2 2 9 Atom Economy page 164 165








- Slides: 25

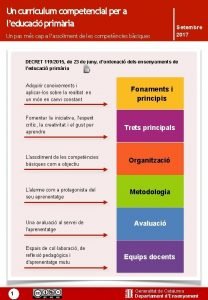
2. 2. 9 Atom Economy page 164 -165 • Define the atom economy of a reaction and describe the benefits of developing chemical processes with a high atom economy. • Explain that addition reactions have an atom economy of 100%, whereas substitution reactions are less efficient. • Carry out calculations to determine the atom economy of a reaction. • Explain that a reaction may have a high percentage yield but a low atom economy. • Perform calculations to determine the percentage yield of a reaction.

Sustainable Development and Atom Economy Today, to reduce the global impact of all industrial processes, scientists aim to design reactions which have the highest possible atom economy, thereby reducing the raw materials used and the amount of energy needed to produce any product.

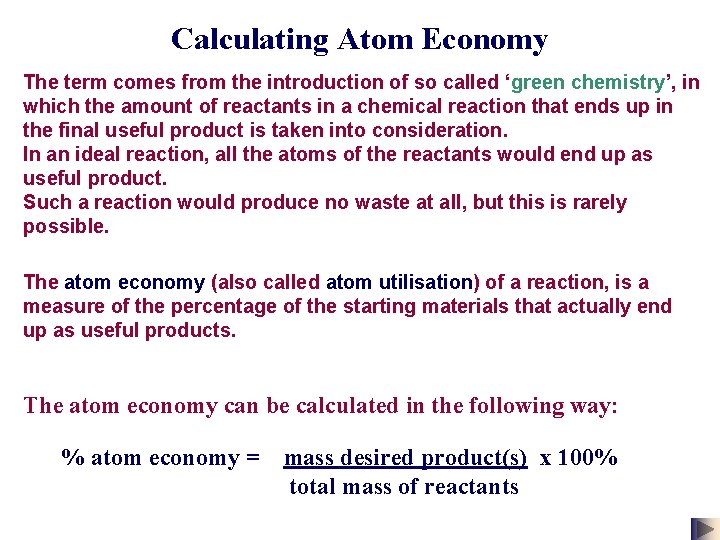
Calculating Atom Economy The term comes from the introduction of so called ‘green chemistry’, in which the amount of reactants in a chemical reaction that ends up in the final useful product is taken into consideration. In an ideal reaction, all the atoms of the reactants would end up as useful product. Such a reaction would produce no waste at all, but this is rarely possible. The atom economy (also called atom utilisation) of a reaction, is a measure of the percentage of the starting materials that actually end up as useful products. The atom economy can be calculated in the following way: % atom economy = mass desired product(s) x 100% total mass of reactants

The atom economy of a reaction is. . . a theoretical measure of the amount of starting materials that ends up as 'desired' reaction product. The greater the atom economy of a reaction the. . more 'efficient' or 'economic' it is likely to be, though this is a gross simplification when complex and costly chemical synthesis are looked at. It can be defined numerically in words in several ways, all of which amount to the same theoretical % number

mass of atoms desired product Atom economy = ------------------- x 100 = total mass of reactant atoms mass of useful product Atom economy = -------------- x 100 = mass of all products total Mr of useful product Atom economy = ---------------- x 100 total Mr of all products

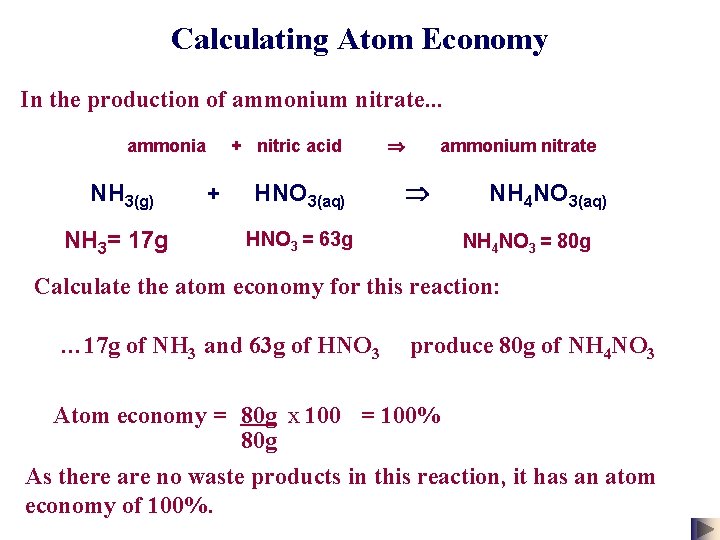
Calculating Atom Economy In the production of ammonium nitrate. . . ammonia + nitric acid ammonium nitrate NH 3(g) + HNO 3(aq) NH 4 NO 3(aq) NH 3= 17 g HNO 3 = 63 g NH 4 NO 3 = 80 g Calculate the atom economy for this reaction: … 17 g of NH 3 and 63 g of HNO 3 produce 80 g of NH 4 NO 3 Atom economy = 80 g x 100 = 100% 80 g As there are no waste products in this reaction, it has an atom economy of 100%.

