2 DIVERSITY OF MATTER Eclipse Pink Floyd The























- Slides: 50

2. DIVERSITY OF MATTER

Eclipse (Pink Floyd –The Dark side of the Moon) All that you touch All that you see All that you taste All you feel All that you love All that you hate All you distrust All you save All that you give All that you deal All that you buy beg, borrow or steal All you create All you destroy All that you do All that you say All that you eat everyone you meet All that you slight everyone you fight All that is now All that is gone All that's to come And everything under the sun is in tune (*) But the sun is eclipsed by the moon. And is made of MATTER!

…Matter is anything that occupies space.

States of Matter The Four States of Matter u. Solid u. Liquid u. Gas u. Plasma 4

States of Matter The Four States of Matter Basis of Classification of the Four Types Ø Based upon particle arrangement Ø Based upon energy of particles Ø Based upon distance between particles 5

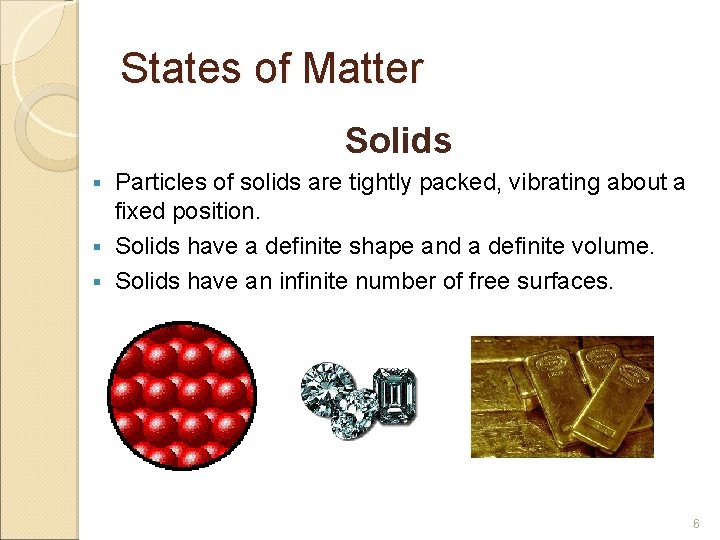
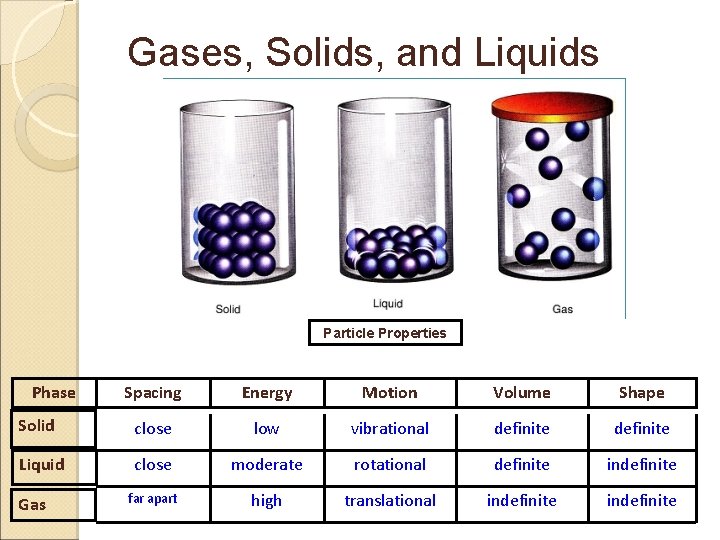
States of Matter Solids Particles of solids are tightly packed, vibrating about a fixed position. § Solids have a definite shape and a definite volume. § Solids have an infinite number of free surfaces. § 6

States of Matter Microscopic Explanation for Properties of Solids § Solids have a definite shape and a definite volume because the particles are locked into place § Solids are not easily compressible because there is little free space between particles § Solids do not flow easily because the particles cannot move/slide past one another 7

States of Matter Liquids Particles of liquids are tightly packed, but are far enough apart to slide over one another. § Liquids have an indefinite shape and a definite volume. § Liquids have one free surface. § 8

States of Matter Microscopic Explanation for Properties of Liquids § Liquids have an indefinite shape because the particles can slide past one another. § Liquids are not easily compressible and have a definite volume because there is little free space between particles. § Liquids flow easily because the particles can move/slide past one another. 9

