WarmUp Calculate Molar Mass 1 K 2 O







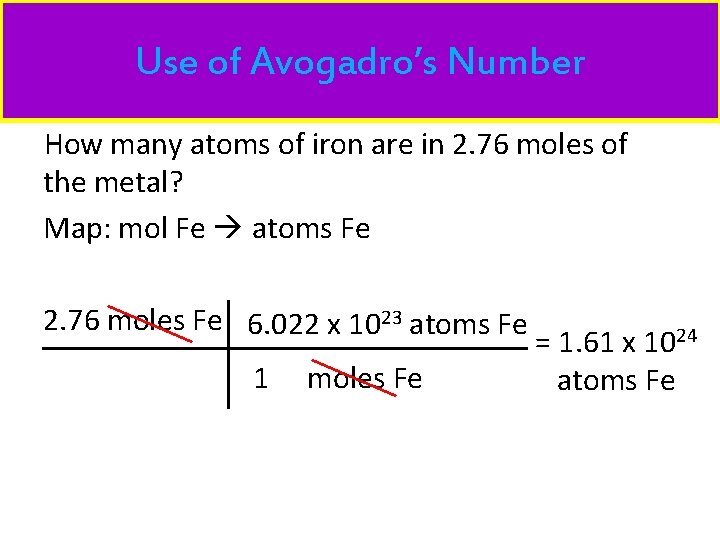

- Slides: 22

Warm-Up Calculate Molar Mass 1. K 2 O 2. PCl 5 3. Li. F

The Mole. . . J. Mc. Leod

Objectives • Describe methods of measuring the amount of something. • Define Avogadro’s number as it relates to a mole of a substance. • Distinguish between the atomic mass of an element and its molar mass. • Describe how the mass of a mole of a compound is calculated. • Describe how to convert the mass of a substance to the number of moles of a substance, and moles to mass. • Describe how to convert the volume of a substance to the number of moles of a substance, and moles to volume.

What is a mole?

The mole… Abbreviated (mol) • It is an amount, such as… o Pair: 1 pair of shoelaces = 2 shoelaces o Dozen: 1 dozen oranges = 12 oranges o Gross: 1 gross of pencils = 144 pencils o Ream: 1 ream of paper = 500 sheets of paper

Definition 1 mole = 6. 02 x 23 10 That’s 602, 000, 000, 000 We call this number Avogadro’s number after Amadeo Avogadro (1766 -1856)

Definition Field Trip!!!!

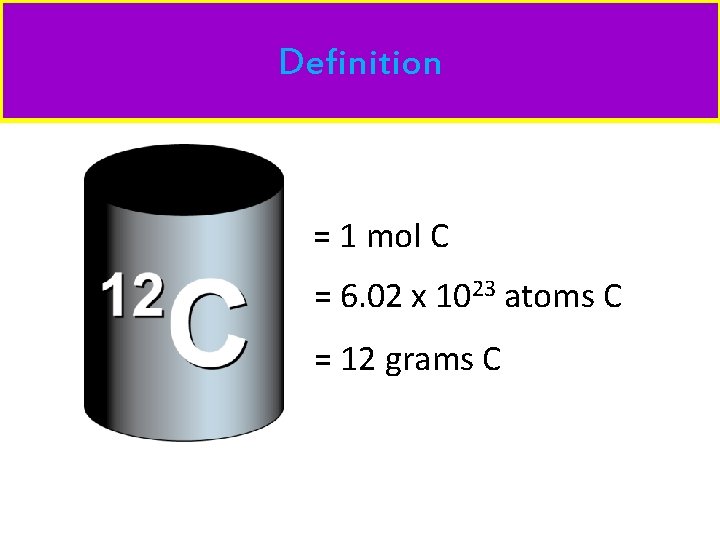
Definition = 1 mol C = 6. 02 x 1023 atoms C = 12 grams C

Review • Atomic Mass is the average of all isotopes of a given element. • 1 amu = 1/12 of a C-12 atom.