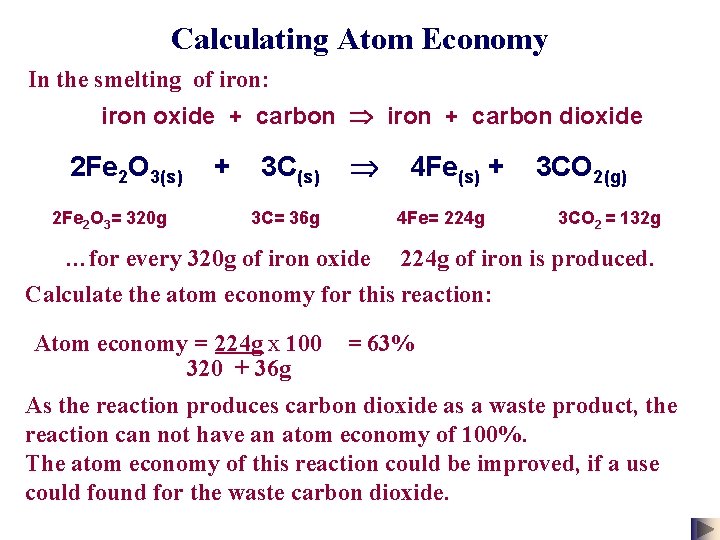
Calculating Atom Economy In the smelting of iron: iron oxide + carbon iron + carbon dioxide 2 Fe 2 O 3(s) + 3 C(s) 4 Fe(s) + 3 CO 2(g) 2 Fe 2 O 3= 320 g 3 C= 36 g 4 Fe= 224 g 3 CO 2 = 132 g …for every 320 g of iron oxide 224 g of iron is produced. Calculate the atom economy for this reaction: Atom economy = 224 g x 100 320 + 36 g = 63% As the reaction produces carbon dioxide as a waste product, the reaction can not have an atom economy of 100%. The atom economy of this reaction could be improved, if a use could found for the waste carbon dioxide.

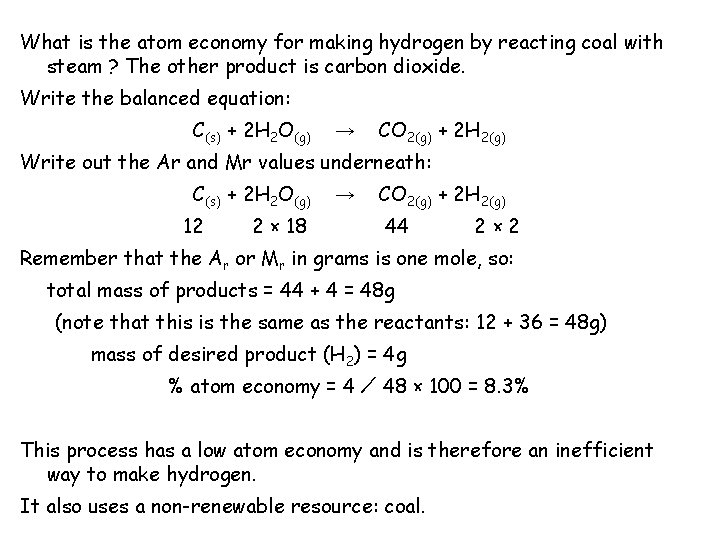
What is the atom economy for making hydrogen by reacting coal with steam ? The other product is carbon dioxide. Write the balanced equation: C(s) + 2 H 2 O(g) → CO 2(g) + 2 H 2(g) Write out the Ar and Mr values underneath: C(s) + 2 H 2 O(g) → CO 2(g) + 2 H 2(g) 12 2 × 18 44 2× 2 Remember that the Ar or Mr in grams is one mole, so: total mass of products = 44 + 4 = 48 g (note that this is the same as the reactants: 12 + 36 = 48 g) mass of desired product (H 2) = 4 g % atom economy = 4 ⁄ 48 × 100 = 8. 3% This process has a low atom economy and is therefore an inefficient way to make hydrogen. It also uses a non-renewable resource: coal.