States of Matter Gases Particles of gases are very far apart and move freely. § Gases have an indefinite shape and an indefinite volume. § Gases have no free surfaces. § 10

States of Matter Microscopic Explanation for Properties of Gases § Gases have an indefinite shape and an indefinite volume because the particles can move past one another. § Gases are easily compressible because there is a great deal of free space between particles. § Gases flow very easily because the particles randomly move past one another. 11

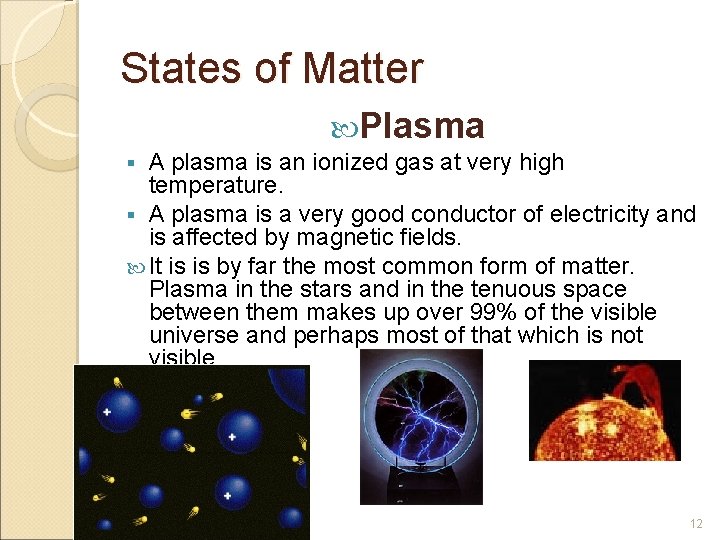
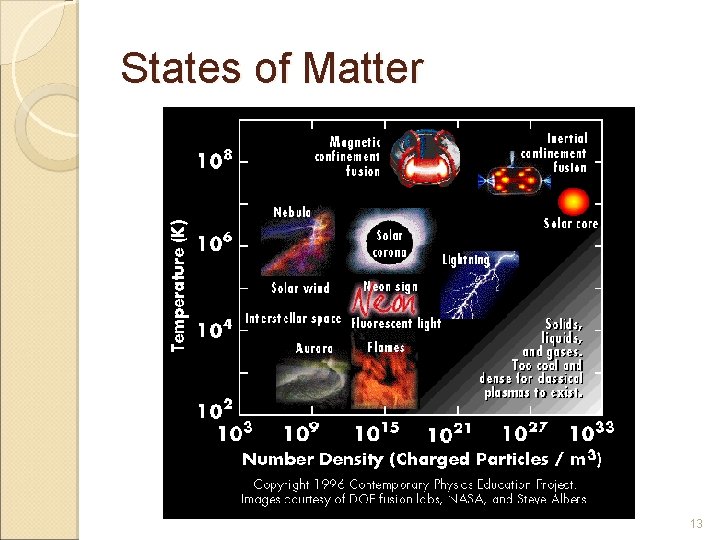
States of Matter Plasma A plasma is an ionized gas at very high temperature. § A plasma is a very good conductor of electricity and is affected by magnetic fields. It is is by far the most common form of matter. Plasma in the stars and in the tenuous space between them makes up over 99% of the visible universe and perhaps most of that which is not visible. § 12

States of Matter 13

States of Matter Microscopic Explanation for Properties of Plasmas § Plasmas have an indefinite shape and an indefinite volume because the particles can move past one another. § Plasmas are easily compressible because there is a great deal of free space between particles. § Plasmas are good conductors of electricity and are affected by magnetic fields because they are composed of ions (negatively charged electrons and positively charged nuclei). 14

States of Matter The Four States of Matter The Classification and Properties of Matter Depend Upon Microscopic Structure Ø Particle arrangement Ø Particle energy Ø Particle to particle distance 15

Gases, Solids, and Liquids Particle Properties Phase Spacing Energy Motion Volume Shape Solid close low vibrational definite Liquid close moderate rotational definite indefinite far apart high translational indefinite Gas

Physical properties are those that we can determine without changing the identity of the substance we are studying.

