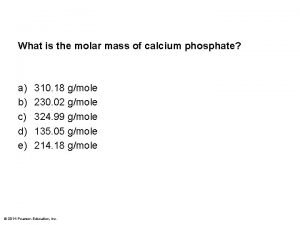
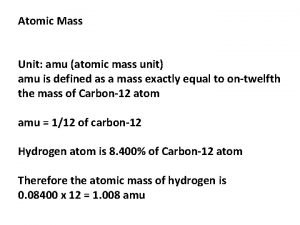Calculating Molar Mass of a Compound Mass in










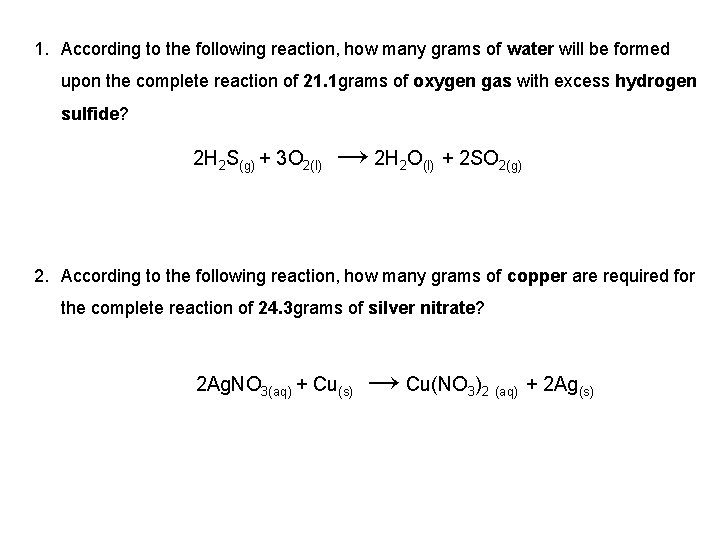








- Slides: 65


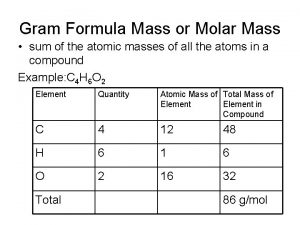
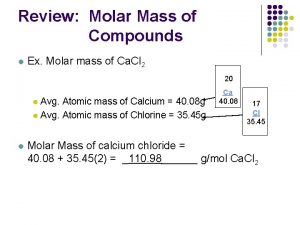
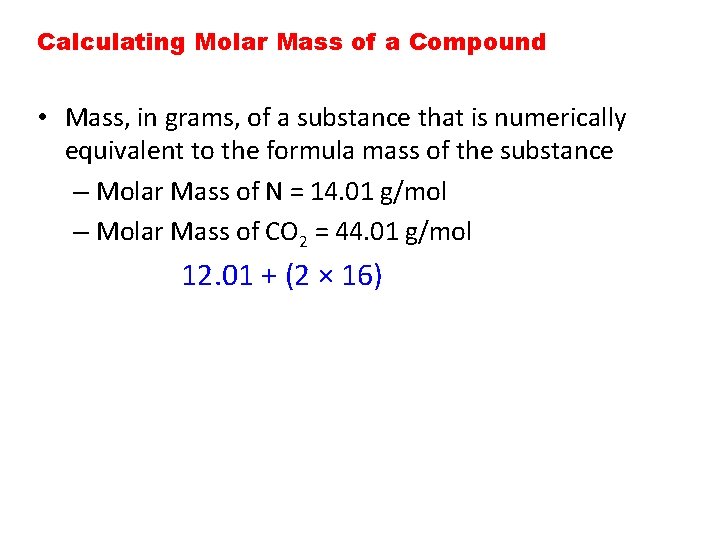
Calculating Molar Mass of a Compound • Mass, in grams, of a substance that is numerically equivalent to the formula mass of the substance – Molar Mass of N = 14. 01 g/mol – Molar Mass of CO 2 = 44. 01 g/mol 12. 01 + (2 × 16)

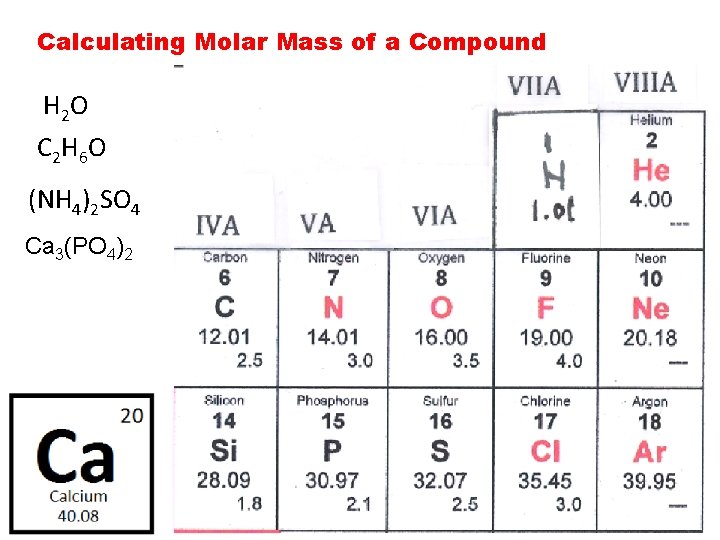
Calculating Molar Mass of a Compound H 2 O C 2 H 6 O (NH 4)2 SO 4 Ca 3(PO 4)2

Mole = the amount of substance whose mass in grams is numerically equal to its molar or formula mass (weight) Mole = is the molecular mass(weight) of the compound in grams

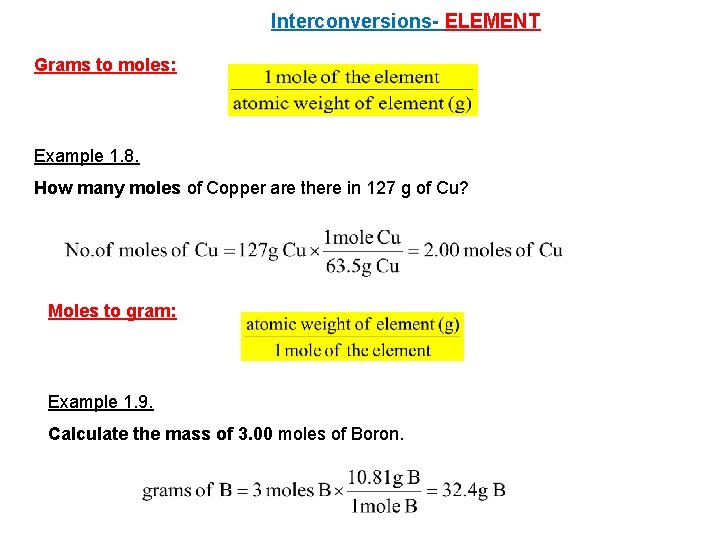
Interconversions- ELEMENT Grams to moles: Example 1. 8. How many moles of Copper are there in 127 g of Cu? Moles to gram: Example 1. 9. Calculate the mass of 3. 00 moles of Boron.