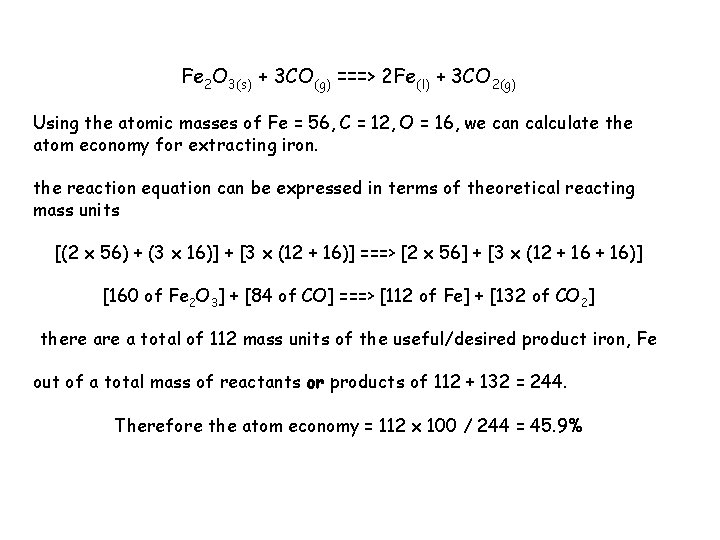
Fe 2 O 3(s) + 3 CO(g) ===> 2 Fe(l) + 3 CO 2(g) Using the atomic masses of Fe = 56, C = 12, O = 16, we can calculate the atom economy for extracting iron. the reaction equation can be expressed in terms of theoretical reacting mass units [(2 x 56) + (3 x 16)] + [3 x (12 + 16)] ===> [2 x 56] + [3 x (12 + 16)] [160 of Fe 2 O 3] + [84 of CO] ===> [112 of Fe] + [132 of CO 2] there a total of 112 mass units of the useful/desired product iron, Fe out of a total mass of reactants or products of 112 + 132 = 244. Therefore the atom economy = 112 x 100 / 244 = 45. 9%

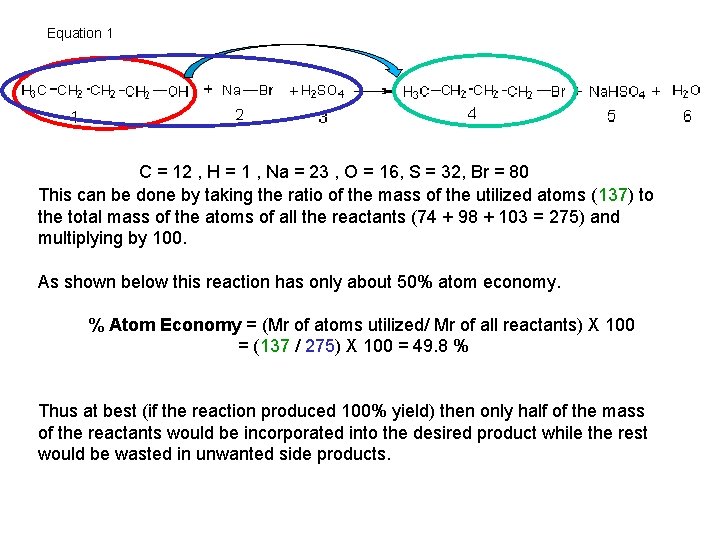
Equation 1 C = 12 , H = 1 , Na = 23 , O = 16, S = 32, Br = 80 This can be done by taking the ratio of the mass of the utilized atoms (137) to the total mass of the atoms of all the reactants (74 + 98 + 103 = 275) and multiplying by 100. As shown below this reaction has only about 50% atom economy. % Atom Economy = (Mr of atoms utilized/ Mr of all reactants) X 100 = (137 / 275) X 100 = 49. 8 % Thus at best (if the reaction produced 100% yield) then only half of the mass of the reactants would be incorporated into the desired product while the rest would be wasted in unwanted side products.

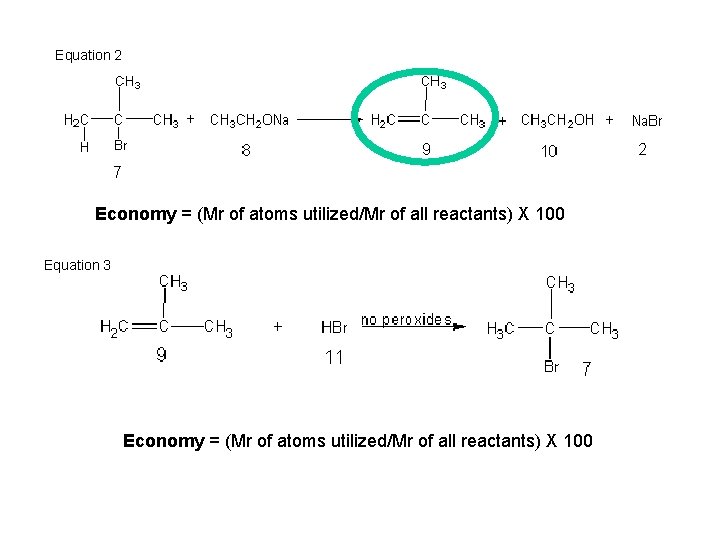
Equation 2 Economy = (Mr of atoms utilized/Mr of all reactants) X 100 = ( 56/ 205 ) X 100 = 27. 3 % Equation 3 Economy = (Mr of atoms utilized/Mr of all reactants) X 100 = ( 137 / 137 ) X 100 = 100 %