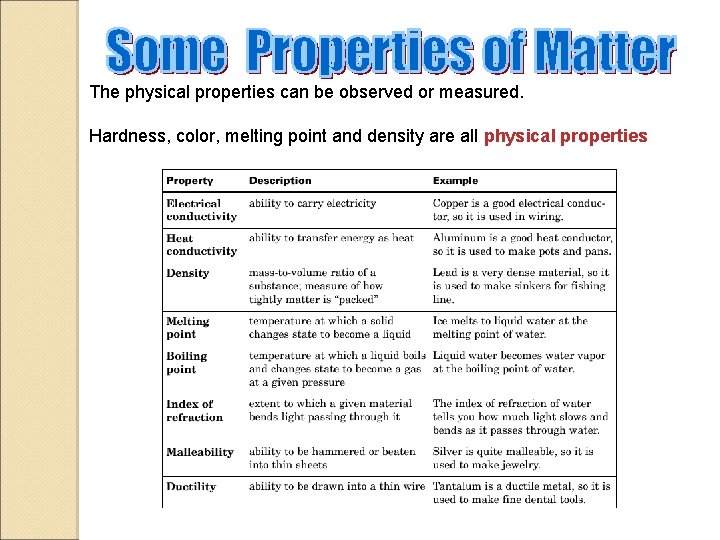
The physical properties can be observed or measured. Hardness, color, melting point and density are all physical properties

Intensive properties do not depend on the size of the sample of matter and can be used to identify substances. density, color, melting and boiling point , …. .

Extensive properties depend on the quantity of the sample. mass , area, volume, …

All matter, regardless of state, undergoes physical and chemical changes.

A physical change occurs when the substance changes state but does not change its chemical composition. For example: • water freezing into ice • cutting a piece of wood into smaller pieces • breaking a paper The form or appearance has changed, but the physical properties of that substance are the same: Melting point Density Boiling point Color Vapor pressure Electrical conductivity Solubility Hardness……

Chemical properties describe the way a substance can change or react to form other substances. These properties, then, must be determined using a process that changes the identity of the substance of interest.

A chemical change occurs when a substance changes into something new. This occurs due to heating, chemical reaction, etc. You can tell a chemical change has occurred if the density, melting point or freezing point of the original substance changes. Many common signs of a chemical change can be seen (bubbles forming, mass or colour changed, etc).

Mixtures are two or more substances that are NOT chemically combined. Mixtures do not: · Have constant boiling points · Have constant melting points

Variable composition Components retain their characteristic properties May be separated into pure substances by physical methods Mixtures of different compositions may have widely different properties

Heterogeneous mixtures are composed of large pieces that are easily separated by physical means (ie. density, polarity, metallic properties).

Do not have same composition throughout Components are distinguishable Examples: fruit salad, vegetable soup, wood, granite, etc.

Solutions are homogenous. Therefore, they are easily separated by physical methods like distillation or evaporation. Examples: • Sugar /water • Stainless steel • Salt / water • Wine • Brass • Air • Cola drink

Separating Mixtures Dirty water Oil and Water Saltwater

Settling Density causes parts of the mixture to settle to the bottom. This process is used during water filtration. Contaminants sink and clearer water is skimmed off. 31

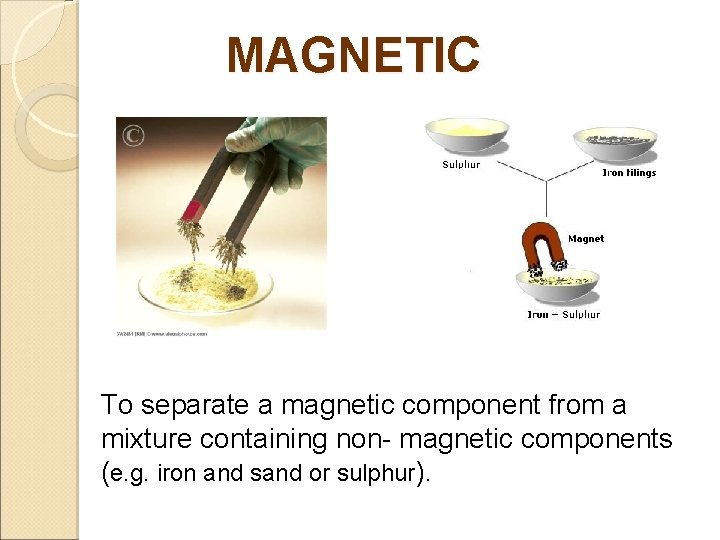
MAGNETIC To separate a magnetic component from a mixture containing non- magnetic components (e. g. iron and sand or sulphur).

