Where does Food marry Physics Physics Food Atomic














- Slides: 52


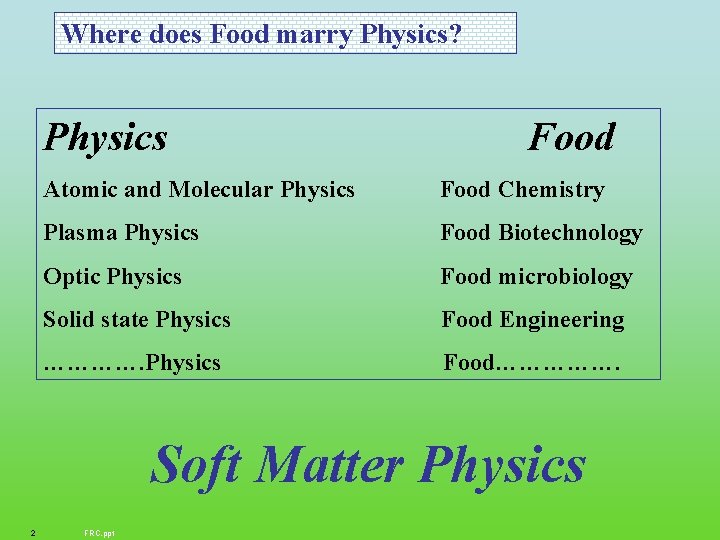
Where does Food marry Physics? Physics Food Atomic and Molecular Physics Food Chemistry Plasma Physics Food Biotechnology Optic Physics Food microbiology Solid state Physics Food Engineering …………. Physics Food……………. Soft Matter Physics 2 FRC. ppt

Soft Matter In Europe: Soft Matter Physics De Gennes: Nobel Lecture In US: Complex Fluid Most people were from Exxon Mobile Including: Polymer (melt, gel, solution etc) Liquid crystals Colloid (micelle, emulsion) Other biomaterials such as tissue etc 3 FRC. ppt

Food Stuff Starch Food: Rice, noodle, bread, chip, fry etc Gel Food: Tofu, cooked egg, yoghurt, Jelly etc Frozen Food: Ice cream, frozen fruit bar etc. Candy: Chocolate Drink: beer, tea, milk, coffee etc. 4 FRC. ppt

Chocolate: food of the gods 5 FRC. ppt 于 熔 只 于 熔 不 口 手

6 FRC. ppt

7 FRC. ppt

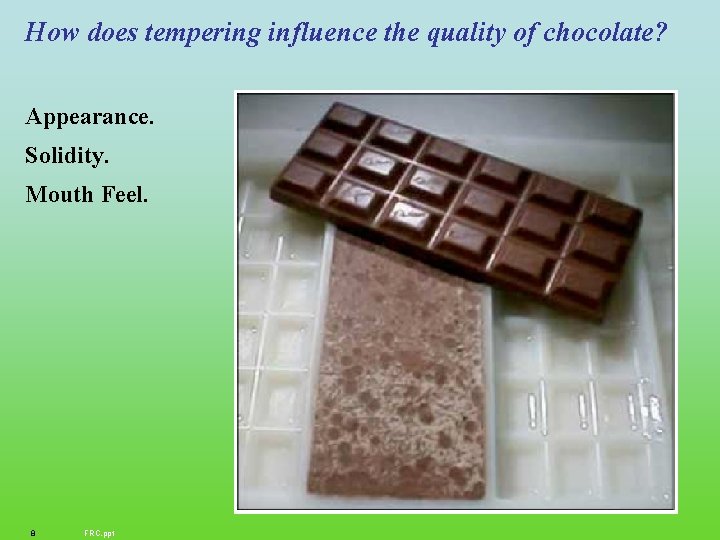
How does tempering influence the quality of chocolate? Appearance. Solidity. Mouth Feel. 8 FRC. ppt

Tempering Four steps (1) complete melting of the chocolate to about 50 o. C, which removes most or all of the crystalline material (2) cooling to the point of crystallization (3) maintaining a holding temperature for crystallization for about a minute (4) reheating to melt out unstable crystals. During this process, temperature control is very critical, and the shear applied within the process is also a crucial parameter. Fryer, P et al. MRS BULLETIN/DECEMBER 2000, 25 -29 9 FRC. ppt

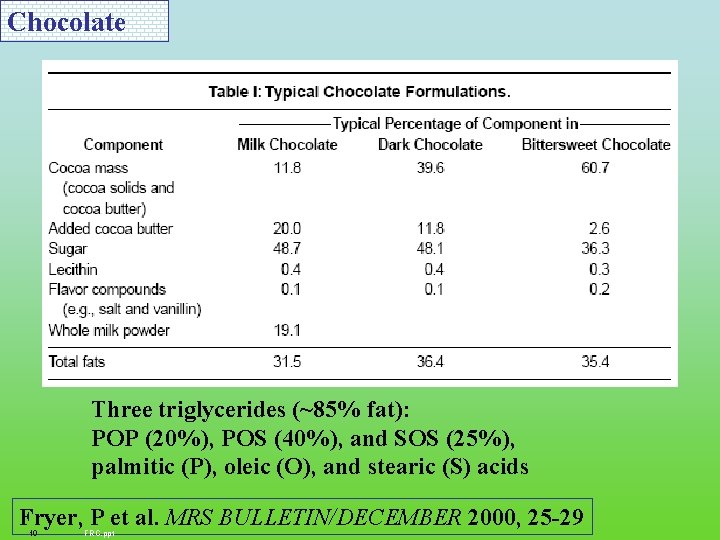
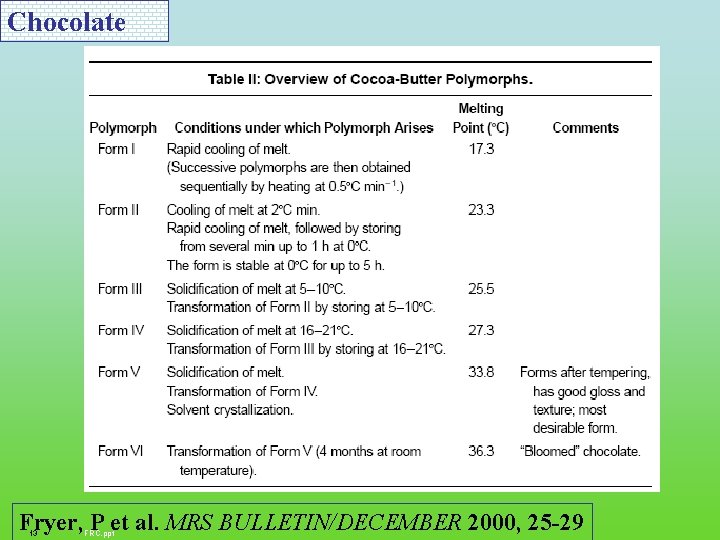
Chocolate Three triglycerides (~85% fat): POP (20%), POS (40%), and SOS (25%), palmitic (P), oleic (O), and stearic (S) acids Fryer, P et al. MRS BULLETIN/DECEMBER 2000, 25 -29 10 FRC. ppt