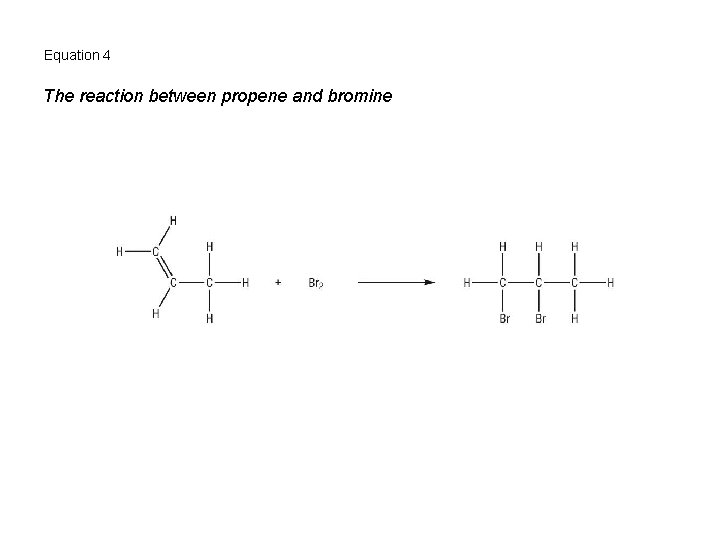
Equation 4 The reaction between propene and bromine

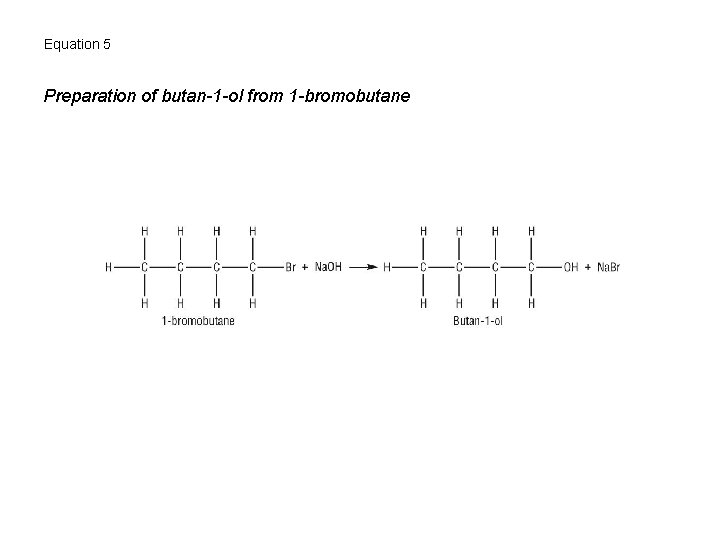
Equation 5 Preparation of butan-1 -ol from 1 -bromobutane