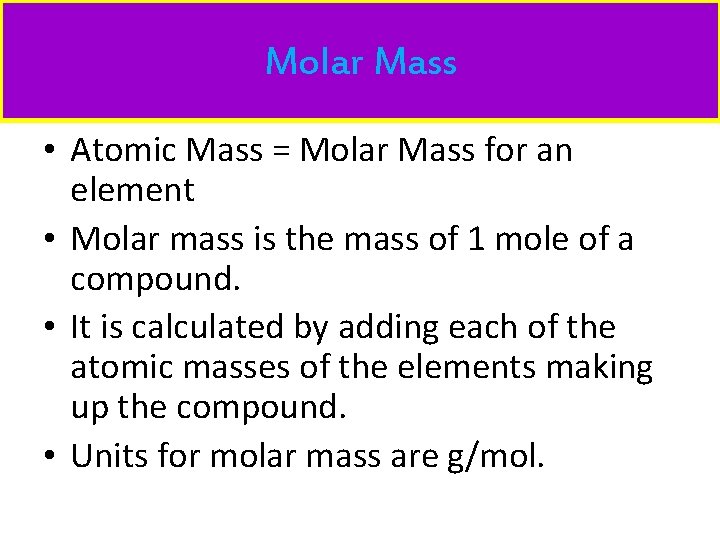
Molar Mass • Atomic Mass = Molar Mass for an element • Molar mass is the mass of 1 mole of a compound. • It is calculated by adding each of the atomic masses of the elements making up the compound. • Units for molar mass are g/mol.

Diatomics • Diatomics…. You need a highlighter!!

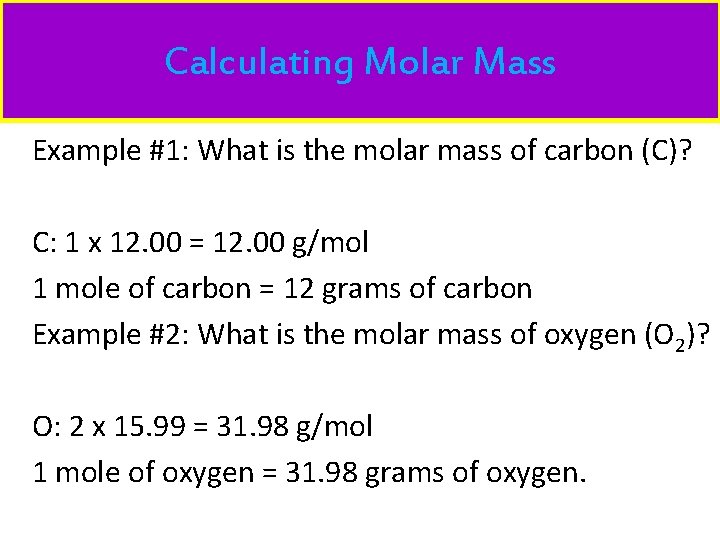
Calculating Molar Mass Example #1: What is the molar mass of carbon (C)? C: 1 x 12. 00 = 12. 00 g/mol 1 mole of carbon = 12 grams of carbon Example #2: What is the molar mass of oxygen (O 2)? O: 2 x 15. 99 = 31. 98 g/mol 1 mole of oxygen = 31. 98 grams of oxygen.

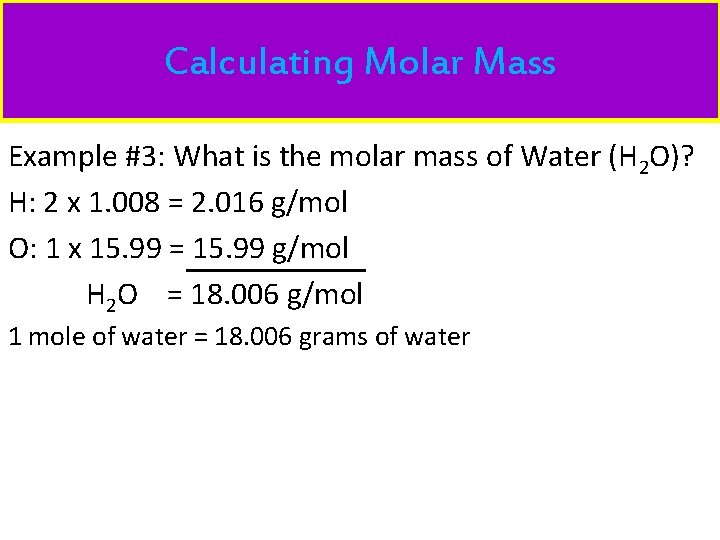
Calculating Molar Mass Example #3: What is the molar mass of Water (H 2 O)? H: 2 x 1. 008 = 2. 016 g/mol O: 1 x 15. 99 = 15. 99 g/mol H 2 O = 18. 006 g/mol 1 mole of water = 18. 006 grams of water

