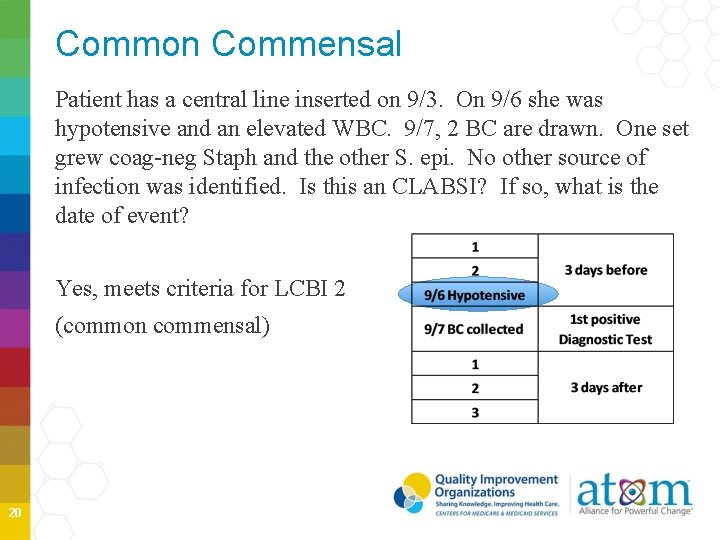
Common Commensal Patient has a central line inserted on 9/3. On 9/6 she was hypotensive and an elevated WBC. 9/7, 2 BC are drawn. One set grew coag-neg Staph and the other S. epi. No other source of infection was identified. Is this an CLABSI? If so, what is the date of event? Yes, meets criteria for LCBI 2 (common commensal) 20

MBI-LCBI Mucosal Barrier Injury Laboratory-Confirmed Bloodstream Infections Subset of LCBI criteria Must meet LCBI 1, 2 or 3 prior to applying the MBI-LCBI criteria If an MBI-LCBI is reported, and a subsequent BC occurs during the RIT of the MBI-LCBI with an organism that is excluded from the MBI criteria, the primary MBI-LCBI event is edited to become an LCBI and the organism is added to the event A single common commensal does not exclude from meeting MBILCBI criteria List of Eligible Enterobacteriaceae is available on the NHSN website MCI-LCBI will be removed from the 2016 CLABSI metrics shared with CMS 21

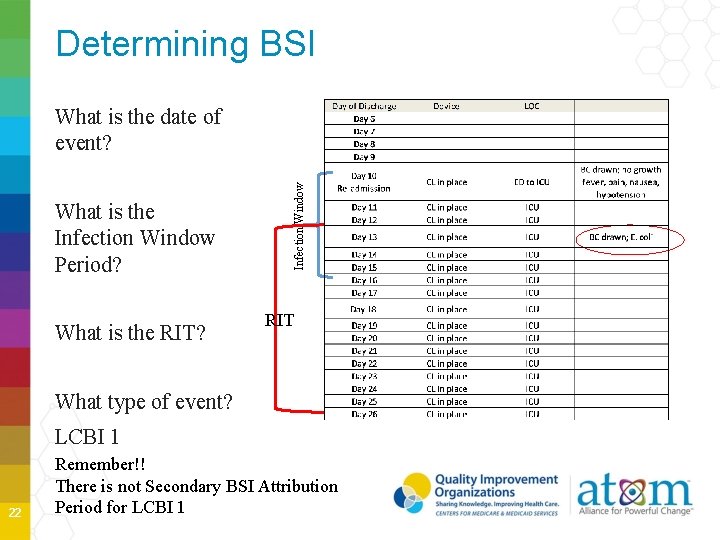
Determining BSI What is the Infection Window Period? What is the RIT? Infection Window What is the date of event? RIT What type of event? LCBI 1 22 Remember!! There is not Secondary BSI Attribution Period for LCBI 1

CLABSI Summary Clinical and surveillance definitions will sometimes differ Accurate data collection is necessary for successful prevention efforts and is dependent on a variety of factors No substantive changes of 2015 CLABSI definitions and protocols New Terms 23

Secondary Bloodstream Infection Secondary BSI Guidance updates Chapter 17 - NHSN Surveillance Definitions for Specific Types of Infections updated Chapter 4 Appendix 1; Secondary Bloodstream Infection Guide is not applicable to Ventilator-Associated Events (VAE). Guidance is central for determining surveillance of primary vs. secondary BSIs. 24

“…and organism cultured from blood is not related to an infection at another site…” BSI that is associated with an infection at another site is referred to as a Secondary BSI and is never reported into NHSN CLABSI may not be secondary to an infection at another site, it is always primary BSI Primary BSI is identified by ruling out all non-blood sites as the source of the BSI 25

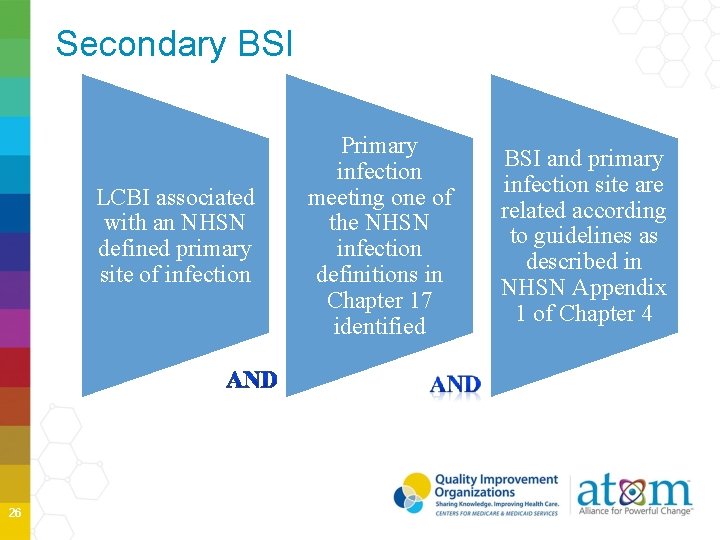
Secondary BSI LCBI associated with an NHSN defined primary site of infection 26 Primary infection meeting one of the NHSN infection definitions in Chapter 17 identified BSI and primary infection site are related according to guidelines as described in NHSN Appendix 1 of Chapter 4