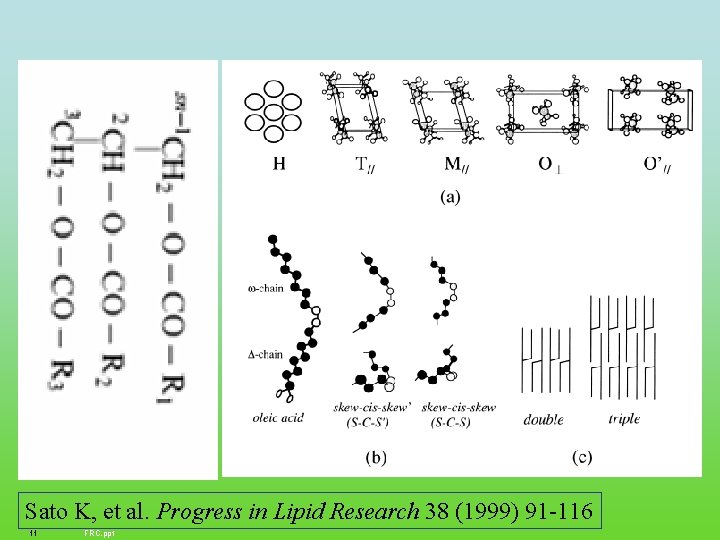
Sato K, et al. Progress in Lipid Research 38 (1999) 91 -116 11 FRC. ppt

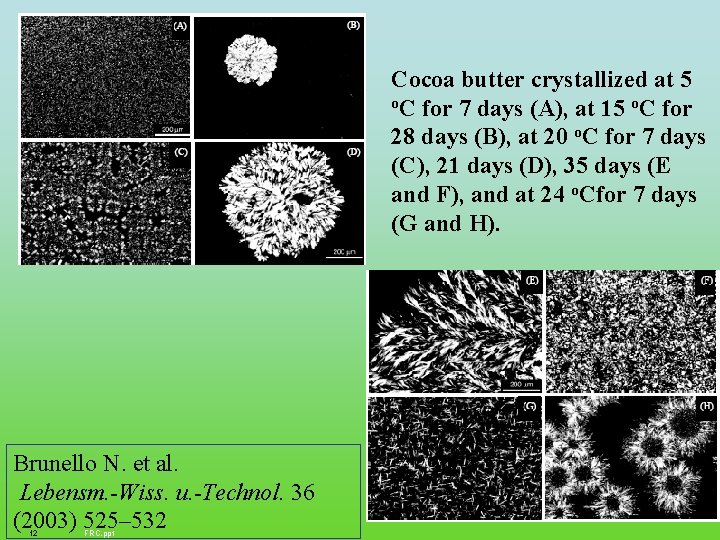
Cocoa butter crystallized at 5 o. C for 7 days (A), at 15 o. C for 28 days (B), at 20 o. C for 7 days (C), 21 days (D), 35 days (E and F), and at 24 o. Cfor 7 days (G and H). Brunello N. et al. Lebensm. -Wiss. u. -Technol. 36 (2003) 525– 532 12 FRC. ppt

Chocolate Fryer, P et al. MRS BULLETIN/DECEMBER 2000, 25 -29 13 FRC. ppt

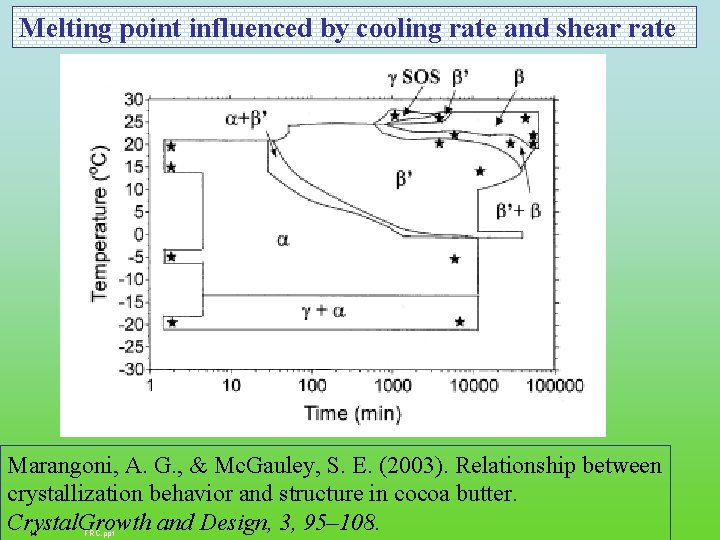
Melting point influenced by cooling rate and shear rate Marangoni, A. G. , & Mc. Gauley, S. E. (2003). Relationship between crystallization behavior and structure in cocoa butter. Crystal. Growth and Design, 3, 95– 108. 14 FRC. ppt

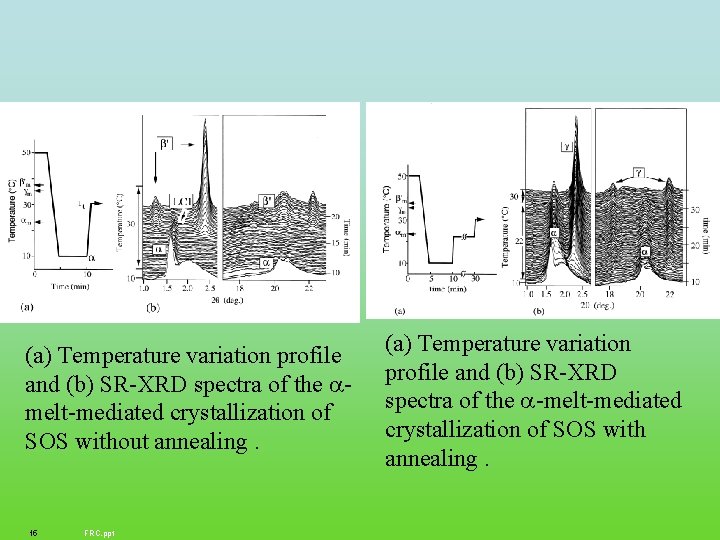
(a) Temperature variation profile and (b) SR-XRD spectra of the melt-mediated crystallization of SOS without annealing. 15 FRC. ppt (a) Temperature variation profile and (b) SR-XRD spectra of the -melt-mediated crystallization of SOS with annealing.