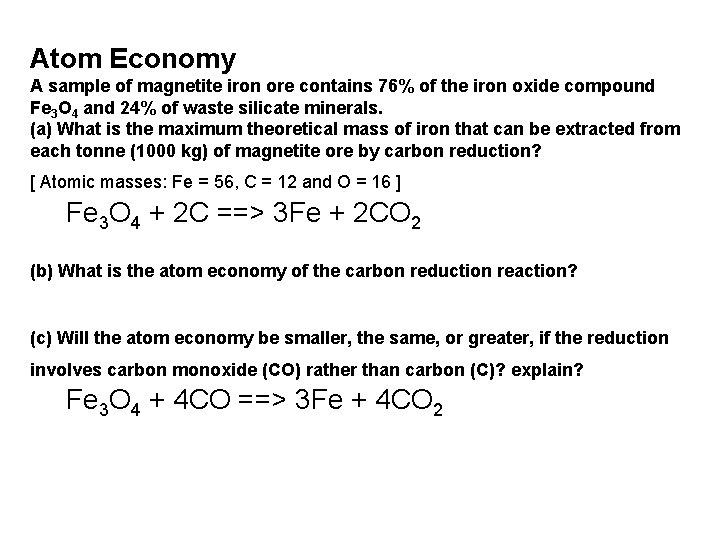
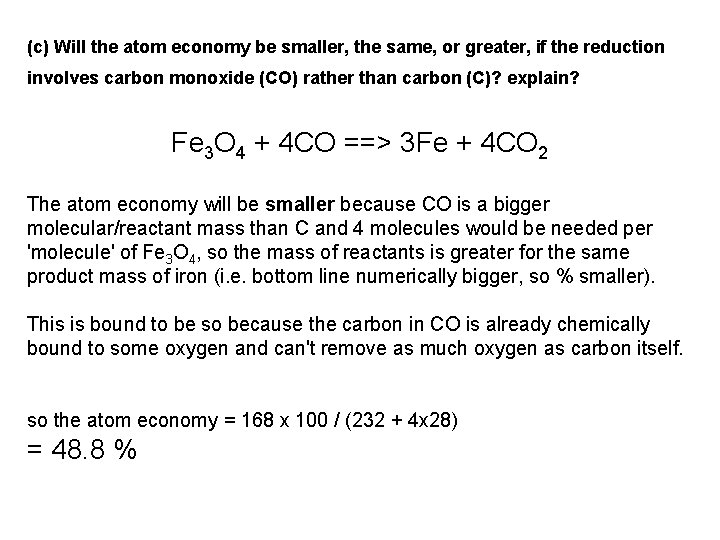
Atom Economy A sample of magnetite iron ore contains 76% of the iron oxide compound Fe 3 O 4 and 24% of waste silicate minerals. (a) What is the maximum theoretical mass of iron that can be extracted from each tonne (1000 kg) of magnetite ore by carbon reduction? [ Atomic masses: Fe = 56, C = 12 and O = 16 ] Fe 3 O 4 + 2 C ==> 3 Fe + 2 CO 2 (b) What is the atom economy of the carbon reduction reaction? (c) Will the atom economy be smaller, the same, or greater, if the reduction involves carbon monoxide (CO) rather than carbon (C)? explain? Fe 3 O 4 + 4 CO ==> 3 Fe + 4 CO 2

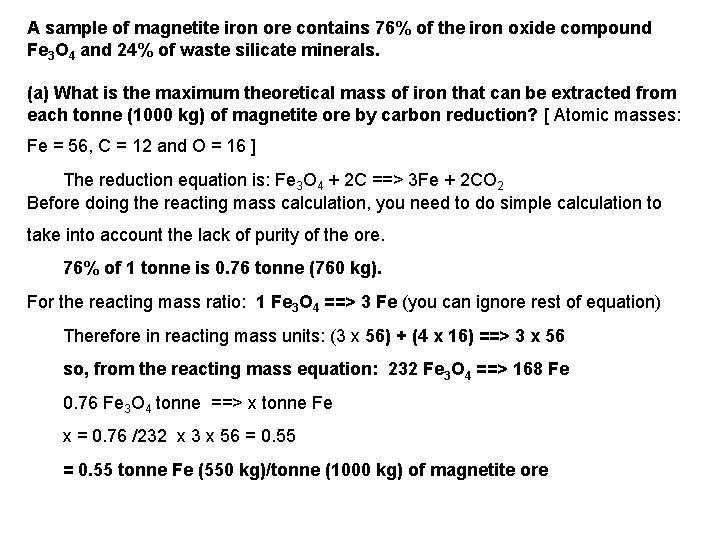
A sample of magnetite iron ore contains 76% of the iron oxide compound Fe 3 O 4 and 24% of waste silicate minerals. (a) What is the maximum theoretical mass of iron that can be extracted from each tonne (1000 kg) of magnetite ore by carbon reduction? [ Atomic masses: Fe = 56, C = 12 and O = 16 ] The reduction equation is: Fe 3 O 4 + 2 C ==> 3 Fe + 2 CO 2 Before doing the reacting mass calculation, you need to do simple calculation to take into account the lack of purity of the ore. 76% of 1 tonne is 0. 76 tonne (760 kg). For the reacting mass ratio: 1 Fe 3 O 4 ==> 3 Fe (you can ignore rest of equation) Therefore in reacting mass units: (3 x 56) + (4 x 16) ==> 3 x 56 so, from the reacting mass equation: 232 Fe 3 O 4 ==> 168 Fe 0. 76 Fe 3 O 4 tonne ==> x tonne Fe x = 0. 76 /232 x 3 x 56 = 0. 55 tonne Fe (550 kg)/tonne (1000 kg) of magnetite ore