Decantation Decanting is the simplest possible way of separating a liquid (pure or a solution) from an insoluble solid which has a density greater than water A mixture of two immiscible liquids can also be separated by decantation (oil and water)

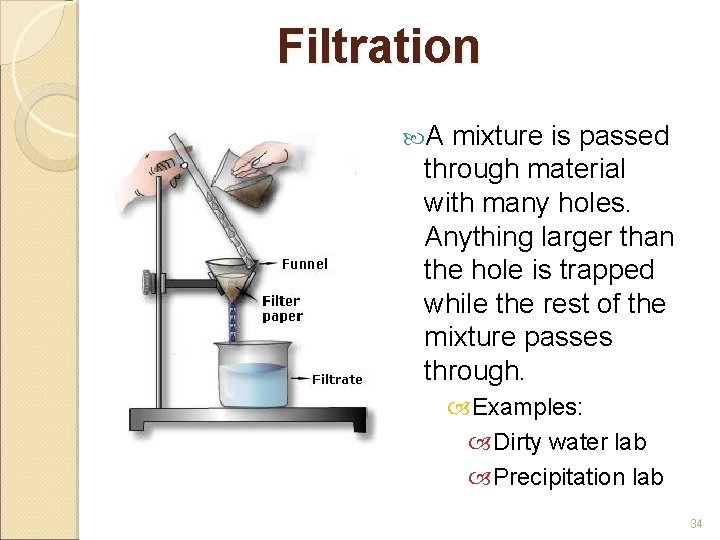
Filtration A mixture is passed through material with many holes. Anything larger than the hole is trapped while the rest of the mixture passes through. Examples: Dirty water lab Precipitation lab 34

Crystallization The separation process in which the solvent is evaporated, leaving crystals of solute behind. ◦ Examples: Rock candy Saltwater lab 35

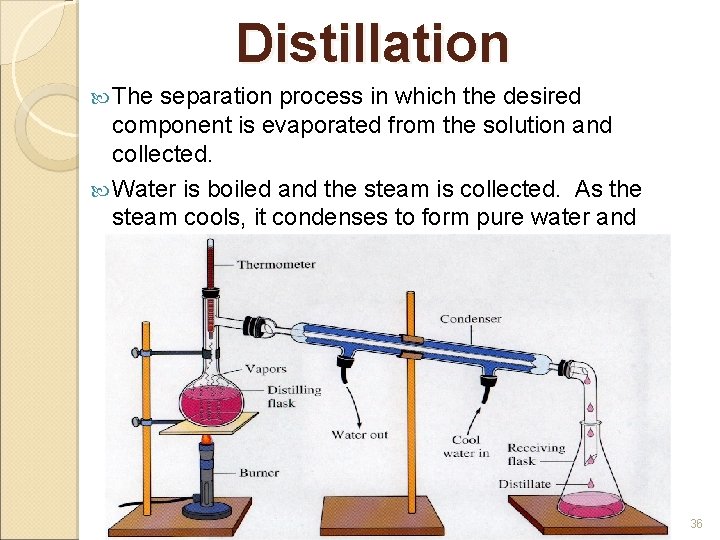
Distillation The separation process in which the desired component is evaporated from the solution and collected. Water is boiled and the steam is collected. As the steam cools, it condenses to form pure water and anything that was dissolved in the water is left behind 36