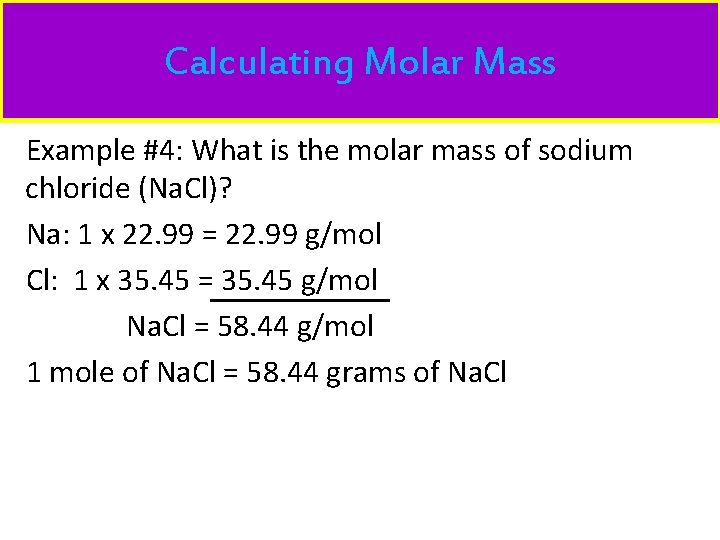
Calculating Molar Mass Example #4: What is the molar mass of sodium chloride (Na. Cl)? Na: 1 x 22. 99 = 22. 99 g/mol Cl: 1 x 35. 45 = 35. 45 g/mol Na. Cl = 58. 44 g/mol 1 mole of Na. Cl = 58. 44 grams of Na. Cl

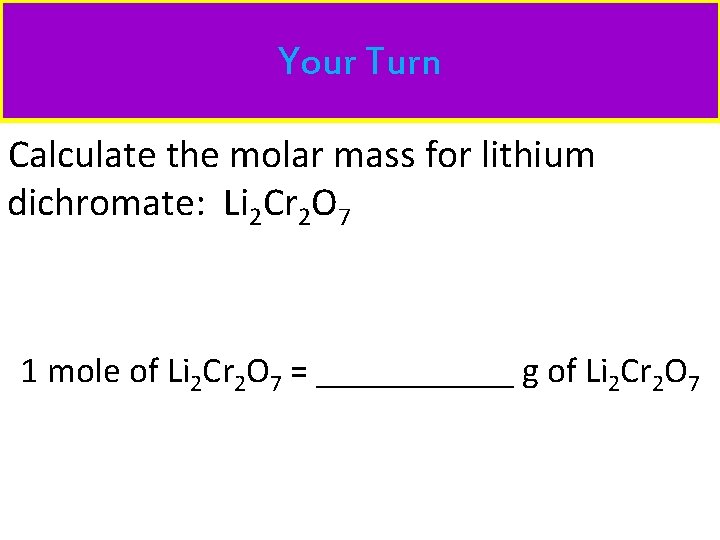
Your Turn Calculate the molar mass for lithium dichromate: Li 2 Cr 2 O 7 1 mole of Li 2 Cr 2 O 7 = ______ g of Li 2 Cr 2 O 7

