Quality Improvement vs Research Can we draw a
















- Slides: 18

Quality Improvement vs. Research: Can we draw a line in the sand? Verena Jorgensen, MD Cheryl Byers, MHA, CIP


Timeline for Investigation • NEJM Publication Letter of complaint to OHRP • OHRP Investigates lack of IRB review and Informed Consent of participants (patients and health care providers) • JHM stated study was exempt from the regulations • Michigan Health made NHSR determinations • OHRP requested corrective action PI has suspended all activities while application was submitted to JHM IRB for review & approval

“The Initiative” • “ We hypothesized that we can improve patient safety; improve safety culture; and reduce ICU mortality, blood stream infections, aspiration pneumonia and ICU LOS. ” • Implement an ICU Safety Reporting System • Improve communication and staffing in ICUs • Intervention to reduce/eliminate catheter related blood stream infections • Intervention to improve the care of ventilated patients • Intervention to reduce mortality • Steering committee to review data annually to ensure “no patient is knowingly subjected to excess risk or prevented from receiving beneficial treatment. ”

The Outcome • “The Initiative” took place between Sept 2003 -Sept 2005. • By 2008, the interventions studied were proven effective and had been implemented as part of clinical practice. • Data collection continued, Michigan hospitals were sending JHM de -identified data so OHRP agreed that the project had evolved to a point where JHM was no longer engaged in human subjects research.

Definition of Quality Improvement (QI) • QI consists of systematic and continuous actions that lead to measurable improvement in health care services and the health status of targeted patient groups. -DHHS Definition • What is considered “quality” in health care? • A direct correlation between the level of improved health services and the desired health outcomes of individuals and populations. -Institute of Medicine

Research • Research: A systematic investigation, including research development, testing, and evaluation designed to develop or contribute to generalizable knowledge. (45 CFR 46. 102(d)) • Under this definition, the project must intend at the outset to generate conclusions which can be applied in, or be predictive of similar circumstances. • Human Subject: a living individual about whom an investigator conducting research obtains (1) data through intervention or interaction with the individual, or (2) obtains identifiable private information. (45 CFR 46. 102(f)).

Key Question! • Is the program implemented for a research purpose, or altered or controlled in some way to answer a research question?

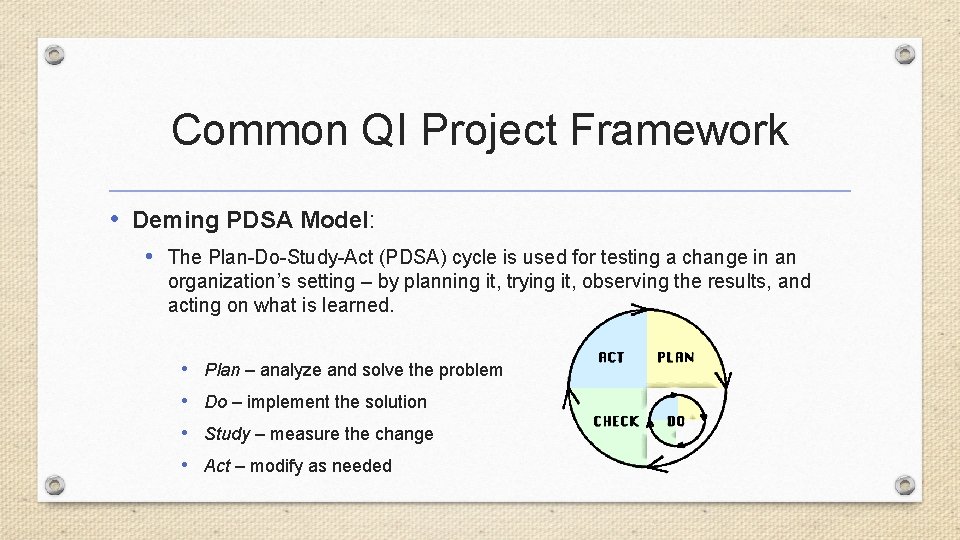
Common QI Project Framework • Deming PDSA Model: • The Plan-Do-Study-Act (PDSA) cycle is used for testing a change in an organization’s setting – by planning it, trying it, observing the results, and acting on what is learned. • Plan – analyze and solve the problem • Do – implement the solution • Study – measure the change • Act – modify as needed

Ask Yourself… • • What are you trying to accomplish? How will you know a change brings about an improvement? What changes can result in improvement? Will the evaluation affect change within the organization?