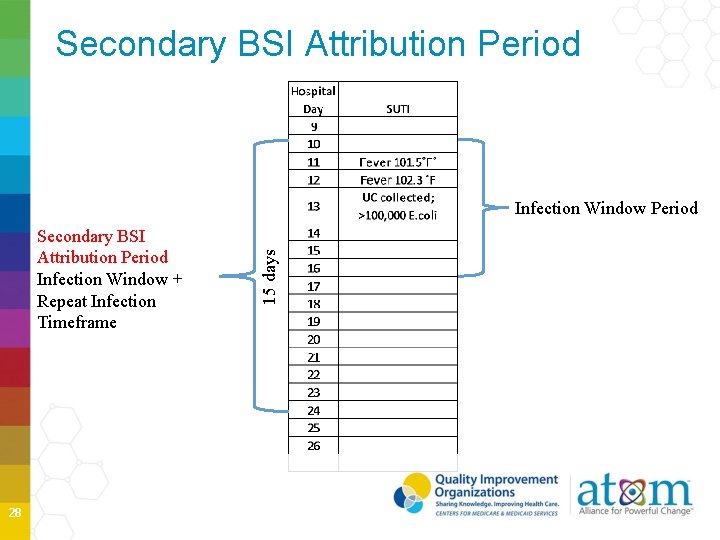
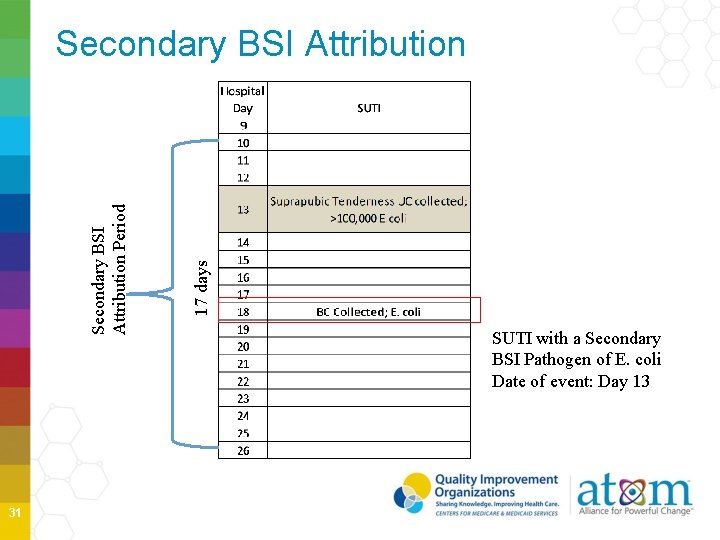
Secondary BSI Attribution Period when a positive blood culture must be collected to be considered a secondary BSI to a primary site infection Period includes the Infection Window Period plus the Repeat Infection Timeframe (RIT) Period is 14 -17 days in length depending on the date of event NOTE: A primary BSI will NOT have a Secondary BSI Attribution Period 27

Secondary BSI Attribution Period Infection Window + Repeat Infection Timeframe 28 15 days Infection Window Period

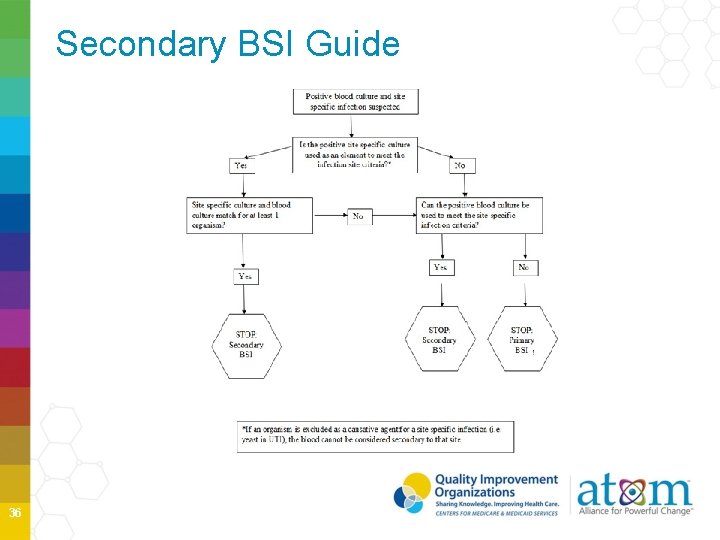
Secondary BSI Attribution Secondary BSI may be attributed to a primary site infection If, • BC pathogen matches at least one organism found in a sitespecific infection culture used to meet the primary site infection criterion OR • Positive BC is an element used to meet the primary site infection criterion 29

Secondary BSI Case Scenario 1 Blood and site-specific cultures match: • At least one organism match • That organism cannot be an excluded organism for that sitespecific infection • Site-specific criteria must be met using the site-specific culture Example Patient meets criterion 1 for a symptomatic UTI (suprapubic tenderness and > 100, 000 CFU/ml of E. coli) and BC collected 5 days later grows E. coli. 30

31 17 days Secondary BSI Attribution Period Secondary BSI Attribution SUTI with a Secondary BSI Pathogen of E. coli Date of event: Day 13

Secondary BSI Scenario 1 Ex. 2 Patient meets criterion 1 for SUTI (suprapubic tenderness and >100, 000 CFU/ml of E. coli and > 100, 000 of Candida glabrata) and BC is collected 5 days later which is within the BSI Attribution period grows Candida glabrata and S. aureus. This is a SUTI with E. coli and a primary BSI with Candida glabrata and S. aureus if no other primary infection site is identified • Candida is an excluded organism for UTIs • S. aureus does not match at least one organism found in the site specific culture (urine) 32

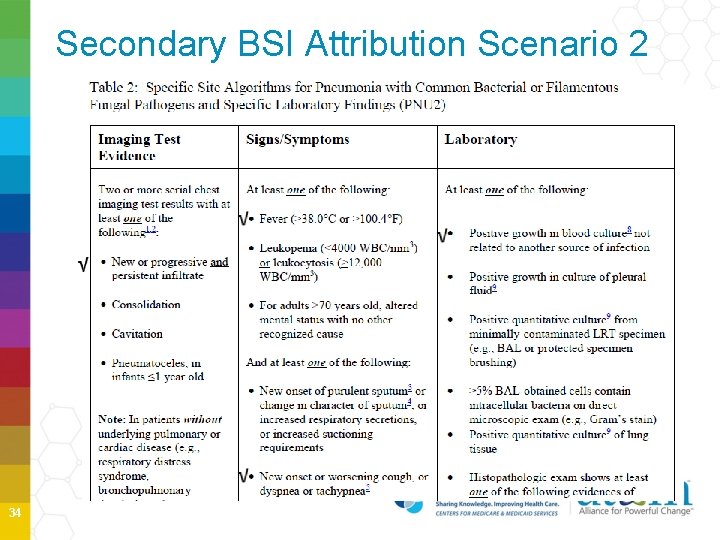
Secondary BSI Scenario 2 Blood and site-specific cultures do NOT match • If blood isolate is an element used to meet the site-specific criterion, then the BSI is reported as a Secondary to that site • Example Patient is afebrile, has a new onset of cough and has positive chest X-ray showing the presence of infiltrate. P. aeruginosa is isolated from the blood. Because the patient meet the PNU 2 definition the blood is considered a secondary BSI to the PNEU. No primary BSI would be reported 33

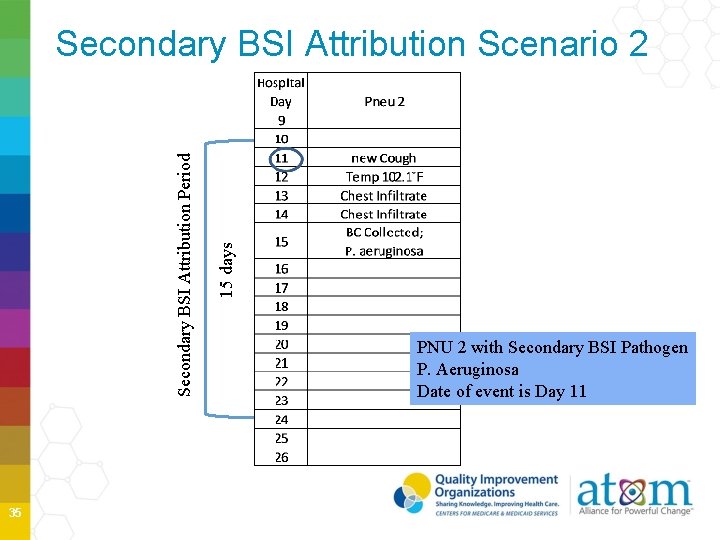
Secondary BSI Attribution Scenario 2 34

35 15 days Secondary BSI Attribution Period Secondary BSI Attribution Scenario 2 PNU 2 with Secondary BSI Pathogen P. Aeruginosa Date of event is Day 11