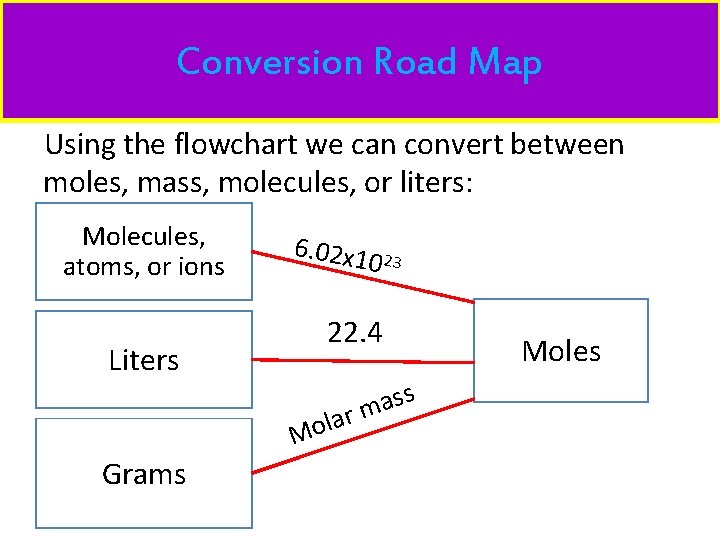
Conversion Road Map Using the flowchart we can convert between moles, mass, molecules, or liters: Molecules, atoms, or ions Liters 6. 02 x 1 22. 4 s s a lar m Mo Grams 0 23 Moles

Rules For Conversion 1. Write Units Map 2. Set Up Problem a. Write Given b. Use a T box for each step of the map c. Make sure your units cancel! 3. Write your answer with correct units

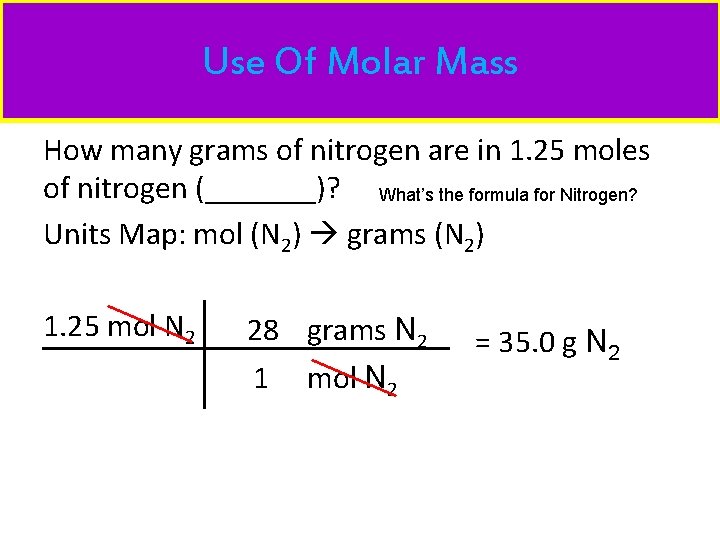
Use Of Molar Mass How many grams of nitrogen are in 1. 25 moles of nitrogen (_______)? What’s the formula for Nitrogen? Units Map: mol (N 2) grams (N 2) 1. 25 mol N 2 28 grams N 2 1 mol N 2 = 35. 0 g N 2
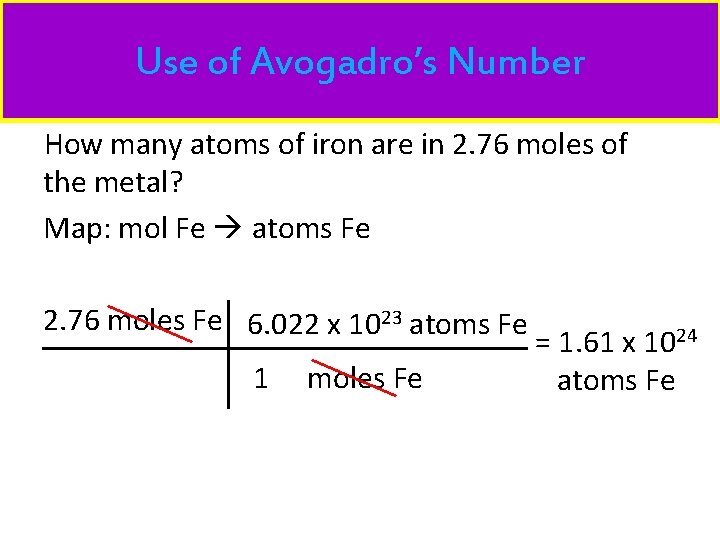
Use of Avogadro’s Number How many atoms of iron are in 2. 76 moles of the metal? Map: mol Fe atoms Fe 2. 76 moles Fe 6. 022 x 1023 atoms Fe 1 moles Fe = 1. 61 x 1024 atoms Fe