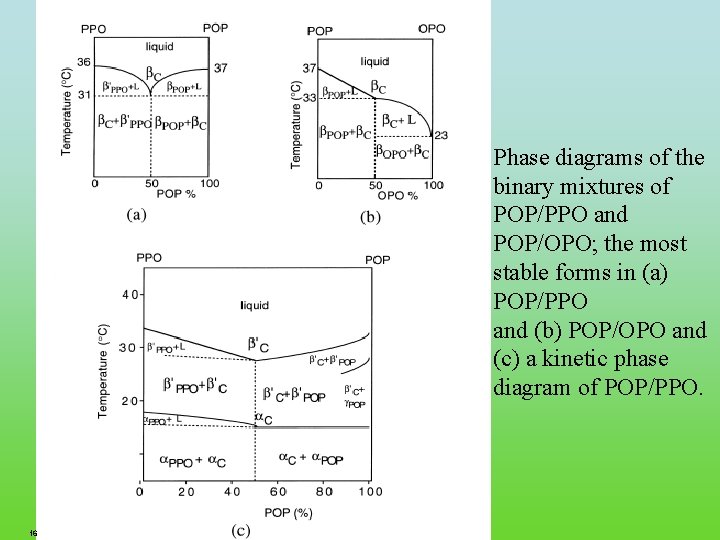
Phase diagrams of the binary mixtures of POP/PPO and POP/OPO; the most stable forms in (a) POP/PPO and (b) POP/OPO and (c) a kinetic phase diagram of POP/PPO. 16 FRC. ppt

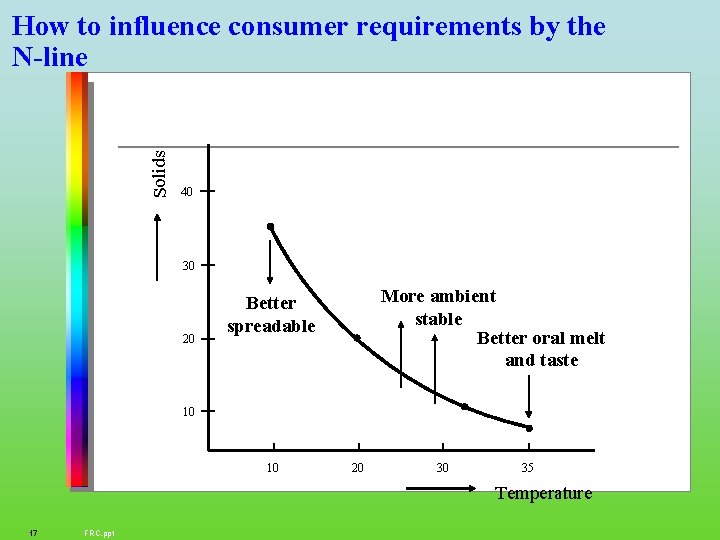
Solids How to influence consumer requirements by the N-line 40 30 20 More ambient stable Better oral melt and taste Better spreadable 10 10 20 30 35 Temperature 17 FRC. ppt


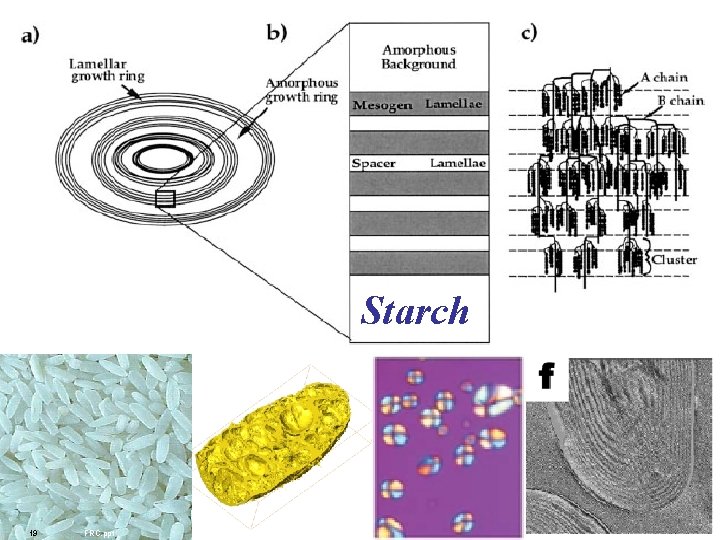
Starch 19 FRC. ppt

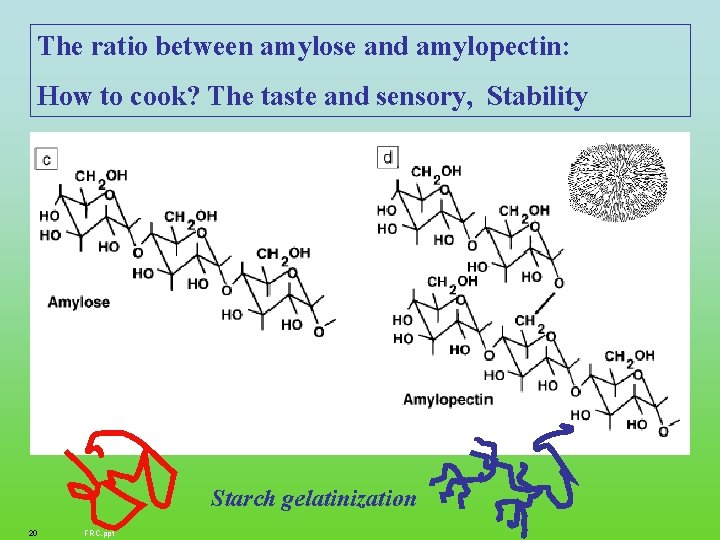
The ratio between amylose and amylopectin: How to cook? The taste and sensory, Stability Starch gelatinization 20 FRC. ppt

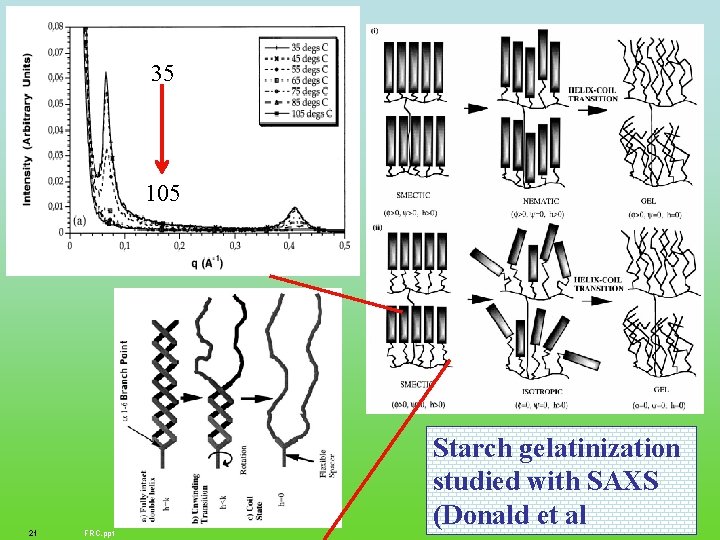
35 105 21 FRC. ppt Starch gelatinization studied with SAXS (Donald et al