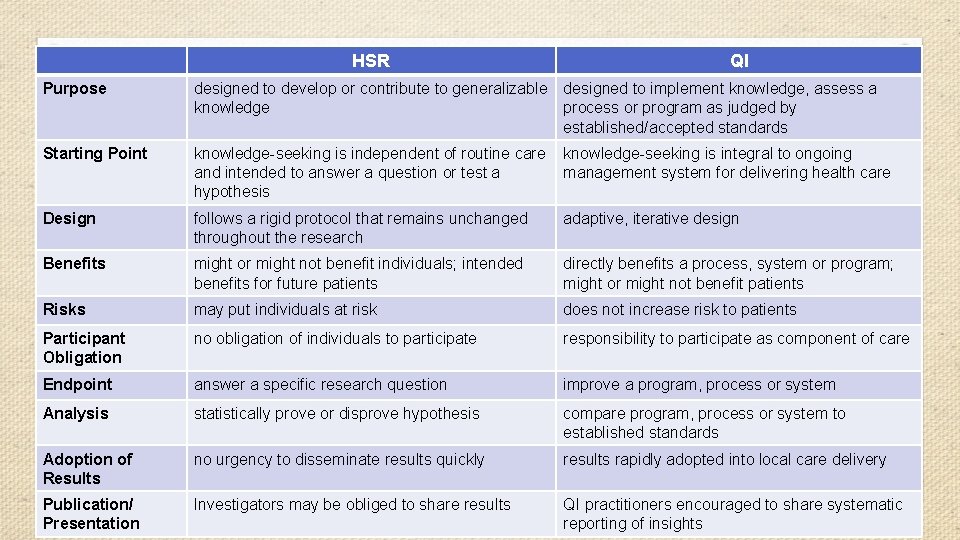
HSR QI Purpose designed to develop or contribute to generalizable designed to implement knowledge, assess a knowledge process or program as judged by established/accepted standards Starting Point knowledge-seeking is independent of routine care and intended to answer a question or test a hypothesis knowledge-seeking is integral to ongoing management system for delivering health care Design follows a rigid protocol that remains unchanged throughout the research adaptive, iterative design Benefits might or might not benefit individuals; intended benefits for future patients directly benefits a process, system or program; might or might not benefit patients Risks may put individuals at risk does not increase risk to patients Participant Obligation no obligation of individuals to participate responsibility to participate as component of care Endpoint answer a specific research question improve a program, process or system Analysis statistically prove or disprove hypothesis compare program, process or system to established standards Adoption of Results no urgency to disseminate results quickly results rapidly adopted into local care delivery Publication/ Presentation Investigators may be obliged to share results QI practitioners encouraged to share systematic reporting of insights

Hastings Center Special Report • The Ethics of Using QI Methods to Improve Health Care Quality and Safety http: //www. thehastingscenter. org/uploaded. Files/Publications/Special_ Reports/using_qi_methods_to_improve_health_care_quality_safety. p df • Includes worksheet to help QI Investigators ascertain whether IRB review is required

Do QI Projects Require IRB Review? • The majority of QI projects do not require review by the USF IRB, as most of these projects are designed solely for internal program evaluation purposes with no external application or generalization (USF HRPP Policy, Section 4. 1). • However, projects which qualify as research and involve human subjects, as defined in the federal regulations, would require IRB review.

IRB Approval is Required if Your Project: • Seeks to develop new knowledge or validate new treatments rather than to assess the implementation of existing knowledge; • Methodology employs a standard research design, such as randomization; • Protocol has a rigid goal, methodology, population, time period, etc. ; • Funding comes from external organization such as the NIH or those with commercial interest in the results; • Includes delays in implementing results; or • Risks from the intervention are greater than minimal.

What about Publishing? • What if I intend to publish the results of the project? • Planning to publish a QI project does not necessarily mean the project meets the definition of research. • People may seek to publish descriptions of non-research activities if they believe others may be interested in learning about those activities. However, QI findings should not be represented as research in publications.

When in Doubt. . . • If you are unsure about your project, please contact a member of the IRB staff for assistance. • If you submit an application, and the project is determined by the IRB Chairperson to be excluded from review, you will be notified and the application will be closed with a notation that the project does not meet the definition of human subjects research and therefore, does not fall within the purview of the USF IRB.

Questions?

Contact Information Jean Winter, CIP Assistant Director for Regulatory Affairs University of South Florida Research Integrity & Compliance 3702 Spectrum Boulevard, Suite 155 Tampa, Florida 33612 tjwinter@usf. edu ARC Help Desk 813 -974 -2880 RSCH-arc@usf. edu