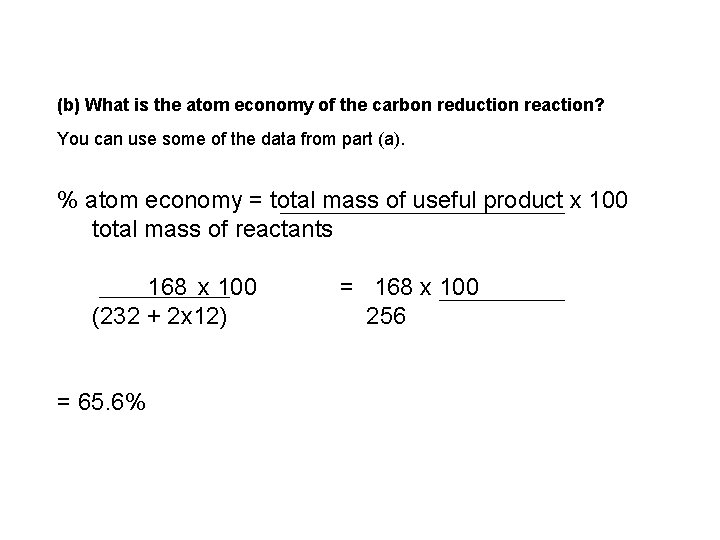
(b) What is the atom economy of the carbon reduction reaction? You can use some of the data from part (a). % atom economy = total mass of useful product x 100 total mass of reactants 168 x 100 (232 + 2 x 12) = 65. 6% = 168 x 100 256

(c) Will the atom economy be smaller, the same, or greater, if the reduction involves carbon monoxide (CO) rather than carbon (C)? explain? Fe 3 O 4 + 4 CO ==> 3 Fe + 4 CO 2 The atom economy will be smaller because CO is a bigger molecular/reactant mass than C and 4 molecules would be needed per 'molecule' of Fe 3 O 4, so the mass of reactants is greater for the same product mass of iron (i. e. bottom line numerically bigger, so % smaller). This is bound to be so because the carbon in CO is already chemically bound to some oxygen and can't remove as much oxygen as carbon itself. so the atom economy = 168 x 100 / (232 + 4 x 28) = 48. 8 %

2. 2 9 Atom Economy Page 164 -165 Questions 1 and 2 Key Definition : atom economy The atom economy of a reaction is. . . a theoretical measure of the amount of starting materials that ends up as 'desired' reaction product

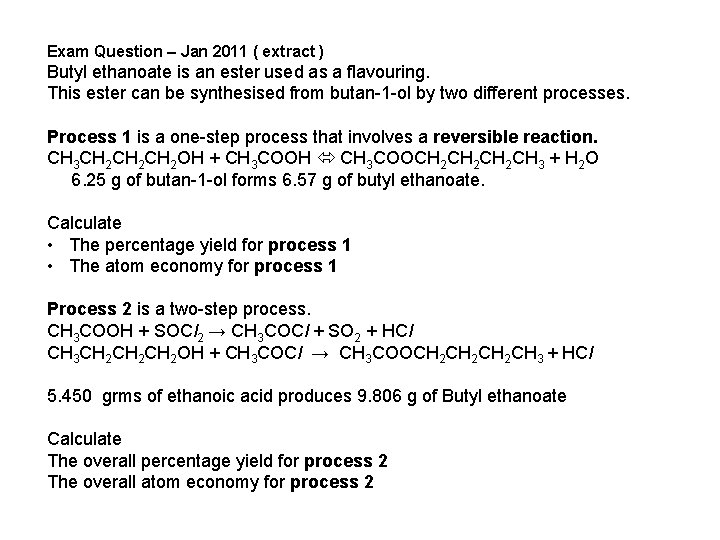
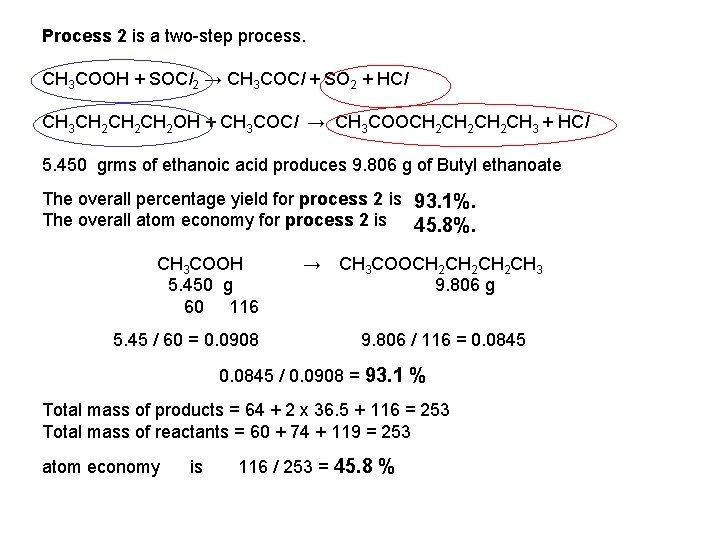
Exam Question – Jan 2011 ( extract ) Butyl ethanoate is an ester used as a flavouring. This ester can be synthesised from butan-1 -ol by two different processes. Process 1 is a one-step process that involves a reversible reaction. CH 3 CH 2 CH 2 OH + CH 3 COOH CH 3 COOCH 2 CH 2 CH 3 + H 2 O 6. 25 g of butan-1 -ol forms 6. 57 g of butyl ethanoate. Calculate • The percentage yield for process 1 • The atom economy for process 1 Process 2 is a two-step process. CH 3 COOH + SOCl 2 → CH 3 COCl + SO 2 + HCl CH 3 CH 2 CH 2 OH + CH 3 COCl → CH 3 COOCH 2 CH 2 CH 3 + HCl 5. 450 grms of ethanoic acid produces 9. 806 g of Butyl ethanoate Calculate The overall percentage yield for process 2 The overall atom economy for process 2