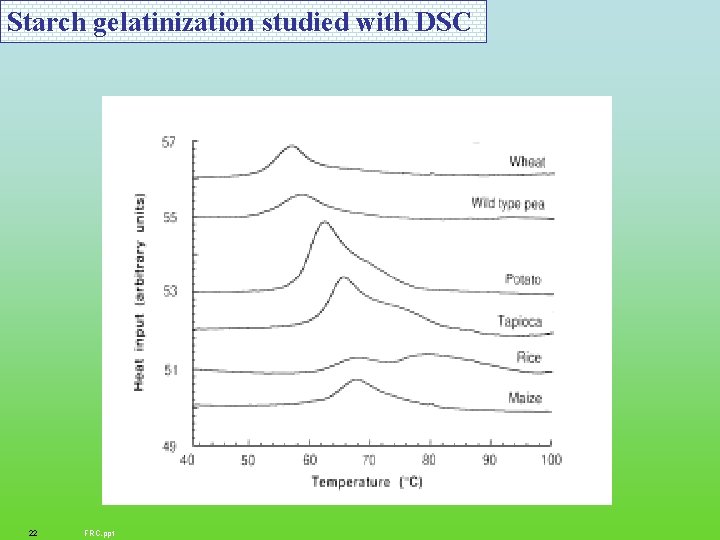
Starch gelatinization studied with DSC 22 FRC. ppt

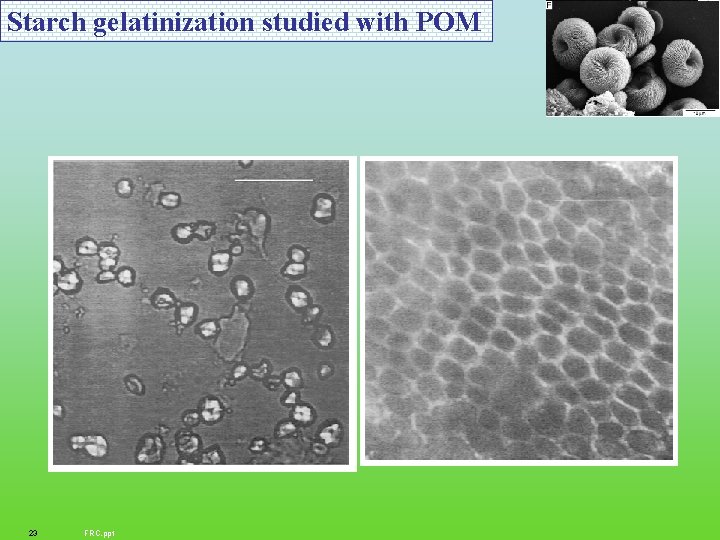
Starch gelatinization studied with POM 23 FRC. ppt

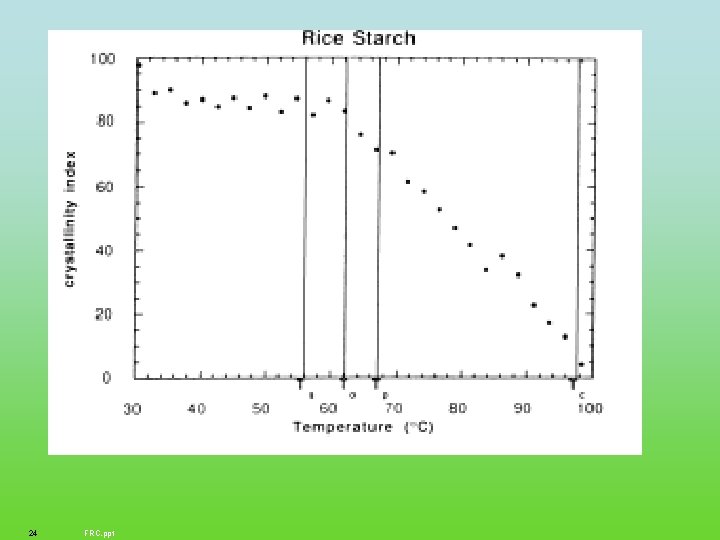
24 FRC. ppt

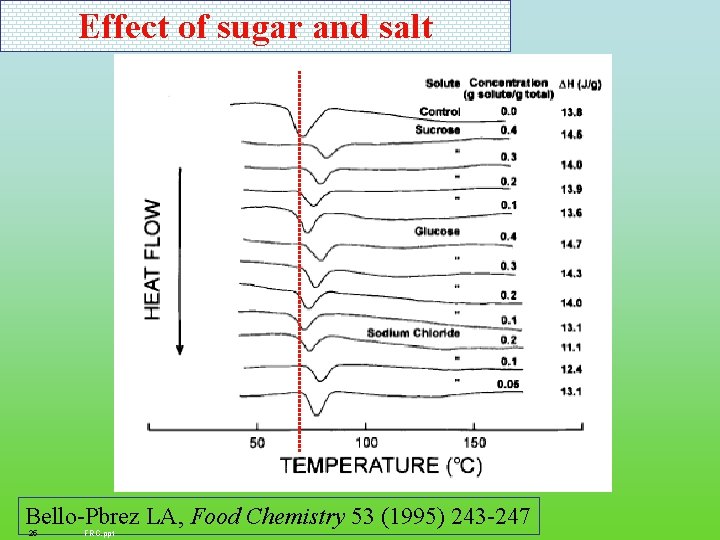
Effect of sugar and salt Bello-Pbrez LA, Food Chemistry 53 (1995) 243 -247 25 FRC. ppt