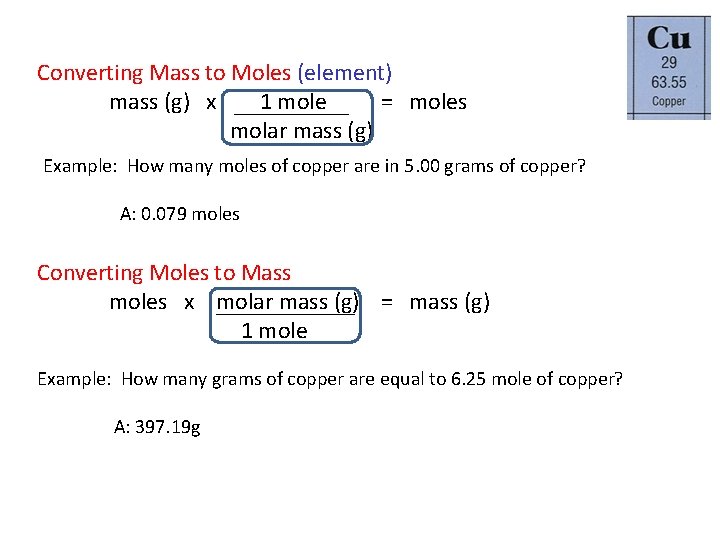
Converting Mass to Moles (element) mass (g) x 1 mole = moles molar mass (g) Example: How many moles of copper are in 5. 00 grams of copper? A: 0. 079 moles Converting Moles to Mass moles x molar mass (g) = mass (g) 1 mole Example: How many grams of copper are equal to 6. 25 mole of copper? A: 397. 19 g

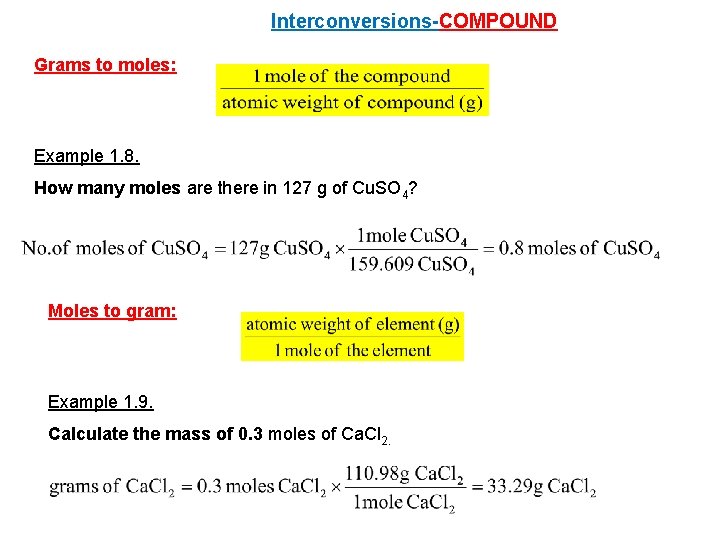
Interconversions-COMPOUND Grams to moles: Example 1. 8. How many moles are there in 127 g of Cu. SO 4? Moles to gram: Example 1. 9. Calculate the mass of 0. 3 moles of Ca. Cl 2.

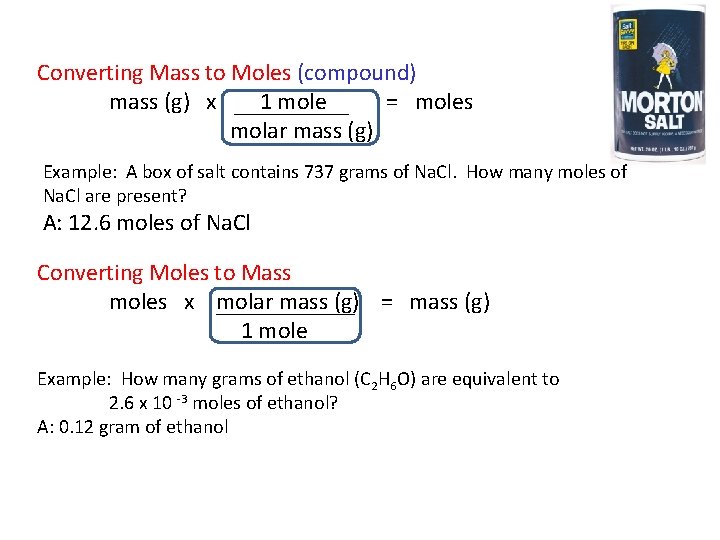
Converting Mass to Moles (compound) mass (g) x 1 mole = moles molar mass (g) Example: A box of salt contains 737 grams of Na. Cl. How many moles of Na. Cl are present? A: 12. 6 moles of Na. Cl Converting Moles to Mass moles x molar mass (g) = mass (g) 1 mole Example: How many grams of ethanol (C 2 H 6 O) are equivalent to 2. 6 x 10 -3 moles of ethanol? A: 0. 12 gram of ethanol

1. How many moles of F are there in 11. 8 grams of F? 2. How many grams of H are there in a sample of H that contains the same number of moles as a 43. 6 gram sample of I? 3. How many grams of barium acetate, Ba(CH 3 COO)2, are present in 1. 03 moles of this compound? How many atoms of barium acetate, Ba(CH 3 COO)2, are present in 1. 03 moles of this compound? 4. How many moles of barium acetate are present in 4. 53 grams of this compound? How many atoms of barium acetate are present in 4. 53 grams of this compound?