Secondary BSI Guide 36

Pathogen Assignment for Secondary BSI Additional eligible pathogens identified within a RIT are added to the index event Pathogen exclusions for specific definitions also apply to secondary BSI pathogen attribution • Excluded pathogens must be attributed to another primary site specific infection as either a secondary BSI or as a primary BSI 37

Secondary BSI Summary Understand new terminology • • • 38 Date of event Infection Window Period Repeat Infection Timeframe (RIT) Consider another primary source for infections Consistently apply Secondary BSI Guidance Assign pathogens appropriately

Surgical Site Infections: SSI 39

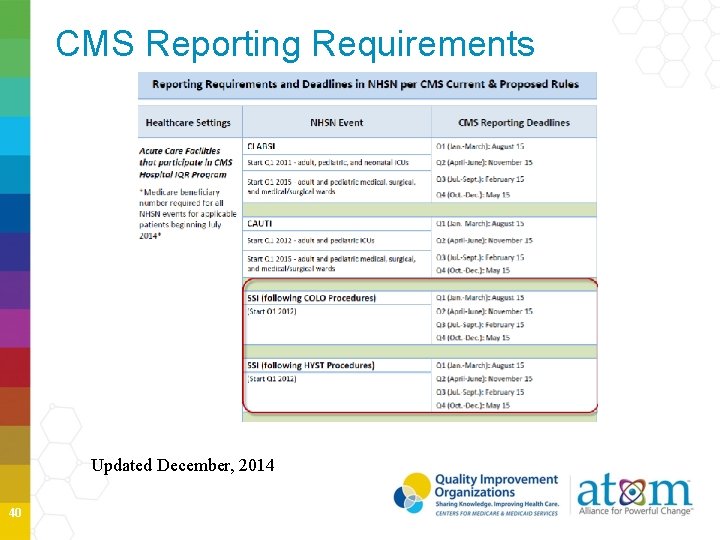
CMS Reporting Requirements Updated December, 2014 40

Supporting Resources 41

Monthly Reporting Plans are very important to your surveillance and CMS Reporting ONLY data included in the Monthly Reporting Plans will be used to aggregate data analysis and submit to CMS appropriately Plans drive much of the business logic of NHSN Must have a plan for every month Must apply all definitions and protocols, reporting all superficial, deep and organ space SSIs 42

Surveillance Methods Active SSI Surveillance Methods Post-discharge SSI Surveillance Method 43

How do the new terms and definitions apply to SSI? Infection Window Period, POA, HAI and RIT and secondary bloodstream infection attribution period definitions DO NOT apply to SSI, VAE or Lab. ID Events. Secondary BSIs may be attributed to SSI events • Guidance specific to SSIs see Secondary BSI Attribution Period and the SSI and BSI protocols 44

NHSN Operative Procedure An NHSN operative procedure is a procedure • that is included in Table 1 and • takes place during an operation where at least one incision (including laparoscopic approach) is made through the skin or mucous membrane, or reoperation via an incision that was left open during a prior operative procedure and • takes place in an operating room [OR], defined as a patient care area that met the Facilities Guidelines Institute’s (FGI) or American Institute of Architects’ (AIA) criteria for an operating room when it was constructed or renovated [9]. This may include an operating room, C-section room, interventional radiology room, or a cardiac catheterization lab. 45

Date of Event Date of event (DOE): For an SSI the date of event is the date when the first element used to meet the SSI infection criterion occurs for the first time during the surveillance period. Synonym: infection 46

SSI Reporting SSIs are always reported at the deepest level that they occur within the surveillance period. If during the surveillance period a patient’s initial SSI meets criteria for a deeper level, then the date of event should be the date for the deepest level. For example: • • 47 Day 1 –COLO procedure Day 6 –DOE for meeting a superficial incisional SSI Day 25 –DOE for the meeting an organ space IAB SSI Only report one SSI with the DOE for the organ space IAB.

Pathogen Assignment The Pathogen Assignment Guidance found in Chapter 2 “Identifying HAIs” is based on Repeat Infection Timeframes (RIT) which is NOT used with SSIs • SSI are procedure based and have long surveillance periods (30 to 90 days) • SSIs can progress to a deeper level during a surveillance period and a new pathogen can be found • Pathogen Assignment for SSI has not changed 48

Primary Closure is defined as closure of the skin level during the original surgery, regardless of the presence of wires, wicks, drains, or other devices or objects extruding through the incision. This category includes surgeries where the skin is closed by some means. Thus, if any portion of the incision is closed at the skin level, by any manner, a designation of primary closure should be assigned to the surgery. NOTE: If a procedure has multiple incision/laparoscopic trocar sites and any of the incisions are closed primarily, then the procedure is entered as having been closed primarily. This change removed the phrase “all tissue levels” from the definition. This definition will be easier to apply and is closer to definitions used by other surgical professional groups. 49

Non-Primary Closure Non-primary Closure is defined as closure that is other than primary and includes surgeries in which the skin level is left completely open during the original surgery and therefore cannot be classified as having primary closure. For surgeries with non-primary closure, the deep tissue layers may be closed by some means (with the skin level left open), or the deep and superficial layers may both be left completely open. An example of a surgery with non-primary closure would be a laparotomy in which the incision was closed to the level of the deep tissue layers, sometimes called “fascial layers” or “deep fascia, ” but the skin level was left open. Another example would be an “open abdomen” case in which the abdomen is left completely open after the surgery. Wounds with nonprimary closure may or may not be described as "packed” with gauze or other material, and may or may not be covered with plastic, “wound vacs, ” or other synthetic devices or materials. One sentence was removed since this scenario would actually meet criteria for a primary closure since the skin was closed. 50

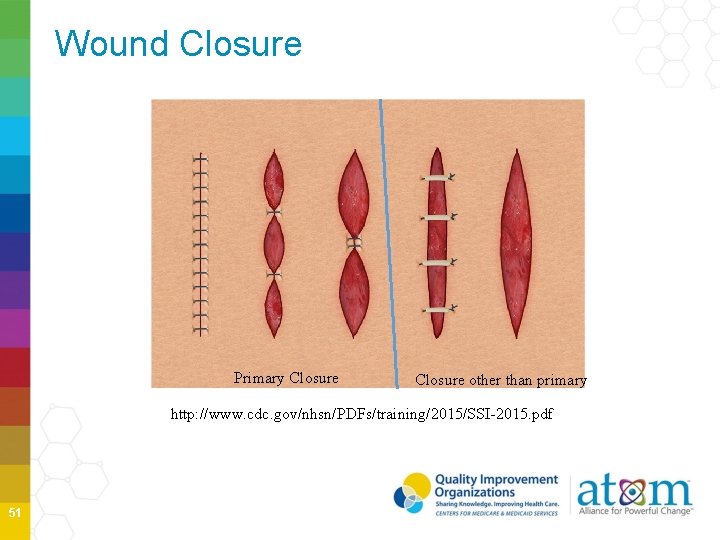
Wound Closure Primary Closure other than primary http: //www. cdc. gov/nhsn/PDFs/training/2015/SSI-2015. pdf 51