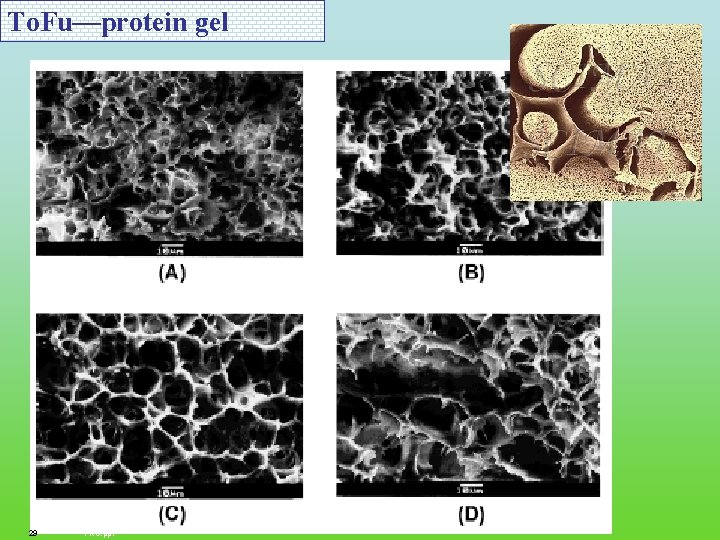
To. Fu—protein gel 27 FRC. ppt

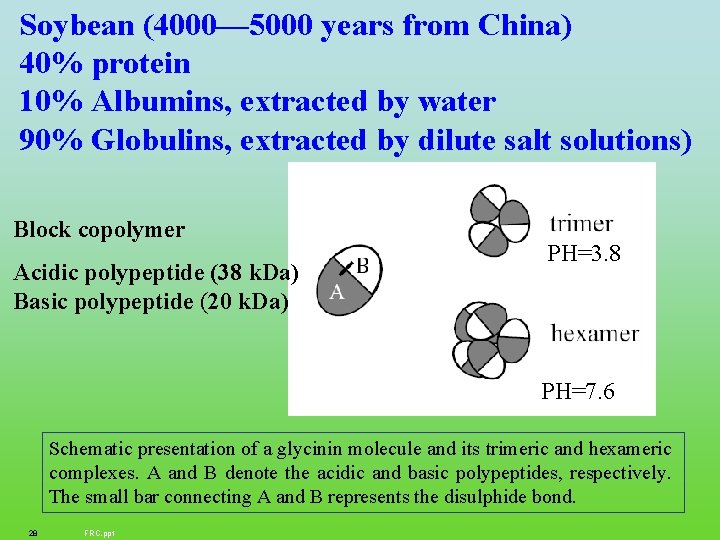
Soybean (4000— 5000 years from China) 40% protein 10% Albumins, extracted by water 90% Globulins, extracted by dilute salt solutions) Block copolymer Acidic polypeptide (38 k. Da) Basic polypeptide (20 k. Da) PH=3. 8 PH=7. 6 Schematic presentation of a glycinin molecule and its trimeric and hexameric complexes. A and B denote the acidic and basic polypeptides, respectively. The small bar connecting A and B represents the disulphide bond. 28 FRC. ppt

To. Fu—protein gel 29 FRC. ppt

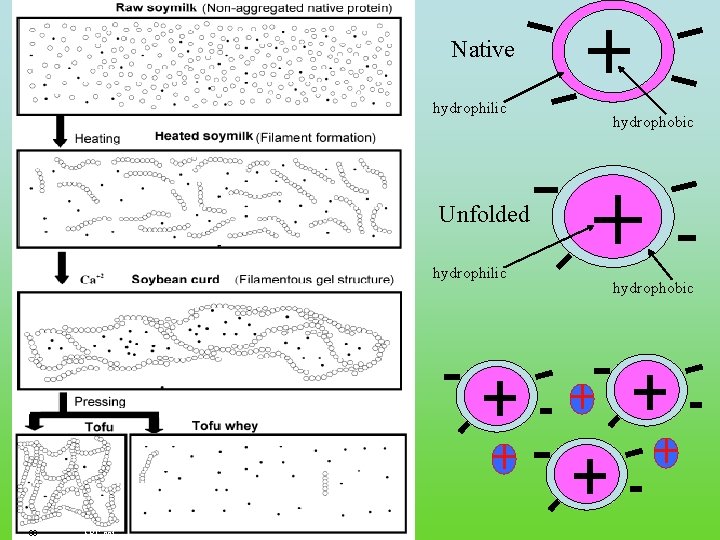
Native hydrophilic hydrophobic Unfolded hydrophilic 30 FRC. ppt hydrophobic

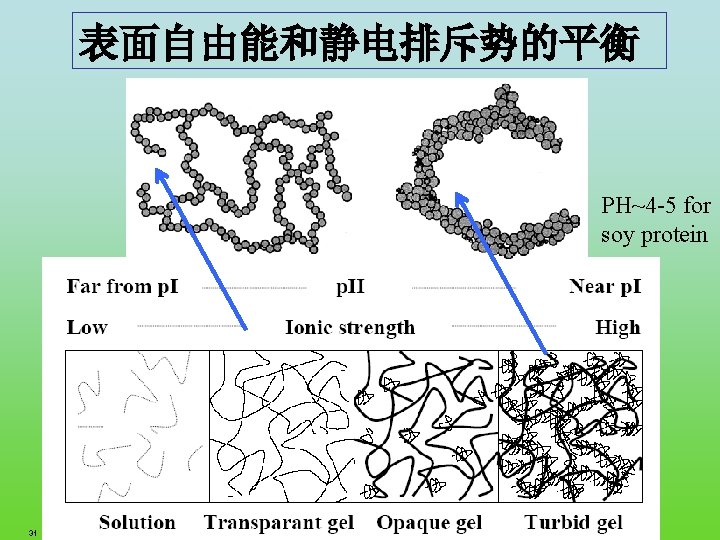
表面自由能和静电排斥势的平衡 PH~4 -5 for soy protein 31 FRC. ppt

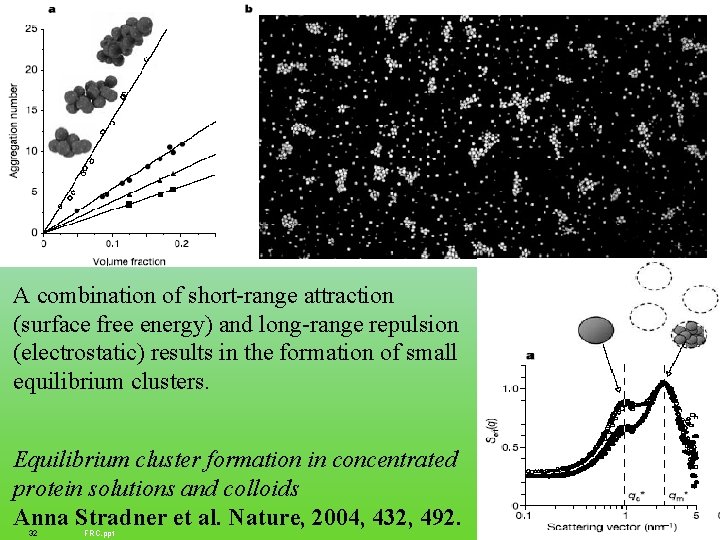
A combination of short-range attraction (surface free energy) and long-range repulsion (electrostatic) results in the formation of small equilibrium clusters. Equilibrium cluster formation in concentrated protein solutions and colloids Anna Stradner et al. Nature, 2004, 432, 492. 32 FRC. ppt

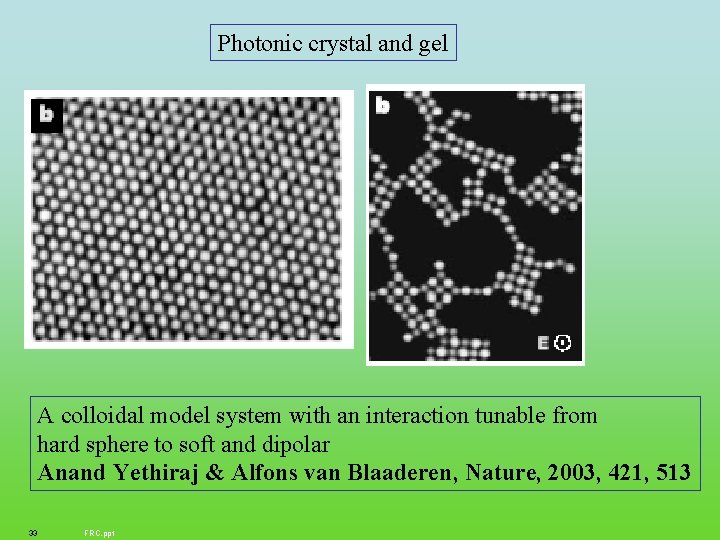
Photonic crystal and gel A colloidal model system with an interaction tunable from hard sphere to soft and dipolar Anand Yethiraj & Alfons van Blaaderen, Nature, 2003, 421, 513 33 FRC. ppt