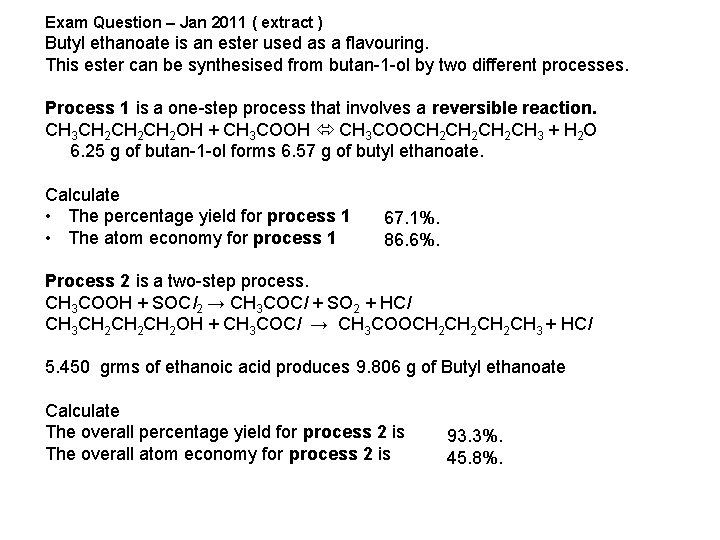
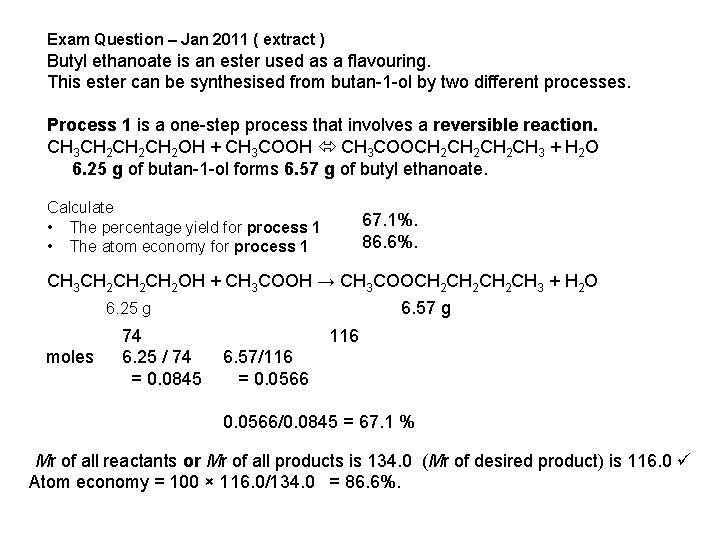
Exam Question – Jan 2011 ( extract ) Butyl ethanoate is an ester used as a flavouring. This ester can be synthesised from butan-1 -ol by two different processes. Process 1 is a one-step process that involves a reversible reaction. CH 3 CH 2 CH 2 OH + CH 3 COOH CH 3 COOCH 2 CH 2 CH 3 + H 2 O 6. 25 g of butan-1 -ol forms 6. 57 g of butyl ethanoate. Calculate • The percentage yield for process 1 • The atom economy for process 1 67. 1%. 86. 6%. Process 2 is a two-step process. CH 3 COOH + SOCl 2 → CH 3 COCl + SO 2 + HCl CH 3 CH 2 CH 2 OH + CH 3 COCl → CH 3 COOCH 2 CH 2 CH 3 + HCl 5. 450 grms of ethanoic acid produces 9. 806 g of Butyl ethanoate Calculate The overall percentage yield for process 2 is The overall atom economy for process 2 is 93. 3%. 45. 8%.