Avogadro’s Number (NA) – the number of units in 1 mole of anything 6. 022 x 1023 Avogadro’s Number (NA) – is the name given to the numerical value 6. 022 x 1023 NA= 6. 022 x 1023 Example 1. 4. 1 atom of sodium = 22. 98977 amu (or u) ≈ 23. 0 g So 23. 0 g of sodium contains 6. 022 x 1023 atoms Avogadro’s Number (NA) number of ions, particles or moles is referred to as 1 mole of the substance. Example 1. 5. 1 mole of HCL = 6. 022 x 1023 HCl molecules = 36. 5 g HCl

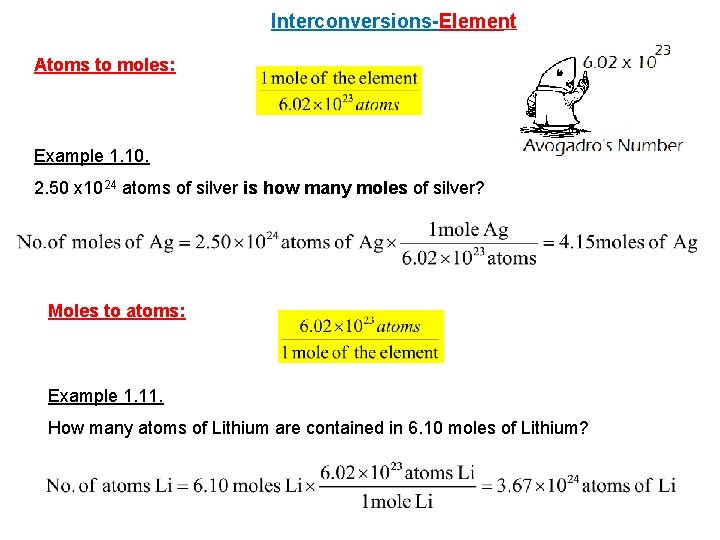
Interconversions-Element Atoms to moles: Example 1. 10. 2. 50 x 1024 atoms of silver is how many moles of silver? Moles to atoms: Example 1. 11. How many atoms of Lithium are contained in 6. 10 moles of Lithium?

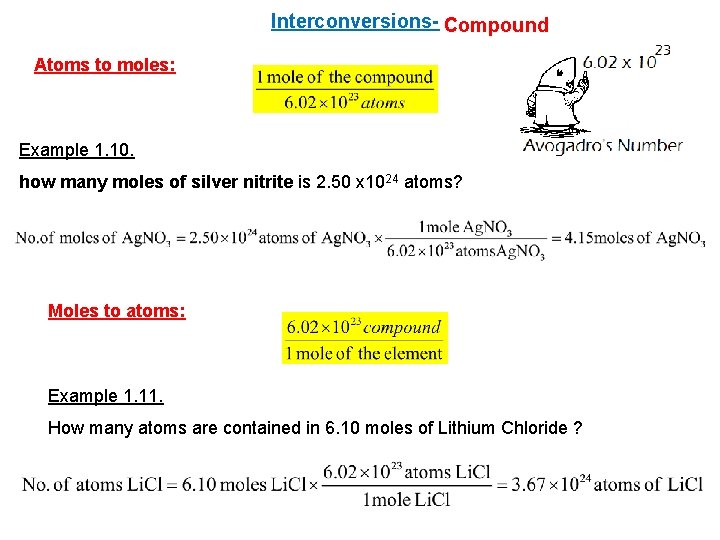
Interconversions- Compound Atoms to moles: Example 1. 10. how many moles of silver nitrite is 2. 50 x 1024 atoms? Moles to atoms: Example 1. 11. How many atoms are contained in 6. 10 moles of Lithium Chloride ?

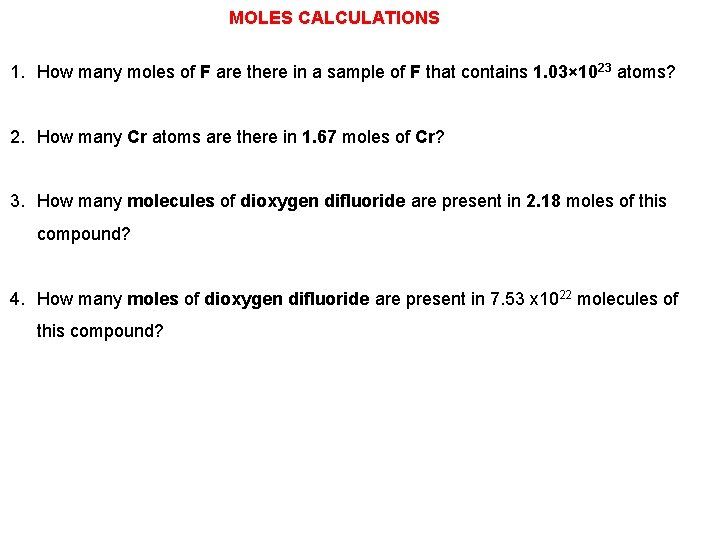
MOLES CALCULATIONS 1. How many moles of F are there in a sample of F that contains 1. 03× 1023 atoms? 2. How many Cr atoms are there in 1. 67 moles of Cr? 3. How many molecules of dioxygen difluoride are present in 2. 18 moles of this compound? 4. How many moles of dioxygen difluoride are present in 7. 53 x 1022 molecules of this compound?

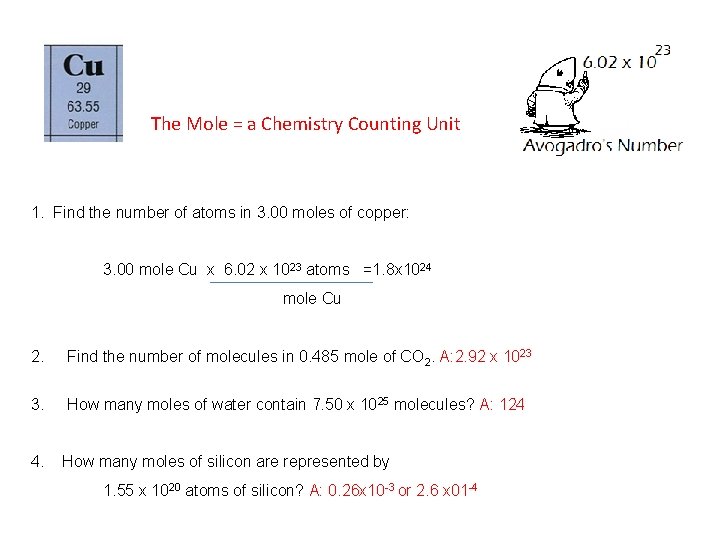
The Mole = a Chemistry Counting Unit 1. Find the number of atoms in 3. 00 moles of copper: 3. 00 mole Cu x 6. 02 x 1023 atoms =1. 8 x 1024 mole Cu 2. Find the number of molecules in 0. 485 mole of CO 2. A: 2. 92 x 1023 3. How many moles of water contain 7. 50 x 1025 molecules? A: 124 4. How many moles of silicon are represented by 1. 55 x 1020 atoms of silicon? A: 0. 26 x 10 -3 or 2. 6 x 01 -4

Interconversions- Element Grams to atoms: Example 1. 6. How many atoms are contained in 7. 15 grams of phosphorus? Atoms to grams: Example 1. 7. What is the molar mass of 18. 06 x 1023 atoms of Carbon?