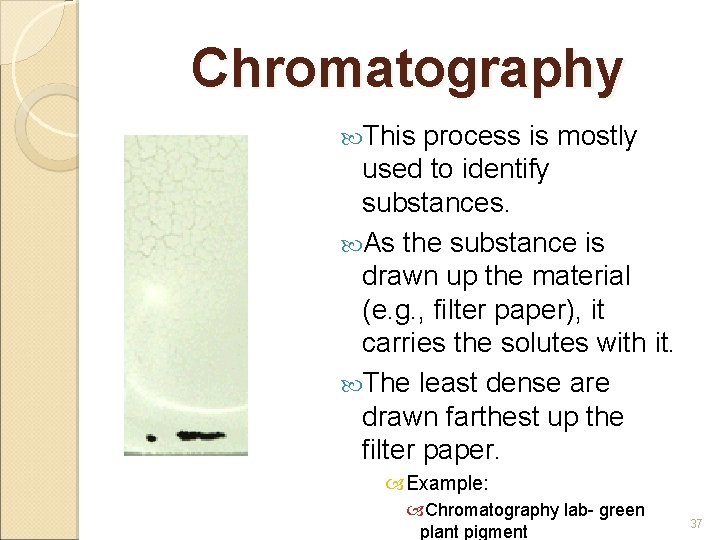
Chromatography This process is mostly used to identify substances. As the substance is drawn up the material (e. g. , filter paper), it carries the solutes with it. The least dense are drawn farthest up the filter paper. Example: Chromatography lab- green plant pigment 37

A substance is matter of a particular kind. A substance cannot be further broken down or purified by physical means. Each substance has its own characteristic properties that are different from the set of properties of any other substance.

Fixed composition Properties do not vary Cannot be separated into simpler substances by physical methods (physical changes) Can only be changed in identity and properties by chemical methods

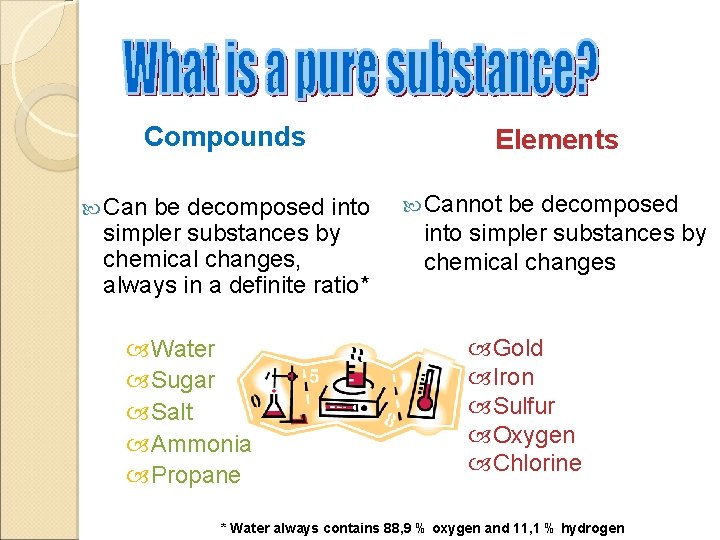
Compounds Can be decomposed into simpler substances by chemical changes, always in a definite ratio* Water Sugar Salt Ammonia Propane Elements Cannot be decomposed into simpler substances by chemical changes Gold Iron Sulfur Oxygen Chlorine * Water always contains 88, 9 % oxygen and 11, 1 % hydrogen

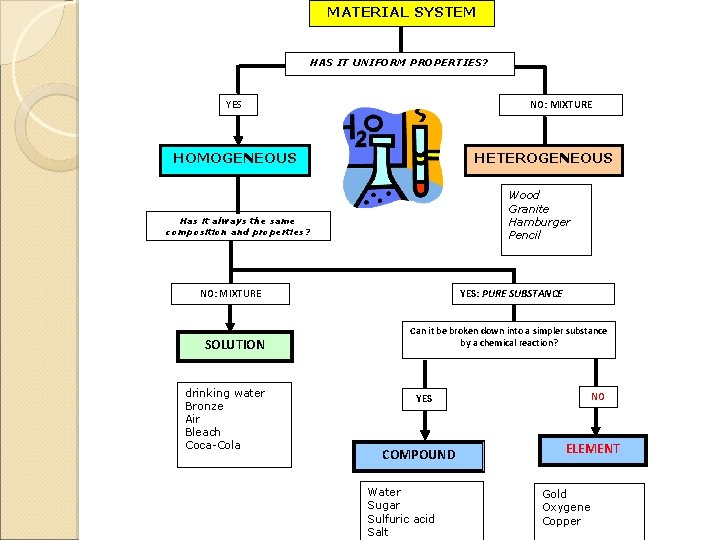
MATERIAL SYSTEM HAS IT UNIFORM PROPERTIES? YES NO: MIXTURE HOMOGENEOUS HETEROGENEOUS Wood Granite Hamburger Pencil Has it always the same composition and properties? NO: MIXTURE SOLUTION drinking water Bronze Air Bleach Coca-Cola YES: PURE SUBSTANCE Can it be broken down into a simpler substance by a chemical reaction? NO YES COMPOUND Water Sugar Sulfuric acid Salt ELEMENT Gold Oxygene Copper