Primary vs Non-primary Closures A patient is admitted with a ruptured diverticulum and a COLO procedure is performed in the inpatient OR. Case is entered as a wound class 3. Specimen is obtained in the OR which later returns (+) for E. coli. Surgeon staples closed the skin at 4 locations with packing placed between the staples. Is this procedure primarily closed in 2015? YES If you are following COLO, should you include this case in your denominator data? YES 52

Diabetes NHSN has added another option for users to answer the question of diabetes on the denominator for procedure form. • NHSN users can chose to use, assignment of the discharge ICD-9 -CM codes in the 250 to 250. 93 range to answer YES to this diabetes field question. • The 2014 definition is also still in place as a choice to answer this field. 53

BSI Secondary to an SSI Secondary BSI Attribution Period: The secondary BSI attribution period for SSI is a 17 -day period that includes the date of event, 3 days prior and 13 days after. Why does SSI have its own secondary BSI attribution period? • For other HAIs the Secondary BSI attribution period is determined by using the Infection Window Period and the Repeat Infection Timeframe. These two definitions do not apply to SSIs. 54

BSI Secondary to an SSI NEW Any blood culture that occurs during the SSI Secondary BSI attribution period will be assessed using Appendix 1 in the BSI protocol to determine if the blood meets Secondary BSI criteria. �If a (+) blood culture occurs after the SSI secondary BSI attribution period it should be fully evaluated to see if at that time it is meeting criteria to be secondary to an ongoing SSI. 55

Infection Present at Time of Surgery Infection present at time of surgery (PATOS) denotes that there is evidence of an infection present at the time of the start of or during the index surgical procedure (in other words, it is present pre-operatively). • This field is a required field and it is found on the SSI event form not on the denominator for procedure form. • PATOS does not apply if there is a period of wellness between the time of a pre-operative condition and the surgery. • The evidence of infection must be noted/documented preoperatively or found intraoperatively in a pre-operative or intra-operative note. 56

PATOS cont’d • Only select PATOS = YES, if it applies to the depth of SSI that is being attributed to the procedure • The patient does not have to meet the NHSN definition of an SSI at the time of the primary procedure but there must be evidence of infection or abscess present at the time of surgery. 57

Re-baselining of SSIs for 2016 NSHN is going to re-baseline all HAIs based on 2015 data. The 2015 data reported to NHSN will provide the baseline for calculating the Standardized Infection Ratio (SIR) for 2016 and subsequent years. SIRs calculated for the 2015 data will use the current baselines. SSIs reported with PATOS = YES will be excluded from the SSI SIR beginning with 2016 data and the new baseline. These excluded SSIs will be analyzed separately. 58

PATOS Patient was admitted with an acute abdomen, to OR for XLAP with findings of an abscess due to ruptured appendix and an APPY is performed. Patient returns 2 weeks later and meets criteria for an organ space IAB SSI. Does this patient meet the criteria for PATOS? YES Since this SSI is related to an infection that was PATOS it does not have to be reported to NHSN ? YES 59

NHSN Inpatient/Outpatient Operative Procedure Based on feedback provided by NHSN users regarding the 2015 changes to the Inpatient and Outpatient OR Procedure definition, NHSN has made a decision to rescind these changes. The SSI protocol and the Table of Instructions in the NHSN manual will be updated to reflect this change. • NHSN users were notified of this update via February NHSN release notes. • Therefore, for 2015, the NHSN SSI protocol will refer to inpatient and outpatient operative procedures, rather than operative procedures that are performed on inpatients or outpatients. • Please disregard earlier guidance to identify OR areas/suites as inpatient or outpatient. 60

Procedures Through Same Incision If procedures in more than one NHSN operative procedure category are done through the same incision during the same trip to the OR, create a record for each procedure that you are monitoring in the Monthly Reporting Plan, and use the total time for the duration for each record. 61

Incisions with 24 - Hours If the patient goes to the OR more than once during the same admission and another procedure of the same or different NHSN operative procedure category is performed through the same incision within 24 hours of the end of the original procedure, report only one Denominator for Procedure form for the original procedure combining the durations for both procedures. • Assign the surgical wound closure that applies when the patient leaves the OR from the principal operative procedure. This instruction should be followed in scenarios where a patient leaves the OR with non-primary closure, but returns to the OR for a subsequent procedure that results in primary closure of the procedure. 62

Additional Fields for Specific Procedures There are 5 procedures for which additional risk factors are collected: • • 63 Cesarean Section –CSEC Spinal Fusion and Refusion –FUSN; RFUSN Hip Arthroplasty –HPRO Knee Arthroplasty –KPRO

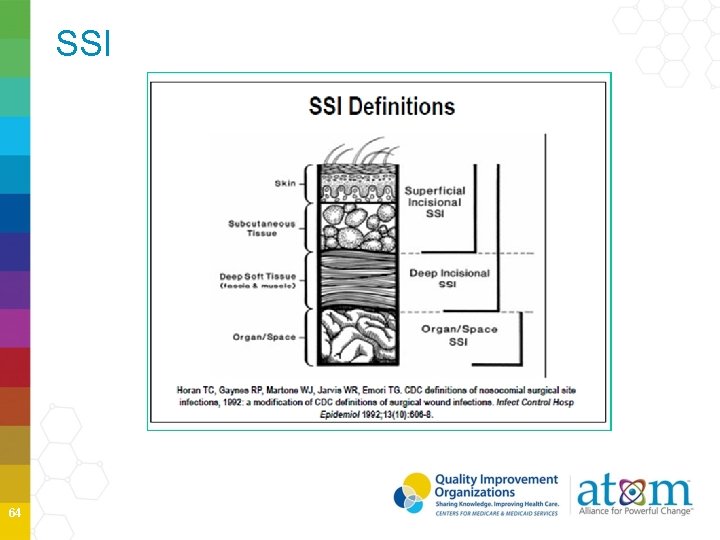
SSI 64

SSI Superficial Diagnosis/treatment of cellulitis (redness/warmth/swelling), by itself, does not meet criterion “d” for superficial incisional SSI. An incision that is draining or culture (+) is not considered a cellulitis. A stitch abscess alone (minimal inflammation and discharge confined to the points of suture penetration) is not considered an SSI. A localized stab wound or pin site infection. While it would be considered either a skin (SKIN) or soft tissue (ST) infection, depending on its depth, it is not reportable under this module. Note: a laparoscopic trocar site for an NHSN operative procedure is not considered a stab wound. 65

If more than one operation is done through a single incision… First, attempt to determine the procedure that is thought to be associated with the infection. Example: If the patient had a CBGC and CARD done at the same time and develops an infected valve, then the SSI will be linked to the CARD. If it’s not clear (as in the case of a superficial incisional SSI), use the NHSN Principal Operative Procedure Selection Lists to select which operative procedure to report. 66