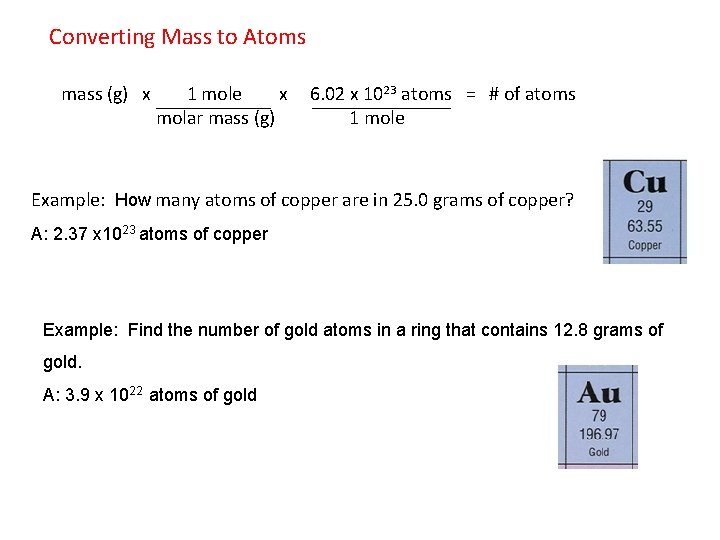
Converting Mass to Atoms mass (g) x 1 mole x 6. 02 x 1023 atoms = # of atoms molar mass (g) 1 mole Example: How many atoms of copper are in 25. 0 grams of copper? A: 2. 37 x 1023 atoms of copper Example: Find the number of gold atoms in a ring that contains 12. 8 grams of gold. A: 3. 9 x 1022 atoms of gold

Interconversions-Compound Grams to atoms: Example 1. 6. How many atoms are contained in 7. 15 grams of phosphoric acid? Atoms to grams: Example 1. 7. What is the molar mass of 18. 06 x 1023 atoms of Carbon Dioxide?

1. What is the mass in grams of 1. 63 x 1023 atoms of gallium? 2. How many gallium atoms are there in 46. 5 grams of gallium? 3. The formula for dioxygen difluoride is O 2 F 2. a) How many molecules are in 3. 56 grams of dioxygen difluoride?

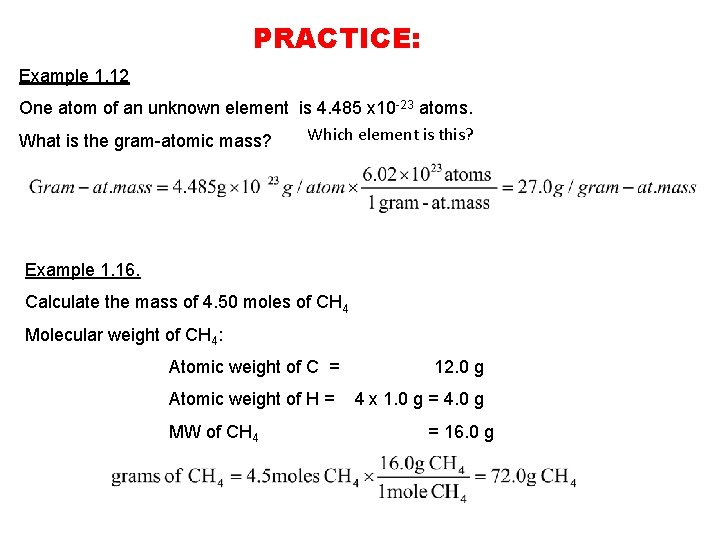
PRACTICE: Example 1. 12 One atom of an unknown element is 4. 485 x 10 -23 atoms. Which element is this? What is the gram-atomic mass? Example 1. 16. Calculate the mass of 4. 50 moles of CH 4 Molecular weight of CH 4: Atomic weight of C = 12. 0 g Atomic weight of H = 4 x 1. 0 g = 4. 0 g MW of CH 4 = 16. 0 g

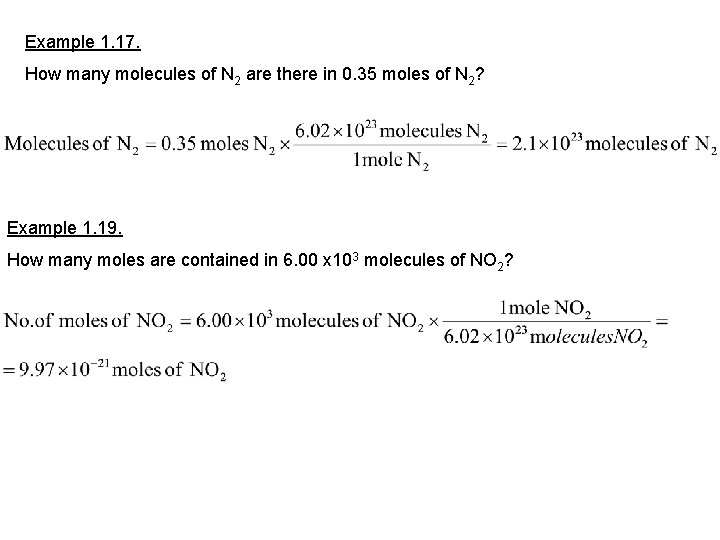
Example 1. 17. How many molecules of N 2 are there in 0. 35 moles of N 2? Example 1. 19. How many moles are contained in 6. 00 x 103 molecules of NO 2?

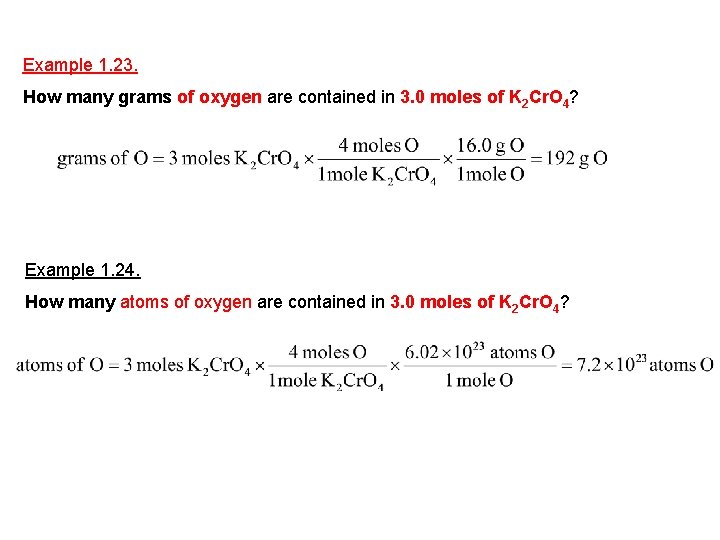
Example 1. 23. How many grams of oxygen are contained in 3. 0 moles of K 2 Cr. O 4? Example 1. 24. How many atoms of oxygen are contained in 3. 0 moles of K 2 Cr. O 4?