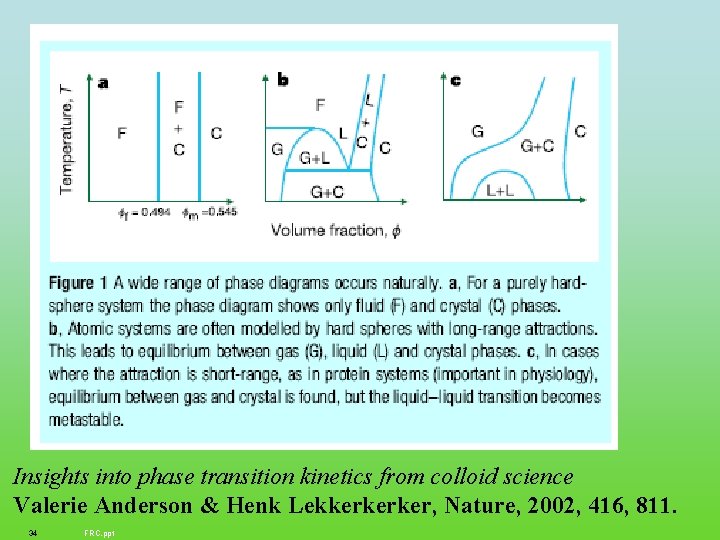
Insights into phase transition kinetics from colloid science Valerie Anderson & Henk Lekkerkerker, Nature, 2002, 416, 811. 34 FRC. ppt

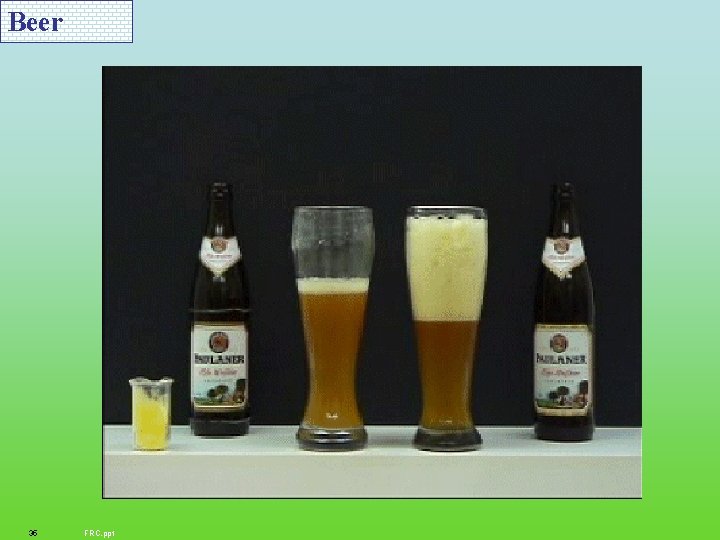
Beer 35 FRC. ppt

36 FRC. ppt

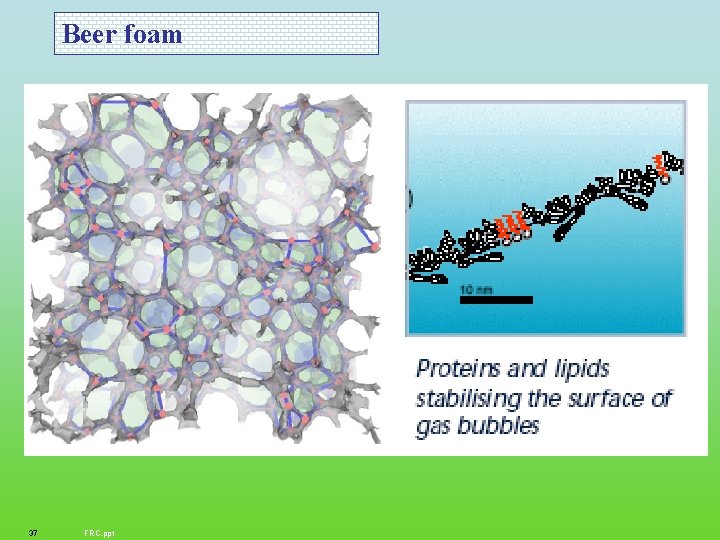
Beer foam 37 FRC. ppt

The physics of beer foam 1) Bubble formation 2) Creaming (bubble rise) 3) Disproportionation (Ostwald ripening) 4) Drainage 38 FRC. ppt

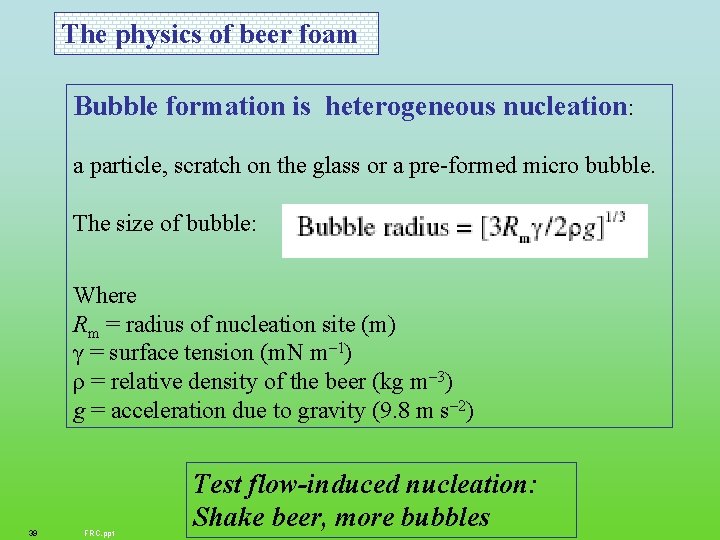
The physics of beer foam Bubble formation is heterogeneous nucleation: a particle, scratch on the glass or a pre-formed micro bubble. The size of bubble: Where Rm = radius of nucleation site (m) γ = surface tension (m. N m– 1) ρ = relative density of the beer (kg m– 3) g = acceleration due to gravity (9. 8 m s– 2) 39 FRC. ppt Test flow-induced nucleation: Shake beer, more bubbles

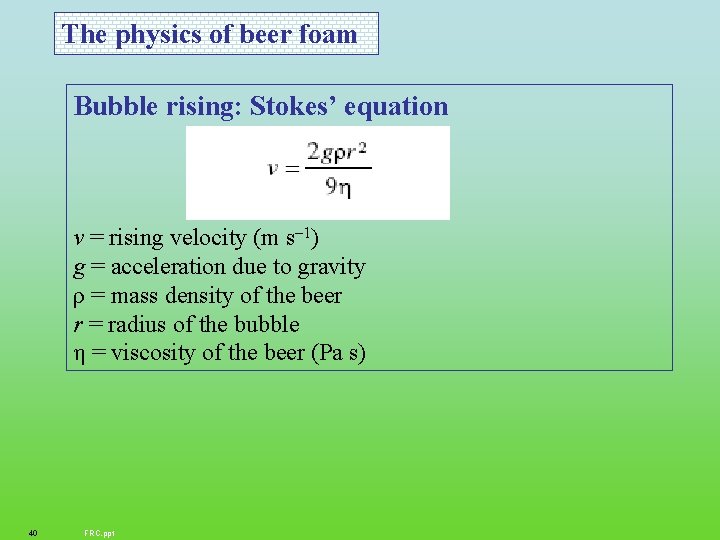
The physics of beer foam Bubble rising: Stokes’ equation v = rising velocity (m s– 1) g = acceleration due to gravity ρ = mass density of the beer r = radius of the bubble η = viscosity of the beer (Pa s) 40 FRC. ppt