SSI following invasive manipulation or accession of the operative site If during the post-operative period the surgical site has an invasive manipulation/accession for diagnostic or therapeutic purposes (e. g. , needle aspiration), and following this manipulation/accession an SSI develops, the infection is not attributed to the operation. • This reporting instruction does NOT apply to closed manipulation (e. g. , closed reduction of a dislocated hip after an orthopedic procedure). • Invasive manipulation does not include wound packing, or changing of wound packing materials as part of postoperative care. 67

When a patient with an SSI has had more than one operation… If a patient has several NHSN operations prior to an SSI, report the operation that was performed most closely in time prior to the infection date. This does not apply when 2 operative procedures are done within the same 24 hour period via the same incision. • Example: Patient underwent a COLO on 1/12/15. One week later on 1/19/15, he returns to OR for an CHOL via the same incision. He developed an incisional SSI on 1/28/15. This SSI is attributed to the second procedure (CHOL), not the COLO. 68

Reporting SSI for Patients who are Readmitted Use the admission date of the surgical admission as the date admitted to facility, not the readmission date • Then the date of procedure and date of event will be in the correct sequence Date Admitted to Facility ≤ Date of Procedure < Date of Event 69

Transition to ICD-10 -CM/PCS and CPT Codes Updated ICD-10 -CM/PCS and CPT mappings to all NHSN operative procedure categories for SSI surveillance. These mappings are anticipated to be available Spring of 2015. ICD-10 -CM/PCS codes will replace ICD-9 -CM codes on October 1, 2015 however NHSN will not have the ability to receive these codes until the January 2016 release. The NHSN guidance for entry of surgical denominator data for the last quarter of 2015 data is to enter the NHSN Procedure Code (e. g. COLO or HYST) but do not enter any ICD-10 CM/PCS 70

Ventilator-Associated Events (VAE) 71

VAE Surveillance Changes for 2015 Modification 1: Consolidation of Third Tier of VAE algorithm • PVAP (possible VAP) replaced the original possible and probable VAP • Uses the same parameters • Culture and non-culture based identification of pathogens, purulent respiratory secretions • Provides simplification and consistent with plan for analysis 72

VAE Surveillance Changes for 2015 Modification 2: Exception to the requirement that a daily minimum PEEP and Fi. O 2 values be maintained for at least 1 hour in select circumstances • When no value documented to have been maintained for at least 1 hour, the minimum value is the lowest value set on the vent during the calendar day Modification 3: Additional pathogen exclusions • Community associated fungal pathogens that are either not know to cause or rarely cause HAIs Modification 4: Introduction of a new denominator: Episodes of Mechanical Ventilation (EMV) 73

NHSN Lower Respiratory Event VAE is the only in-plan option available for ventilated patients in adult locations VAP (Ventilator-associated Pneumonia) is the only in-plan option for ventilated patients in pediatric locations (ped. VAC) 74

VAE Surveillance Changes for 2015 Modification 4: Introduction of a new denominator: Episodes of Mechanical Ventilation (EMV) • Total number of episodes occurring during month • Evidence based practices that improve outcomes in vent patient focusing on getting patient off • Need to explore use of other denominator with this new surveillance method • Vent days (required) and APRV (required) • EMV (optional) 75

NHSN Surveillance NHSN Lower Respiratory Event Surveillance • PNEU definitions are still available • Off-plan of VAP in adults, children, neonates and non-ventilated PNEU in adults, children and neonates PNEU definitions are in Chapter 6, titled Pneumonia (Ventilator-Associated (VAP) and Non-ventilator-Associated Pneumonia (PNEU) Event Purulent sputum is determined by direct exam/ Gram stain results PNEU Pathogen exclusions are the same as for VAE protocol • Candida species or yeast not otherwise specified, Coag-neg Staph species, and Enterococcus species are excluded unless from lung tissue or pleural fluid • Cryptococcus, Histoplasma, Coccidioides, Paraoccidioides, Blastomyces, Pneumocystis are excluded for use in PNEU definition (PNEU 2, PNEU 3) 76

PNEU/VAP Modification 3 Pathogen reporting and secondary BSI attribution for the PNEU 1 is not permitted • NHSN application will default to NO for secondary BSI and NO for pathogens when PNEU 1 is selected • Modification of the specific event to PNU 1 to PNU 2 or PNU 3 is permitted if PNU 2 or 3 definitions are met within the RIT Matching pathogens recovered from a respiratory culture and blood culture alone do not allow for assignment of a BSI as secondary to PNEU 77

PNEU/VAP Endotracheal aspirate and sputum specimens are not minimally contaminated LRT specimens Quantitative and semi-quantitative equivalent reporting of pathogens recovered from minimally contaminated LRT specimens (BAL, protected specimen brush) are now acceptable 78

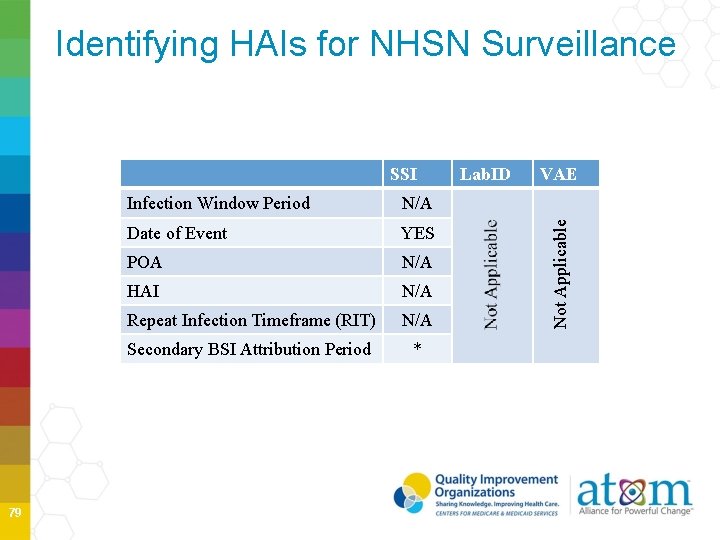
Identifying HAIs for NHSN Surveillance 79 Infection Window Period N/A Date of Event YES POA N/A HAI N/A Repeat Infection Timeframe (RIT) N/A Secondary BSI Attribution Period * Lab. ID VAE Not Applicable SSI

Who is eligible for VAE? Inpatients of acute care hospitals, long term acute care hospitals, inpatient rehabilitation facilities Patients in adult locations are eligible for VAE surveillance • Pediatric patients in adult locations are included in surveillance • Adult patients in pediatric locations are included in ped. VAP surveillance Patients ventilated for ≥ 3 days Patients not on high frequency ventilation (HFV) or extracorporeal life support (ECLS) 80

What about other alternative modes of mechanical ventilation? Include patients who are receiving a conventional mode of ventilation and: • • Prone positioning Nitric oxide therapy Helium-oxygen mixture Epoprostenol therapy Include patients on Airway Pressure Release Ventilation (APRV) or related modes 81

APRV and VAC Determinations Evaluation for VAC will be limited to the Fi. O 2 parameter when the patient is on APRV for the entire calendar day, since changes in PEEP as indicated in this surveillance algorithm may not apply to APRV • Do not use Hi/Lo values • Do not designate PEEP as “ 0” on data collection tool or VAE calculator • PEEP is N/A When patient is on APRV for parts of calendar day PEEP values recorded during periods of time when the patient in on conventional mode of ventilation is used to determine daily minimum PEEP 82