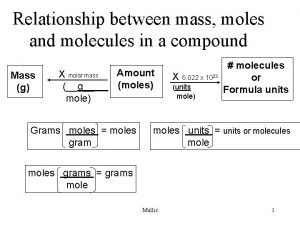
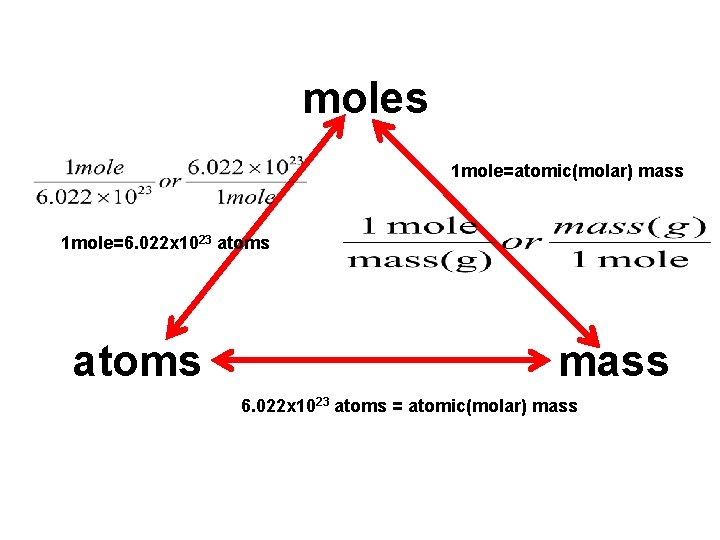
moles 1 mole=atomic(molar) mass 1 mole=6. 022 x 1023 atoms mass 6. 022 x 1023 atoms = atomic(molar) mass

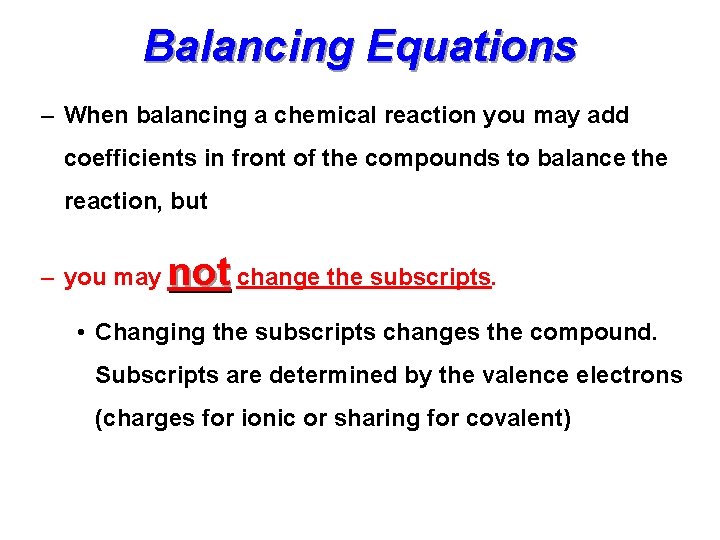
Balancing Equations – When balancing a chemical reaction you may add coefficients in front of the compounds to balance the reaction, but – you may not change the subscripts. • Changing the subscripts changes the compound. Subscripts are determined by the valence electrons (charges for ionic or sharing for covalent)

Steps to Balancing Equations There are four basic steps to balancing a chemical equation. 1. Write the correct formula for the reactants and the products. DO NOT TRY TO BALANCE IT YET! You must write the correct formulas first. And most importantly, once you write them correctly DO NOT CHANGE THE FORMULAS! 2. Find the number of atoms for each element on the left side. Compare those against the number of the atoms of the same element on the right side 3. Determine where to place coefficients in front of formulas so that the left side has the same number of atoms as the right side for EACH element in order to balance the equation. 4. Check your answer to see if: – The numbers of atoms on both sides of the equation are now balanced. – The coefficients are in the lowest possible whole number ratios. (reduced)

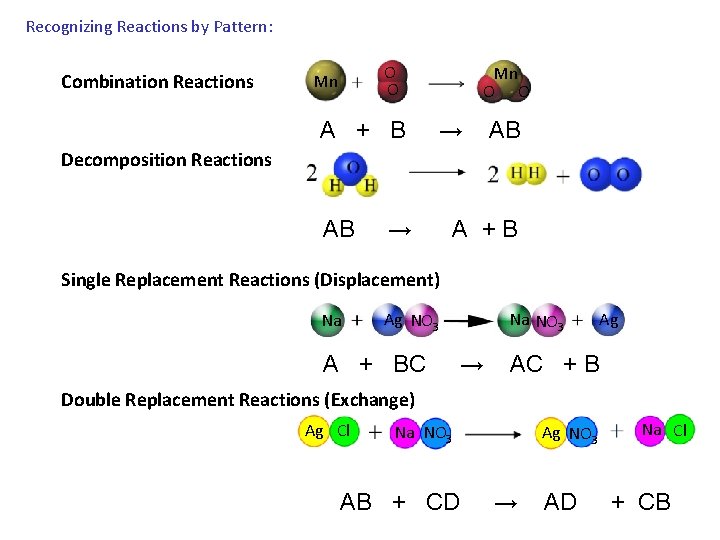
Recognizing Reactions by Pattern: O O Combination Reactions Mn Mn O O A + B → AB Decomposition Reactions AB → A + B Single Replacement Reactions (Displacement) Na Ag NO 3 Na NO 3 Ag A + BC → AC + B Double Replacement Reactions (Exchange) Ag Cl Na NO 3 Ag NO 3 Na Cl AB + CD → AD + CB

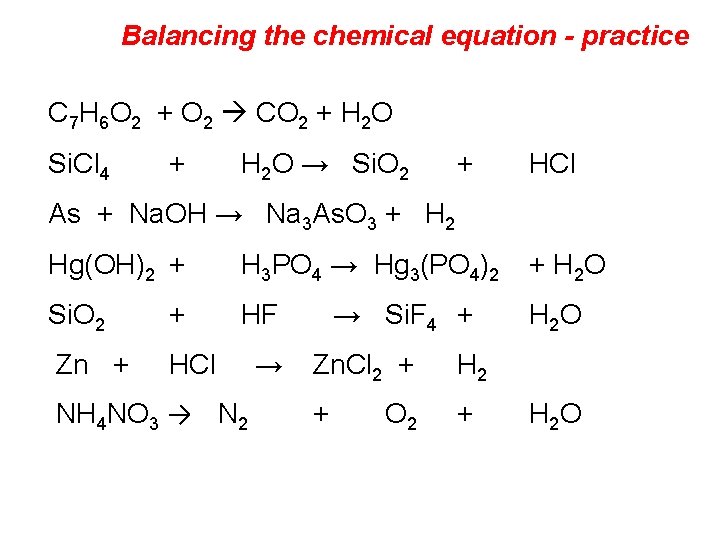
Balancing the chemical equation - practice C 7 H 6 O 2 + O 2 CO 2 + H 2 O Si. Cl 4 + H 2 O → Si. O 2 + HCl As + Na. OH → Na 3 As. O 3 + H 2 Hg(OH)2 + H 3 PO 4 → Hg 3(PO 4)2 + H 2 O Si. O 2 + HF → Si. F 4 + Zn + HCl → NH 4 NO 3 → N 2 Zn. Cl 2 + H 2 + + O 2 H 2 O