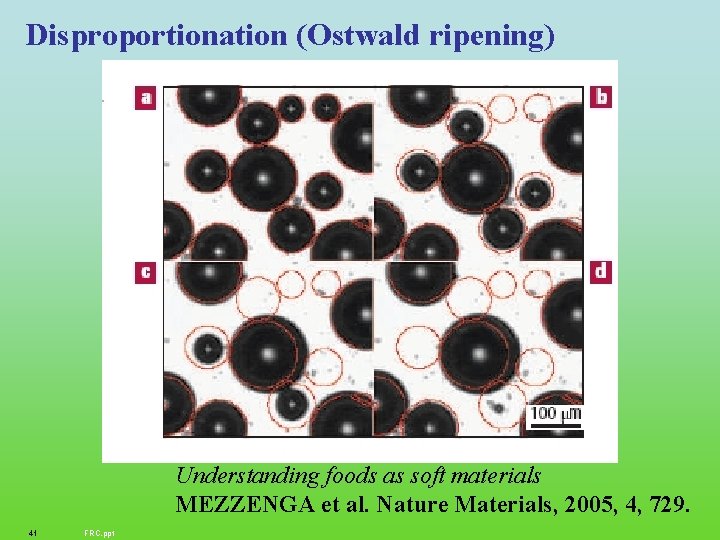
Disproportionation (Ostwald ripening) Understanding foods as soft materials MEZZENGA et al. Nature Materials, 2005, 4, 729. 41 FRC. ppt

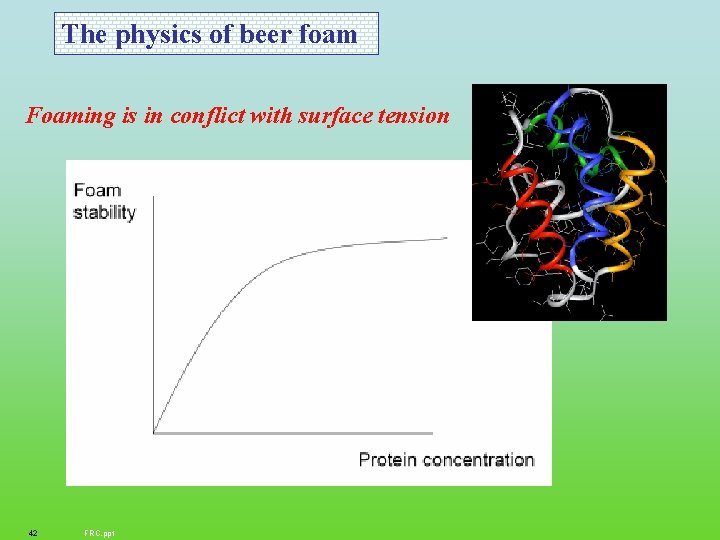
The physics of beer foam Foaming is in conflict with surface tension 42 FRC. ppt

43 FRC. ppt

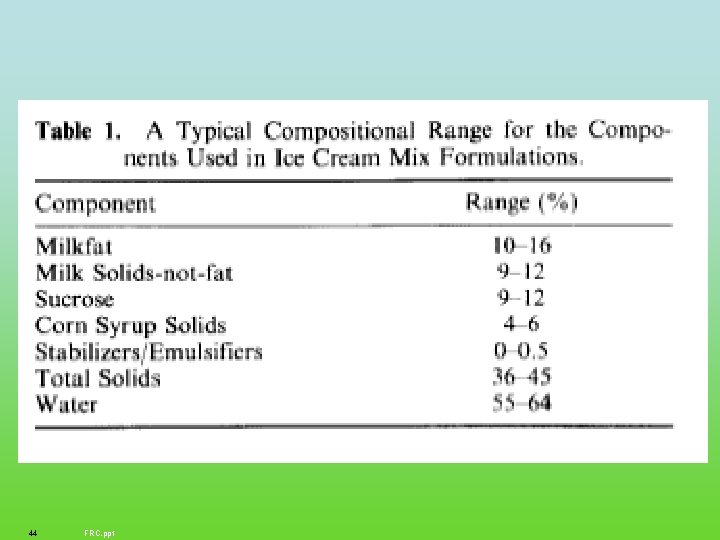
44 FRC. ppt

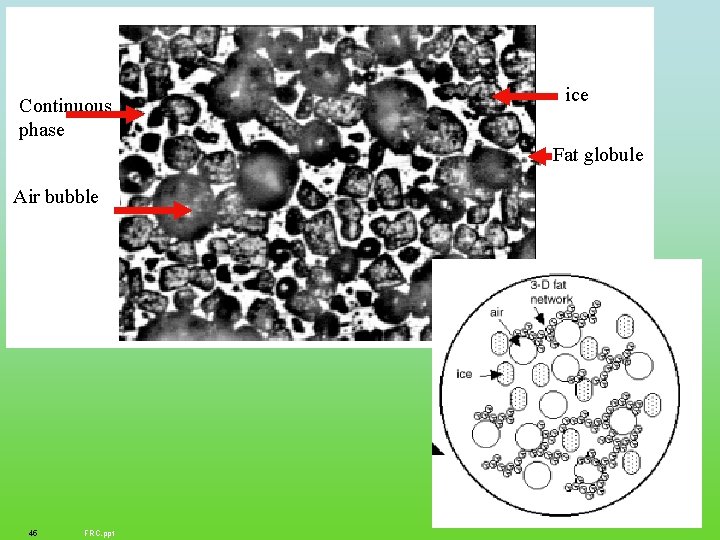
Continuous phase ice Fat globule Air bubble 45 FRC. ppt