PEEP and FIO 2 A sustained increase in the daily minimum PEEP of ≥ 3 cm. H 20 following a period of stability or improvement on the ventilator is one of two criteria that can be used to meet the VAC definition A sustained increase in the daily minimum FIO 2 of ≥ 0. 20 (20%) following a period of stability or improvement on the ventilator is the second of the two criteria that can be used to meet the VAC definition 83

Daily Minimum PEEP and FIO 2 settings are documented across the calendar day are used to identify daily minimum PEEP and FIO 2 Typically both are recorded in the paper or electronic medical record on respiratory therapy and/or nursing flowsheets Always use a calendar day Choose the lowest PEEP and FIO 2 setting during the calendar day that was maintained for at least 1 hour, if no value was maintained for 1 hour, select the lowest value regardless of timeframe Exception to 1 hour maintenance • Ventilation began late in calendar day • Ventilation stopped early in calendar day • Ventilation setting unstable throughout calendar day 84

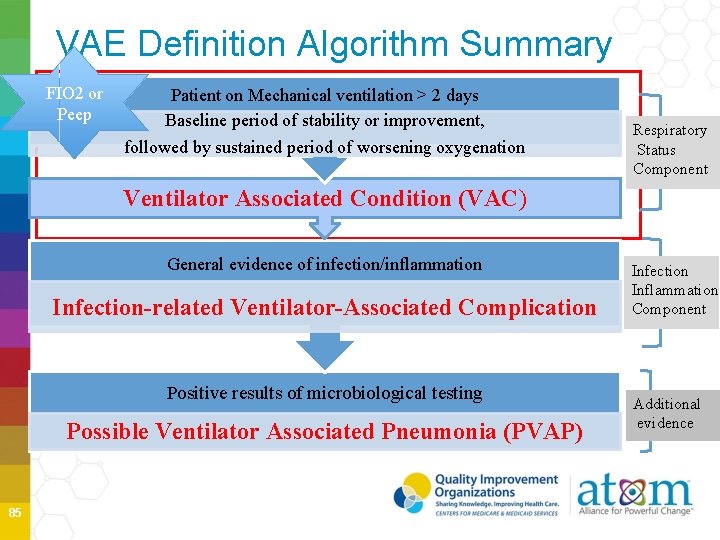
VAE Definition Algorithm Summary FIO 2 or Peep Patient on Mechanical ventilation > 2 days Baseline period of stability or improvement, followed by sustained period of worsening oxygenation Respiratory Status Component Ventilator Associated Condition (VAC) General evidence of infection/inflammation Infection-related Ventilator-Associated Complication Positive results of microbiological testing Possible Ventilator Associated Pneumonia (PVAP) 85 Infection Inflammation Component Additional evidence

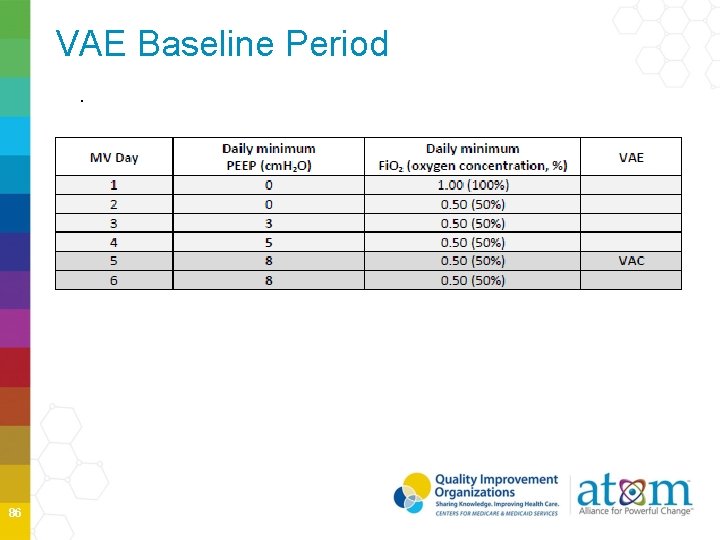
VAE Baseline Period. 86

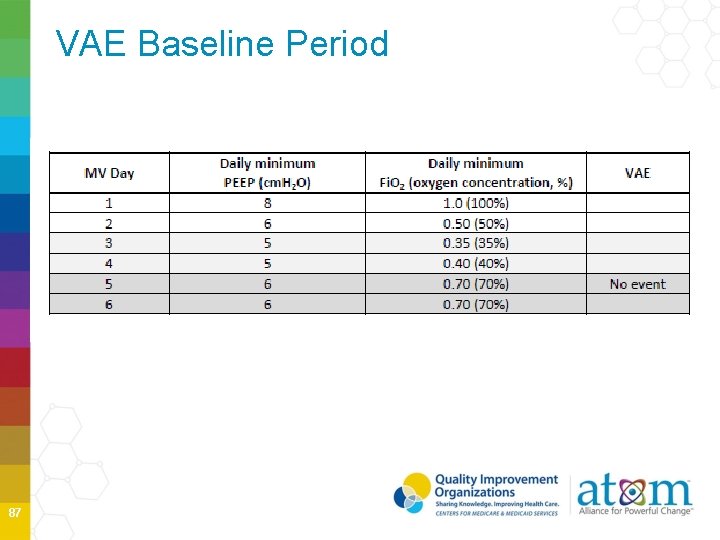
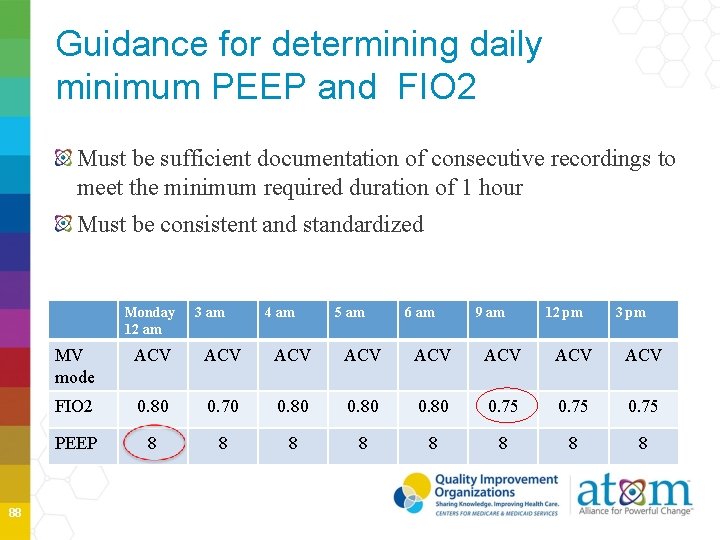
VAE Baseline Period 87

Guidance for determining daily minimum PEEP and FIO 2 Must be sufficient documentation of consecutive recordings to meet the minimum required duration of 1 hour Must be consistent and standardized Monday 12 am 88 3 am 4 am 5 am 6 am 9 am 12 pm 3 pm MV mode ACV ACV FIO 2 0. 80 0. 70 0. 80 0. 75 PEEP 8 8 8 8