Stoichiometry

Steps Involved in Solving Mass-Mass Stoichiometry Problems • Balance the chemical equation correctly • Using the molar mass of the given substance, convert the mass given to moles. • Construct a molar proportion (two molar ratios set equal to each other) • Using the molar mass of the unknown substance, convert the moles just calculated to mass.

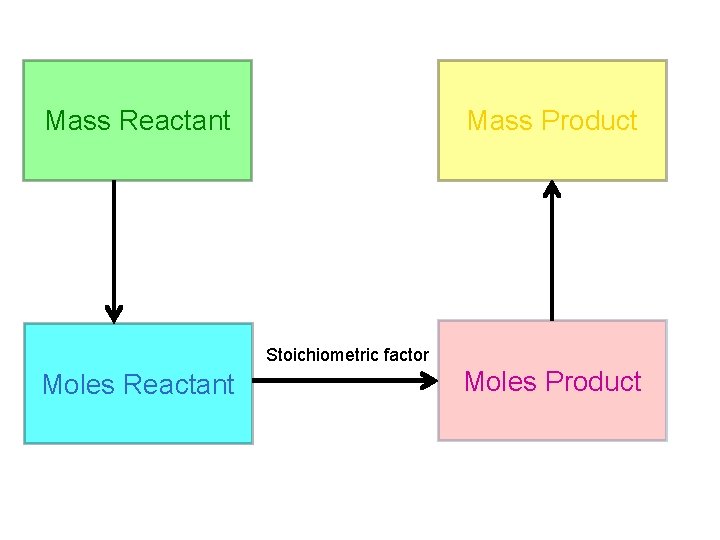
Mass Reactant Mass Product Stoichiometric factor Moles Reactant Moles Product

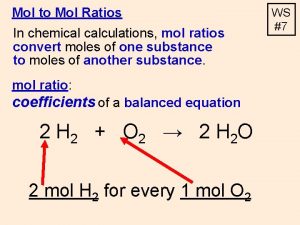
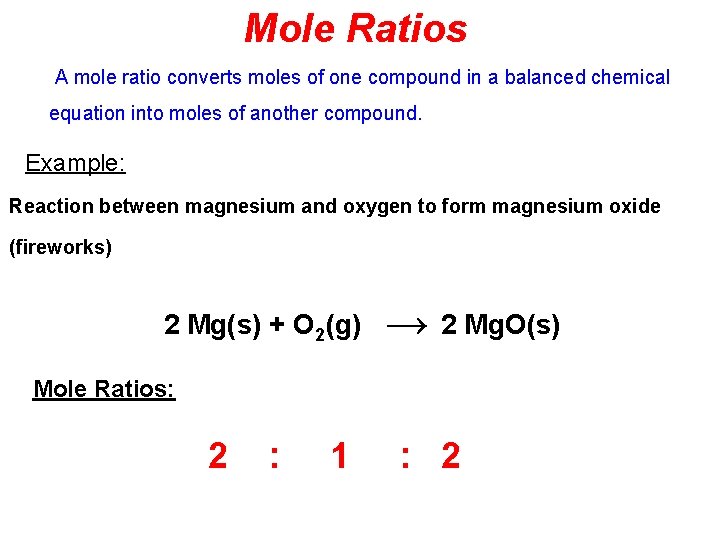
Mole Ratios A mole ratio converts moles of one compound in a balanced chemical equation into moles of another compound. Example: Reaction between magnesium and oxygen to form magnesium oxide (fireworks) 2 Mg(s) + O 2(g) → 2 Mg. O(s) Mole Ratios: 2 : 1 : 2

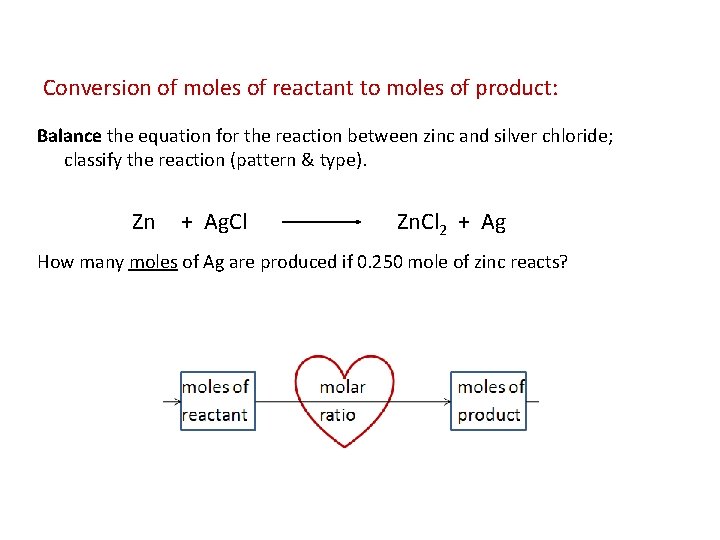
Conversion of moles of reactant to moles of product: Balance the equation for the reaction between zinc and silver chloride; classify the reaction (pattern & type). Zn + Ag. Cl Zn. Cl 2 + Ag How many moles of Ag are produced if 0. 250 mole of zinc reacts?
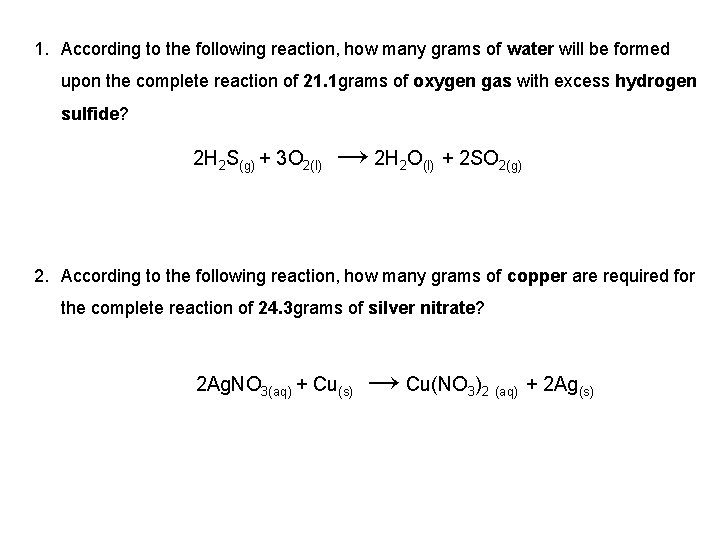
1. According to the following reaction, how many grams of water will be formed upon the complete reaction of 21. 1 grams of oxygen gas with excess hydrogen sulfide? → 2 H 2 O(l) + 2 SO 2(g) 2 H 2 S(g) + 3 O 2(l) 2. According to the following reaction, how many grams of copper are required for the complete reaction of 24. 3 grams of silver nitrate? → Cu(NO 3)2 (aq) + 2 Ag(s) 2 Ag. NO 3(aq) + Cu(s)