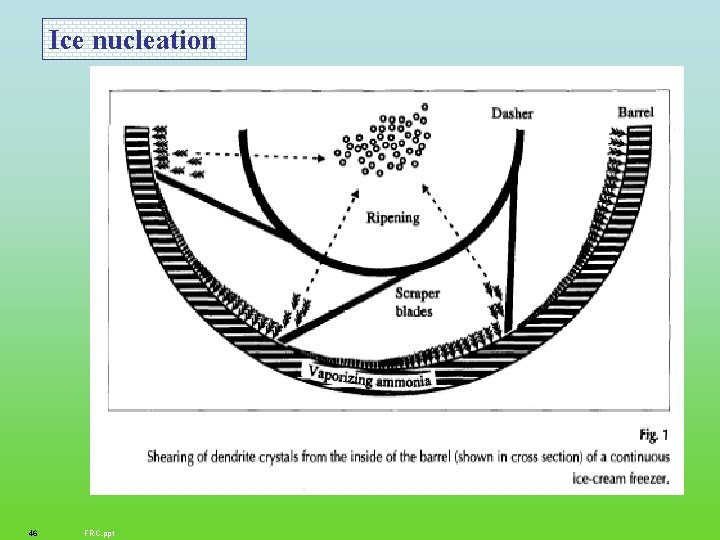
Ice nucleation 46 FRC. ppt

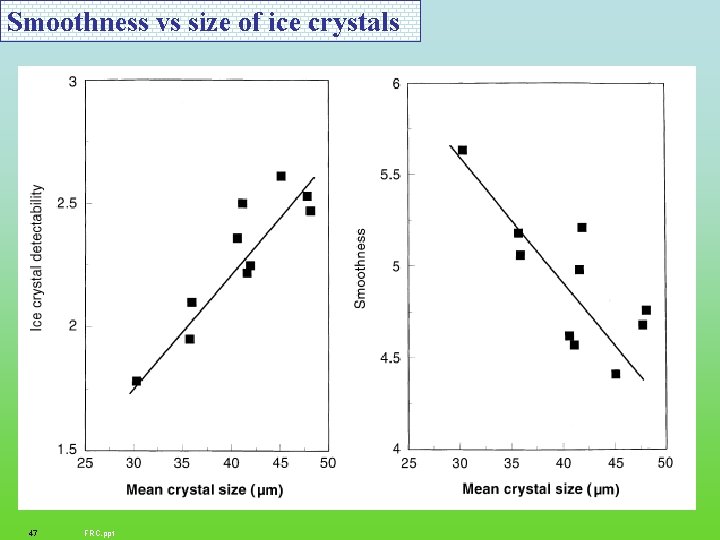
Smoothness vs size of ice crystals 47 FRC. ppt

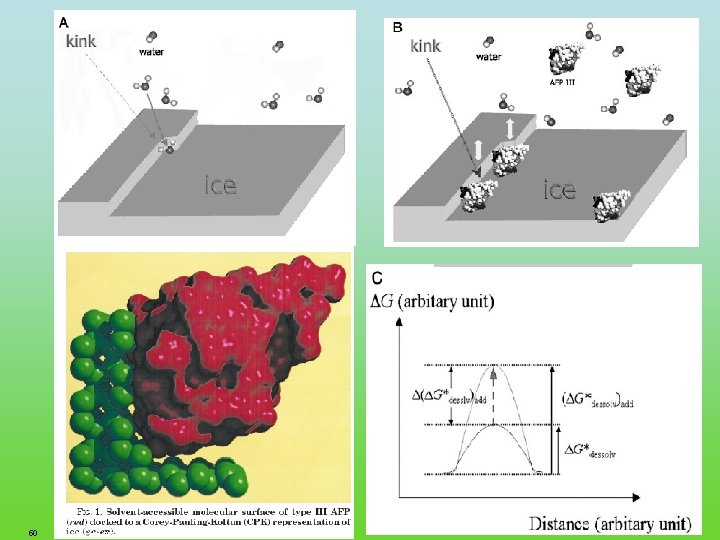
Biopolymer retarding ice re-crystallization Antifreeze glycoproteins and antifreeze proteins comprise several structurally diverse classes of molecules that have in common the ability to inhibit the growth of ice. The antifreeze glycoproteins are carbohydrate rich 2. 6 -34 k. Da proteins containing an (Ala-Thr)n repeat with a disaccharide attached to threonine. Four classes: Type I, alanine-rich, a-helical 3. 3 to 4. 5 -k. Da proteins; Type II, cysteine rich globular proteins that contain five disulfide bonds; Type III, approximately 6 k. Da globular proteins Type IV, glutamate- and glutamine-rich proteins that contain a-helices but appear to be unrelated to other proteins. 48 FRC. ppt Haring et al. Eur. J. Biochem. 264, 653 -665 (1999)

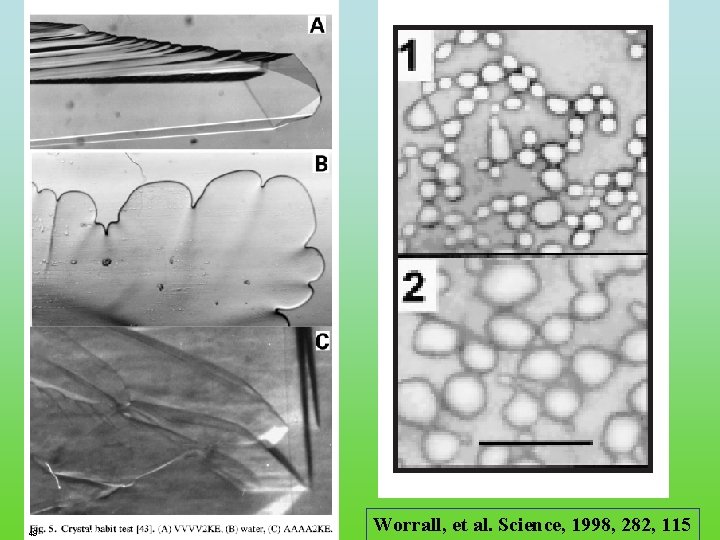
49 FRC. ppt Worrall, et al. Science, 1998, 282, 115

50 FRC. ppt

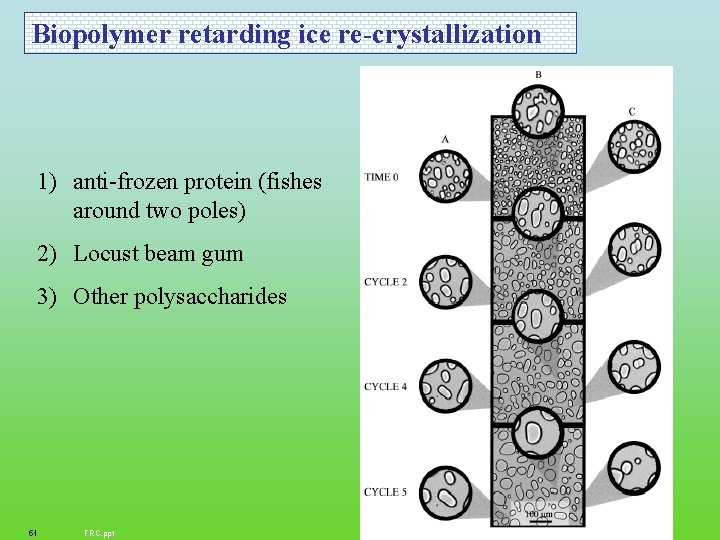
Biopolymer retarding ice re-crystallization 1) anti-frozen protein (fishes around two poles) 2) Locust beam gum 3) Other polysaccharides 51 FRC. ppt

Enjoy Science Enjoy Food Enjoy Health Thank you!! 52 FRC. ppt