Explain why process 2 has a high percentage yield but a low atom economy. . . . . . . . [2] Suggest two reasons why butyl ethanoate is manufactured by process 1 rather than by process 2. . . . . . . . . [2]

Exam Question – Jan 2011 ( extract ) Butyl ethanoate is an ester used as a flavouring. This ester can be synthesised from butan-1 -ol by two different processes. Process 1 is a one-step process that involves a reversible reaction. CH 3 CH 2 CH 2 OH + CH 3 COOH CH 3 COOCH 2 CH 2 CH 3 + H 2 O 6. 25 g of butan-1 -ol forms 6. 57 g of butyl ethanoate. Calculate • The percentage yield for process 1 • The atom economy for process 1 67. 1%. 86. 6%. CH 3 CH 2 CH 2 OH + CH 3 COOH → CH 3 COOCH 2 CH 2 CH 3 + H 2 O 6. 57 g 6. 25 g 74 116 ( moles 6. 25 / 74 6. 57/116 = 0. 0845 = 0. 0566/0. 0845 = 67. 1 % (Mr of all reactants or Mr of all products is 134. 0 (Mr of desired product) is 116. 0 Atom economy = 100 × 116. 0/134. 0 = 86. 6%.

Process 2 is a two-step process. CH 3 COOH + SOCl 2 → CH 3 COCl + SO 2 + HCl CH 3 CH 2 CH 2 OH + CH 3 COCl → CH 3 COOCH 2 CH 2 CH 3 + HCl 5. 450 grms of ethanoic acid produces 9. 806 g of Butyl ethanoate The overall percentage yield for process 2 is 93. 1%. The overall atom economy for process 2 is 45. 8%. CH 3 COOH 5. 450 g 60 116 5. 45 / 60 = 0. 0908 → CH 3 COOCH 2 CH 2 CH 3 9. 806 g 9. 806 / 116 = 0. 0845 / 0. 0908 = 93. 1 % Total mass of products = 64 + 2 x 36. 5 + 116 = 253 Total mass of reactants = 60 + 74 + 119 = 253 atom economy is 116 / 253 = 45. 8 %

Explain why process 2 has a high percentage yield but a low atom economy. . . . . . . . [2] Link between yield AND explanation required: (high percentage) yield shows a high % conversion (of reactants into products) Link between atom economy AND explanation required: (low) atom economy shows a lot of waste (product) OR (low) atom economy shows not much desired product

Suggest two reasons why butyl ethanoate is manufactured by process 1 rather than by process 2. . . . . . . . . [2] ANY TWO FROM - Comparison essential throughout Less waste (products) OR higher atom economy Less toxic reactants OR less toxic (waste) products OR less corrosive reactants OR less corrosive (waste) products OR less harmful reactants OR less harmful (waste) products OR less hazardous reactants OR less hazardous (waste) products Cheaper starting materials OR more readily available starting materials Fewer steps OR one step rather than two steps
 Athens vs sparta differences
Athens vs sparta differences Concurrent validity
Concurrent validity Cs 164
Cs 164 Psms164
Psms164 International format phone number
International format phone number Ordre ens/164/2016
Ordre ens/164/2016 Krs 164
Krs 164 Si a+aa+aaa+aaaa=7404
Si a+aa+aaa+aaaa=7404 Yajurveda chapter 20 verse 37
Yajurveda chapter 20 verse 37 Kj 164
Kj 164 Art 164
Art 164 Atom economy formula
Atom economy formula Atom economy vs percent yield
Atom economy vs percent yield Atom economy
Atom economy Atom economy in green chemistry
Atom economy in green chemistry Atom economy
Atom economy Atom economy formula
Atom economy formula Percentage atom economy definition
Percentage atom economy definition Atom economy formula
Atom economy formula Atom economy
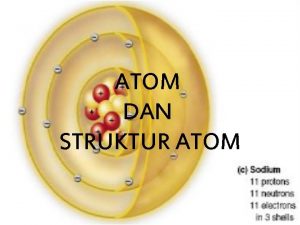
Atom economy The structure of the atom section 2 defining the atom
The structure of the atom section 2 defining the atom Democritus atom modeli
Democritus atom modeli Décomposer 210 en produit de facteurs premiers
Décomposer 210 en produit de facteurs premiers Uredba eu 165/14
Uredba eu 165/14 Ms 165
Ms 165 165 as a mixed number
165 as a mixed number