1. For the following reaction, 3. 03 grams of water are mixed with excess sulfur dioxide. The reaction yields 11. 3 grams of sulfurous acid (H 2 SO 3). SO 2(g) + H 2 O (l) → H 2 SO 3(g) a) What is theoretical yield of sulfurous acid (H 2 SO 3)? b) What is the percent yield for this reaction? 2. For the following reaction, 4. 88 grams of nitrogen gas are mixed with excess hydrogen gas. The reaction yields 4. 62 grams of ammonia. N 2(g) + 3 H 2(g) → 2 NH 3(g) a) What is theoretical yield of ammonia? b) What is the percent yield for this reaction?

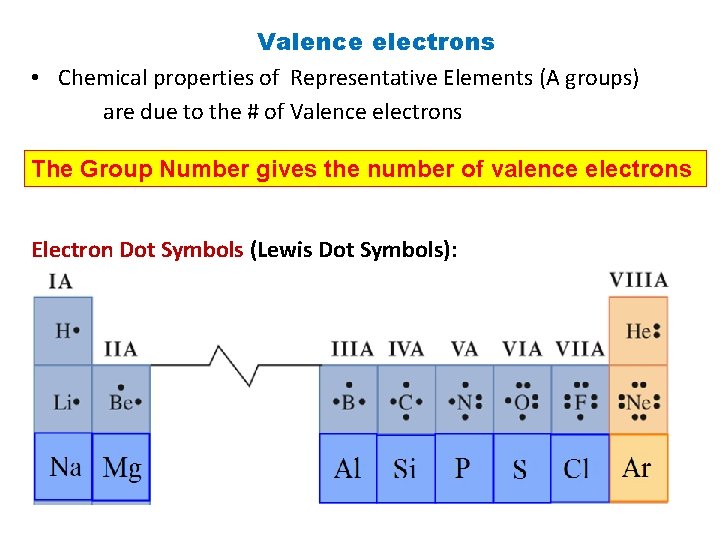
Valence electrons • Chemical properties of Representative Elements (A groups) are due to the # of Valence electrons The Group Number gives the number of valence electrons Electron Dot Symbols (Lewis Dot Symbols):

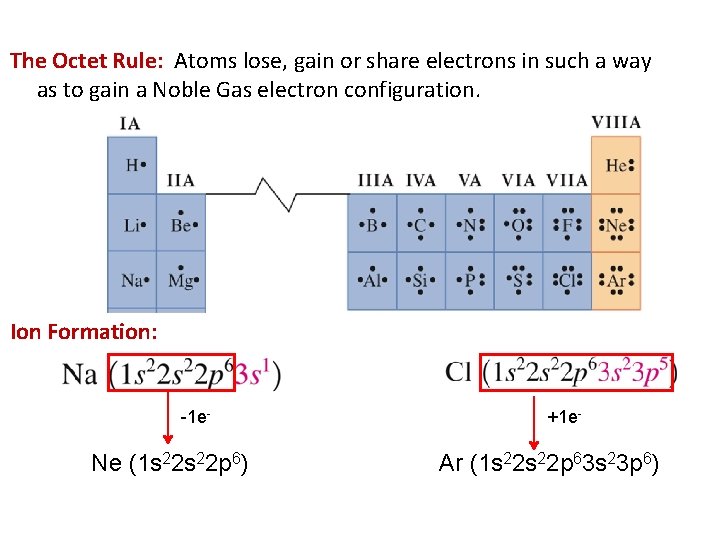
The Octet Rule: Atoms lose, gain or share electrons in such a way as to gain a Noble Gas electron configuration. Ion Formation: -1 e- Ne (1 s 22 p 6) +1 e- Ar (1 s 22 p 63 s 23 p 6)

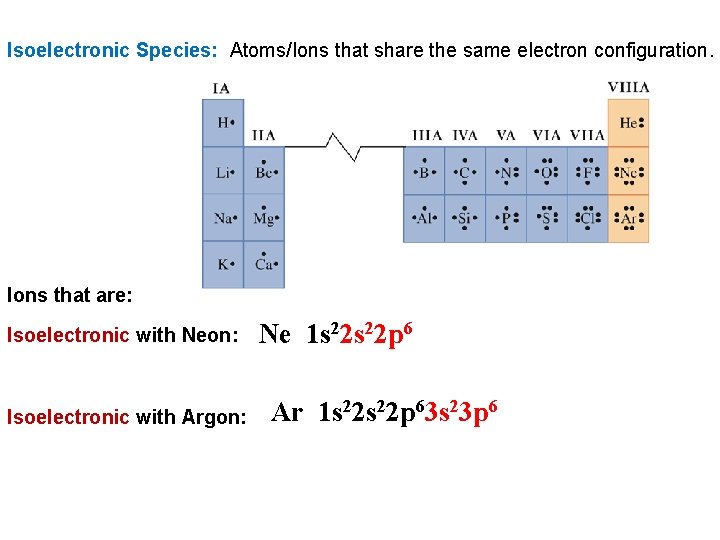
Isoelectronic Species: Atoms/Ions that share the same electron configuration. Ions that are: Isoelectronic with Neon: Ne 1 s 22 p 6 Isoelectronic with Argon: Ar 1 s 22 p 63 s 23 p 6