Solutions

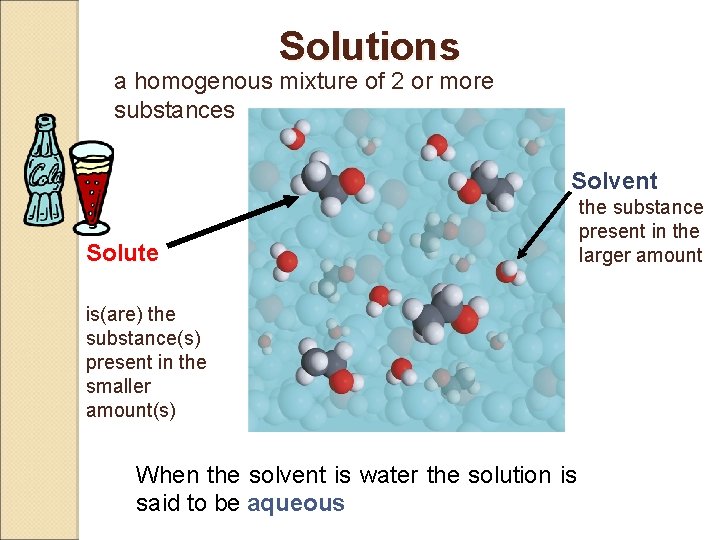
Definitions A solution is a homogeneous mixture A solute is dissolved in a solvent. Solute is present in the smaller amount The solvent is present in the larger amount. an aqueous solution has water as solvent *ey-kwee-uhs

Solutions a homogenous mixture of 2 or more substances Solvent Solute the substance present in the larger amount is(are) the substance(s) present in the smaller amount(s) When the solvent is water the solution is said to be aqueous

Types of solutions �� Gases dissolved in gases (air) Liquids dissolved in gases (humid air) Solids dissolved in gases (moth balls *) �� Liquids Gases in liquids (air dissolved in water) Liquids in liquids (ethanol in water) Solids in Liquids (salt in water) �� Solids Gases in solids (H 2 in Pt) Liquids in solids (Hg/Ag amalgam) Solids in solids (Cr in Fe alloy) (*) bolas antipolilla

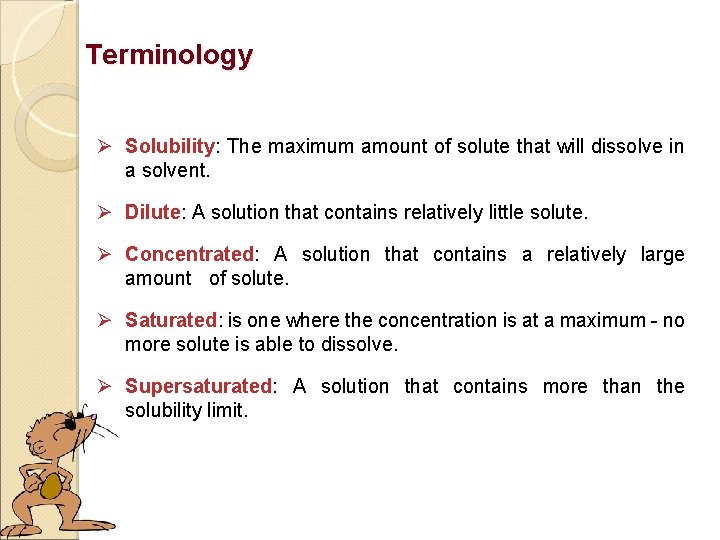
Terminology Ø Solubility: The maximum amount of solute that will dissolve in a solvent. Ø Dilute: A solution that contains relatively little solute. Ø Concentrated: A solution that contains a relatively large amount of solute. Ø Saturated: is one where the concentration is at a maximum - no more solute is able to dissolve. Ø Supersaturated: A solution that contains more than the solubility limit.