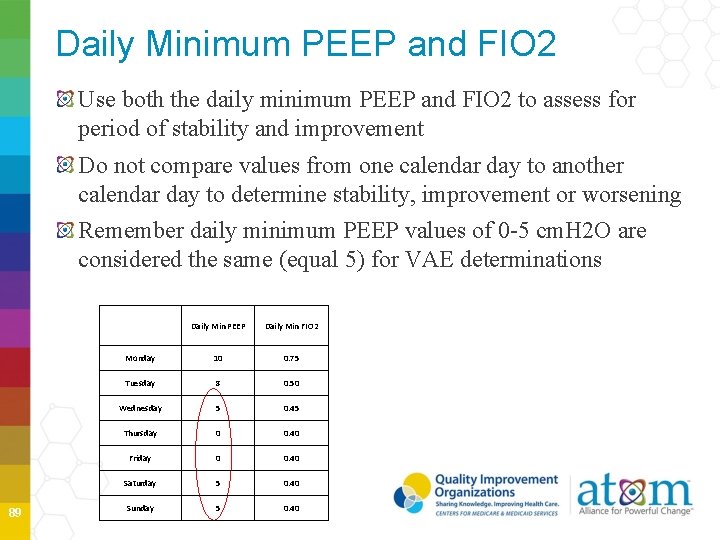
Daily Minimum PEEP and FIO 2 Use both the daily minimum PEEP and FIO 2 to assess for period of stability and improvement Do not compare values from one calendar day to another calendar day to determine stability, improvement or worsening Remember daily minimum PEEP values of 0 -5 cm. H 2 O are considered the same (equal 5) for VAE determinations 89 Daily Min PEEP Daily Min FIO 2 Monday 10 0. 75 Tuesday 8 0. 50 Wednesday 5 0. 45 Thursday 0 0. 40 Friday 0 0. 40 Saturday 5 0. 40 Sunday 5 0. 40

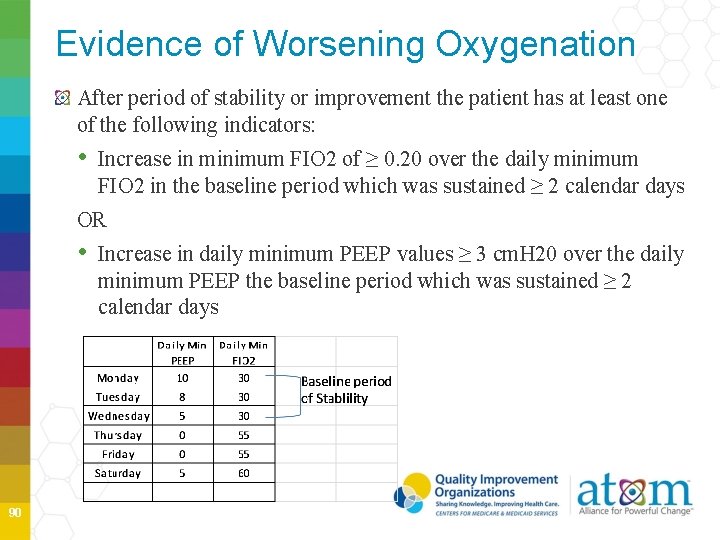
Evidence of Worsening Oxygenation After period of stability or improvement the patient has at least one of the following indicators: • Increase in minimum FIO 2 of ≥ 0. 20 over the daily minimum FIO 2 in the baseline period which was sustained ≥ 2 calendar days OR • Increase in daily minimum PEEP values ≥ 3 cm. H 20 over the daily minimum PEEP the baseline period which was sustained ≥ 2 calendar days 90

Date of Event Date of event of worsening oxygenation (day 1 of the required ≥ 2 day period) • Earliest date of event for VAE is mechanical ventilation day 3 (first day of worsening Oxygenation) • First possible day that VAC criteria can be fulfilled is mechanical ventilation day 4 91

14 -Day VAE Event Period Date of event sets VAE Event Period is the timeframe during which criteria for other events--- IVAC, PVAP----must be met • Each VAE Event Period is a 14 -day in duration • Day 1 is the date of event • Cannot upgrade VAE on data collected outside of VAE Event Period • Blood cultures must be collected during the 14 -day event period for a BSI to be secondary to VAE 92