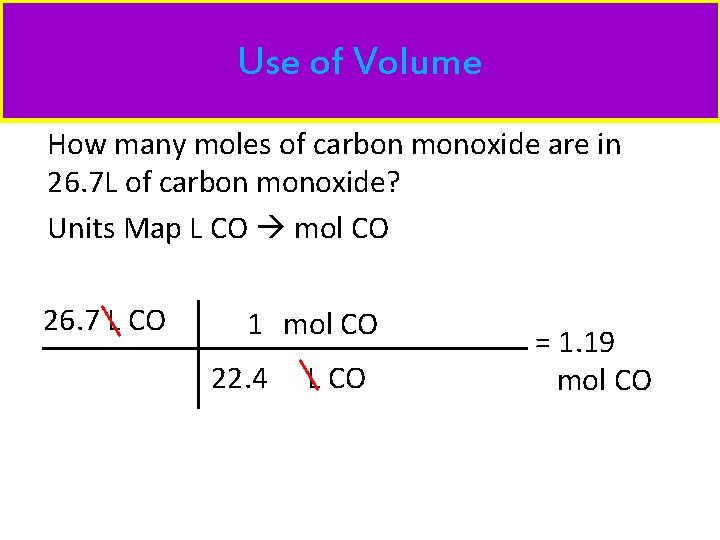
Use of Volume How many moles of carbon monoxide are in 26. 7 L of carbon monoxide? Units Map L CO mol CO 26. 7 L CO 1 mol CO 22. 4 L CO = 1. 19 mol CO

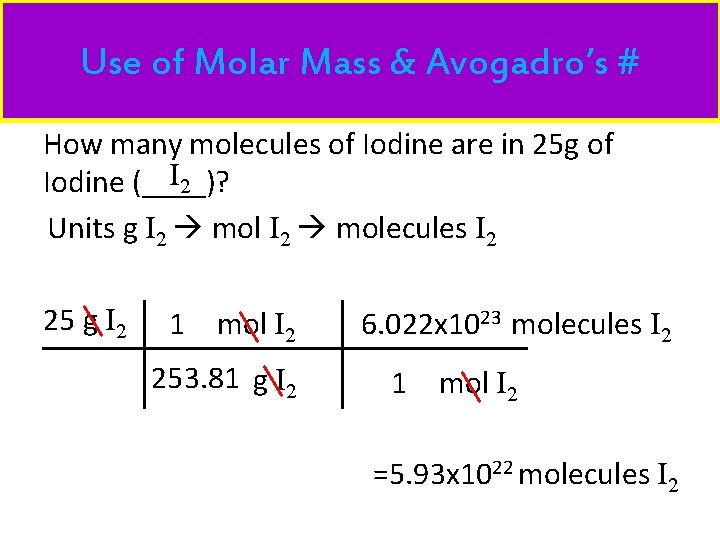
Use of Molar Mass & Avogadro’s # How many molecules of Iodine are in 25 g of I 2 Iodine (____)? Units g I 2 molecules I 2 25 g I 2 1 mol I 2 253. 81 g I 2 6. 022 x 1023 molecules I 2 1 mol I 2 =5. 93 x 1022 molecules I 2

Ticket Out The Door • In your groups, compare and contrast the process to convert moles to grams and that to convert moles to atoms or particles. • WHAT’S SIMILAR? • WHAT’S DIFFERENT?