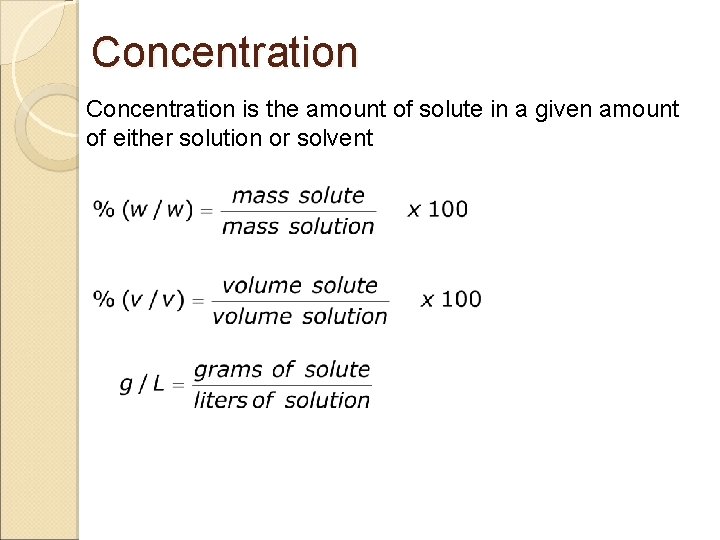
Concentration is the amount of solute in a given amount of either solution or solvent

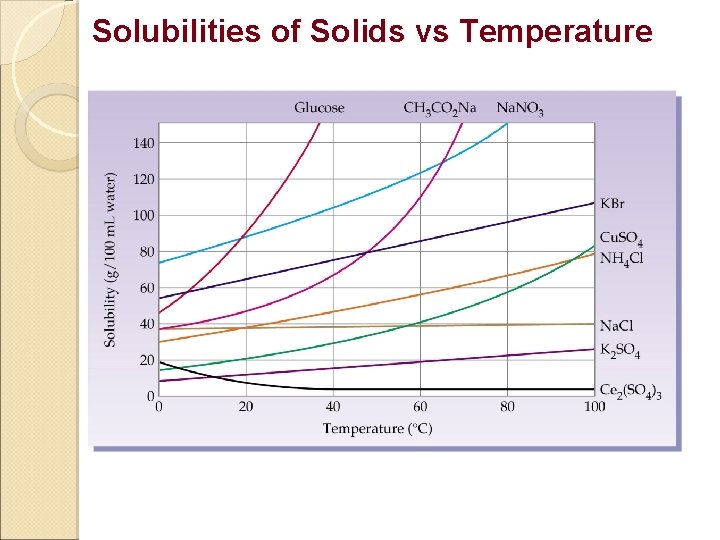
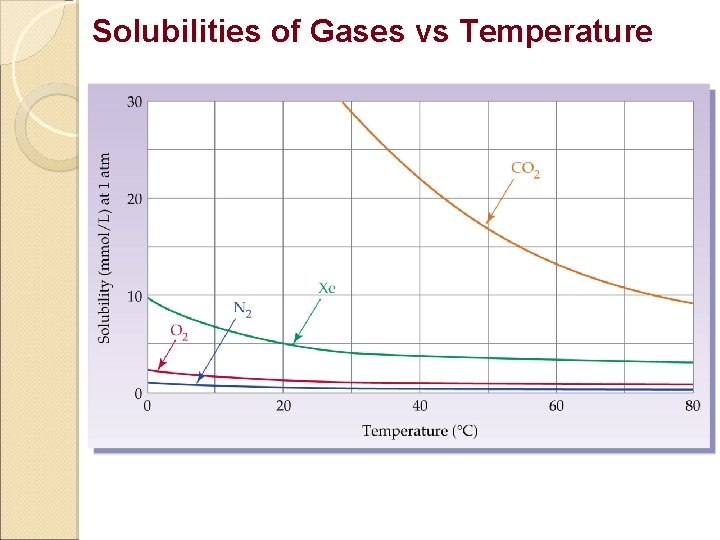
Factors Affecting Solubility 1. Nature of Solute / Solvent. - Like dissolves like 2. Temperature Solids/Liquids- Solubility increases with Temperature Gases - Solubility decreases with Temperature 3. Pressure Factor Solids/Liquids - Very little effect Gas - Solubility increases with Pressure.

Solubilities of Solids vs Temperature

Solubilities of Gases vs Temperature