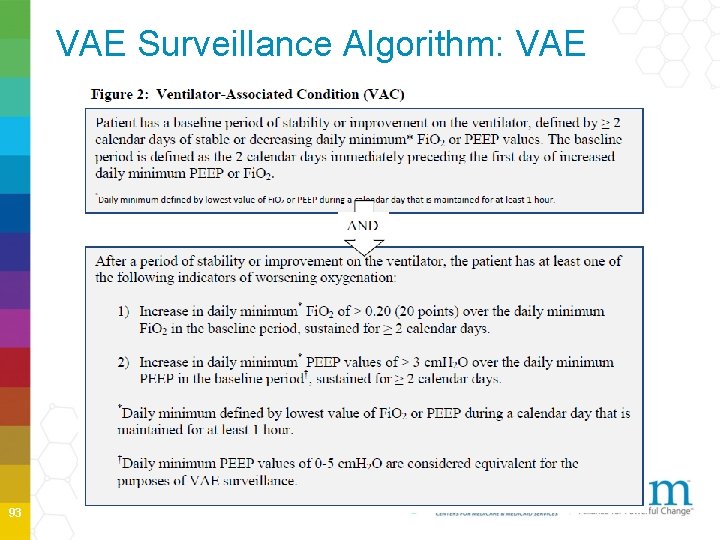
VAE Surveillance Algorithm: VAE 93

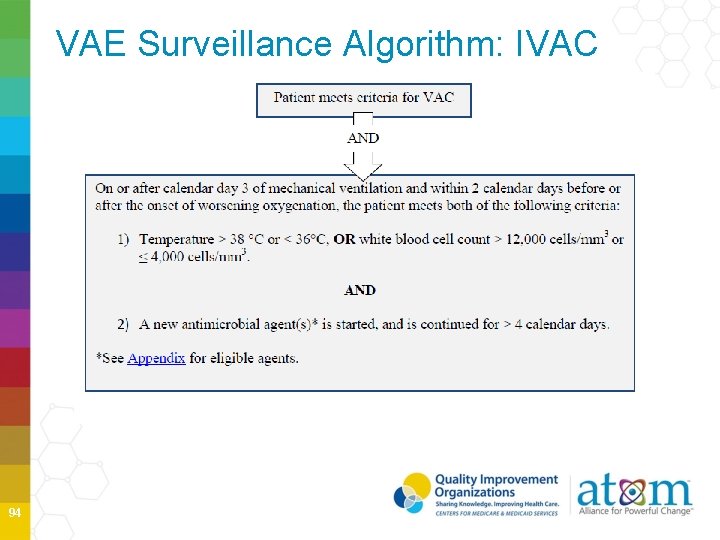
VAE Surveillance Algorithm: IVAC 94

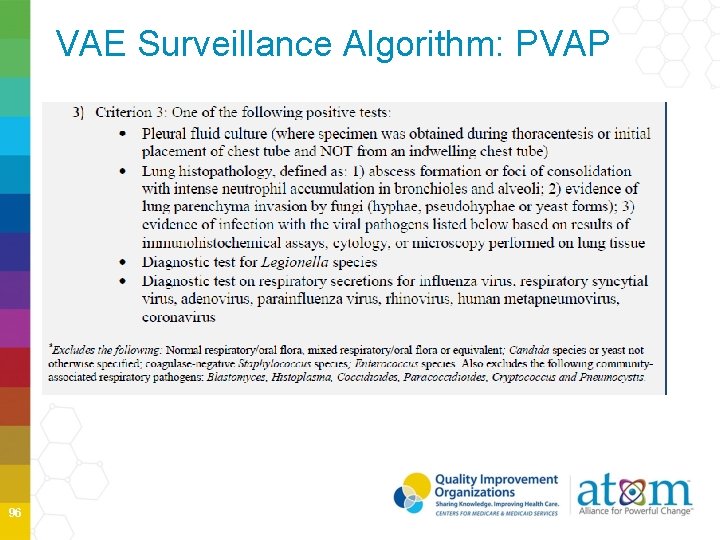
VAE Surveillance Algorithm: PVAP 95

VAE Surveillance Algorithm: PVAP 96

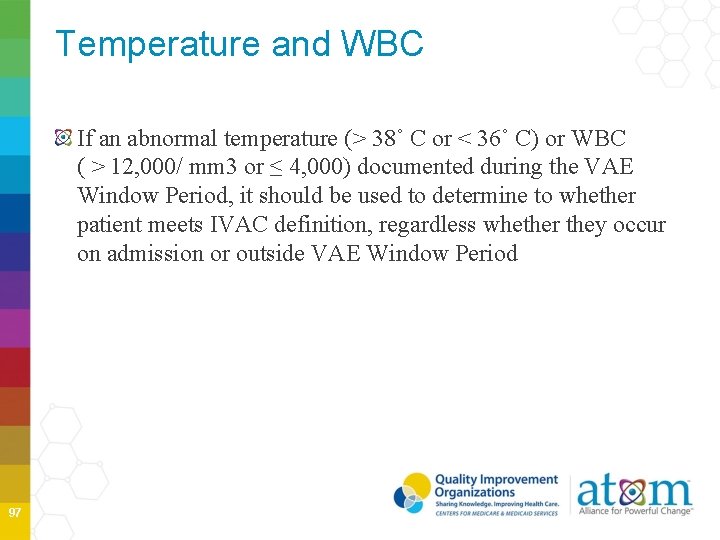
Temperature and WBC If an abnormal temperature (> 38˚ C or < 36˚ C) or WBC ( > 12, 000/ mm 3 or ≤ 4, 000) documented during the VAE Window Period, it should be used to determine to whether patient meets IVAC definition, regardless whether they occur on admission or outside VAE Window Period 97

IVAC New Antimicrobials New antimicrobial is any agent listed in the protocol Appendix that is initiated on or after the third calendar day of mechanical ventilation and in the VAE Window Period • Considered new if it was not given to patient on either days preceding the current start date • Must be continued for ≥ 4 consecutive days • No requirement that the same antimicrobial be given on all 4 consecutive days • Administered IV, IM digestive tract or respiratory tract 98

PVAP VAC and IVAC must be met Laboratory test collection dates must occur • On or after calendar day 3 of ventilation and within 2 calendar days before or after onset of worsening oxygenation (VAE Window Period) • Organism exclusions must be considered • Normal respiratory/oral flora, mixed respiratory/oral flora • Candid species or yeast not otherwise specified, coag-neg Staph species, Enterococcus species, unless isolated from lung tissue or pleural fluid • Community-associated pathogens 99

Pathogen Reporting Pathogens are not reported for VAC or for IVAC Pathogens may be reported for PVAP, according to usual pathogen and antimicrobial susceptibility reporting methods 10 0

Secondary BSI to PVAP Secondary BSI may only be reported for PVAP • When at least one eligible organism from the blood culture specimen matches an eligible organism from an appropriate respiratory tract specimen collected during the VAE Window Period • And when the blood culture was collected during the 14 -day event period 10 1

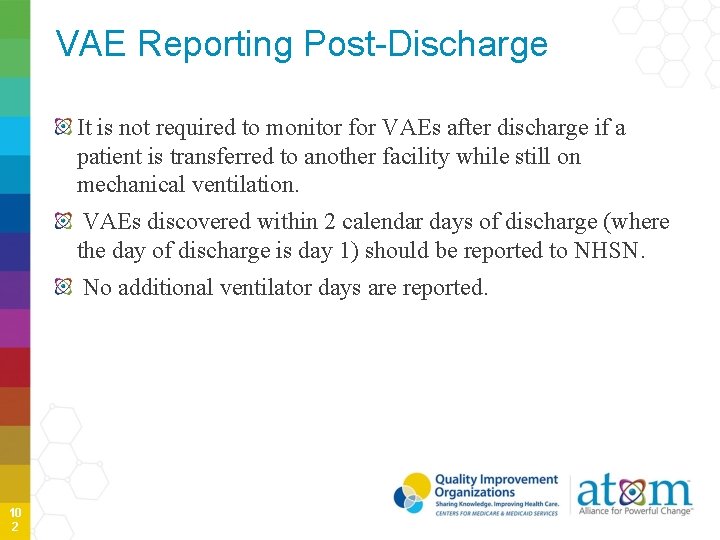
VAE Reporting Post-Discharge It is not required to monitor for VAEs after discharge if a patient is transferred to another facility while still on mechanical ventilation. VAEs discovered within 2 calendar days of discharge (where the day of discharge is day 1) should be reported to NHSN. No additional ventilator days are reported. 10 2

Using VAE Calculator http: //www. cdc. gov/nhsn/VAE-calculator/ 10 3

VAE Summary Patient must be ventilated for more than 2 calendar days Patient must have ≥ 2 calendar days of stability or improvement of oxygenation immediately followed by ≥ 2 calendar days of worsening oxygenation Earliest date of event for VAE is mechanical ventilation day 3 10 4

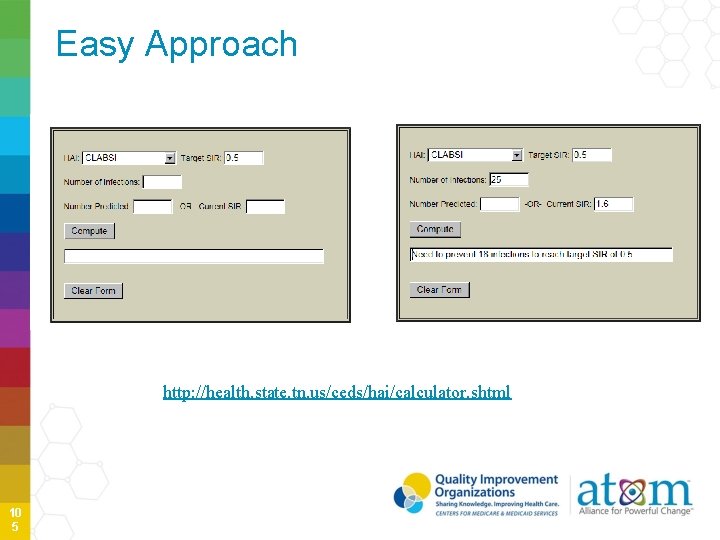
Easy Approach http: //health. state. tn. us/ceds/hai/calculator. shtml 10 5

Thank you Slides and information prepared using CDC/NHSN Training February 2015 slides and materials 10 6