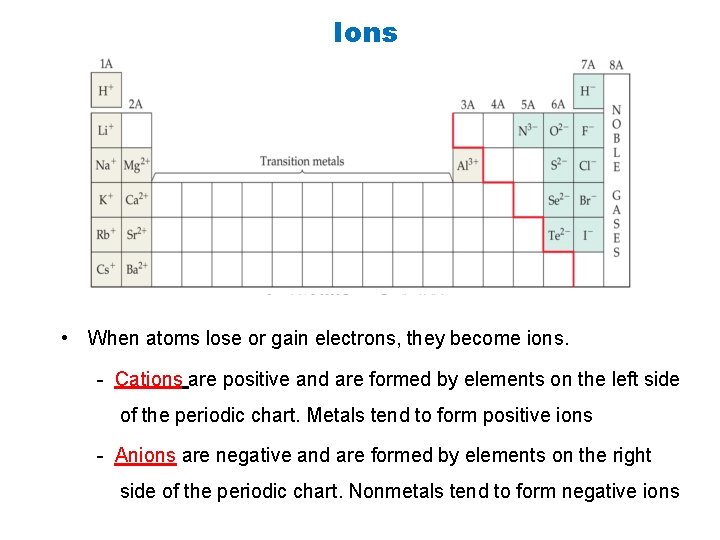
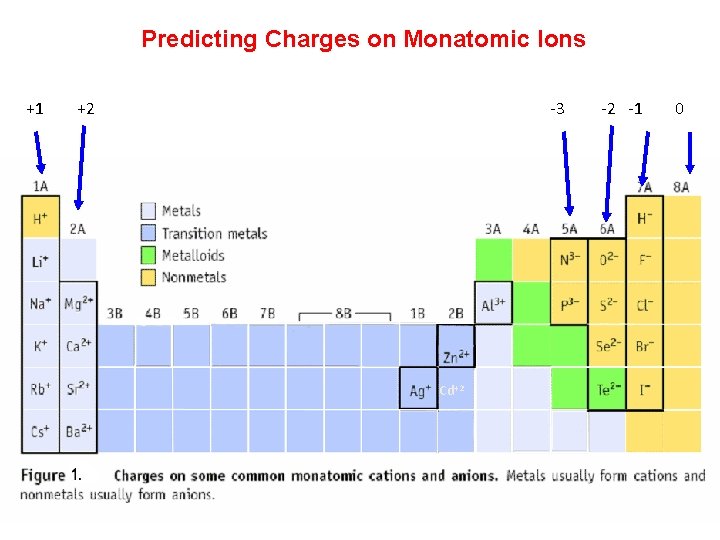
Ions • When atoms lose or gain electrons, they become ions. - Cations are positive and are formed by elements on the left side of the periodic chart. Metals tend to form positive ions - Anions are negative and are formed by elements on the right side of the periodic chart. Nonmetals tend to form negative ions

Predicting Charges on Monatomic Ions +1 +2 -3 -2 -1 0 Cd+2

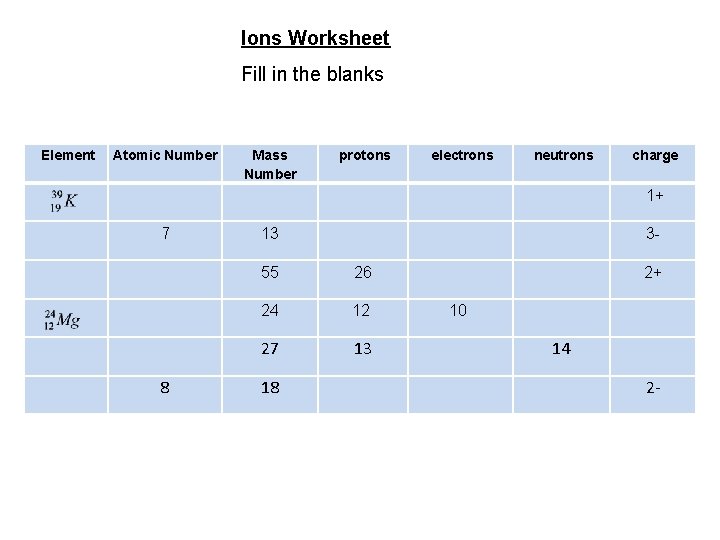
Ions Worksheet Fill in the blanks Element Atomic Number protons electrons neutrons charge 1+ 7 Mass Number 8 13 3 - 55 26 24 12 27 13 18 2+ 10 14 2 -

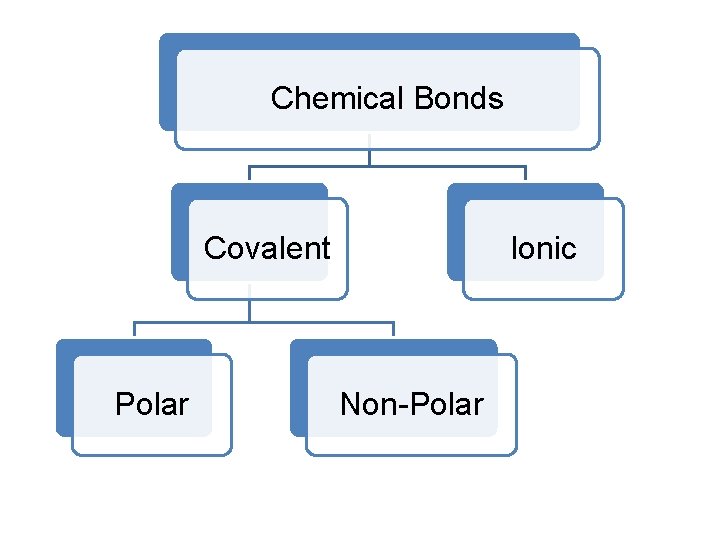
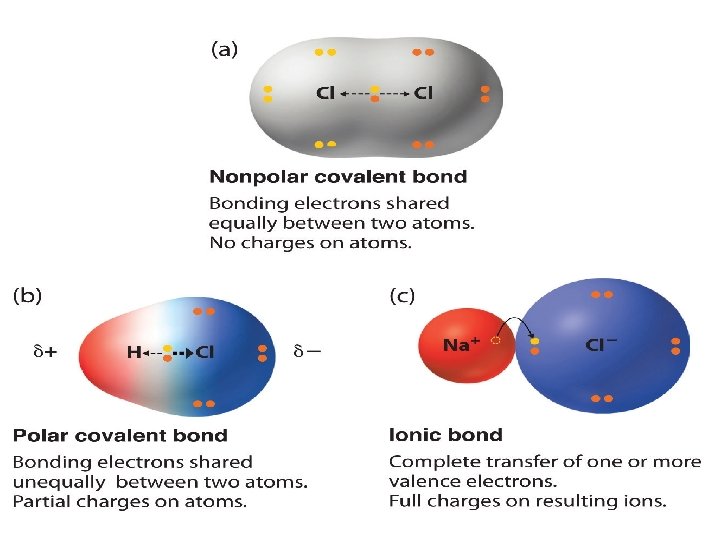
Chemical Bonds Covalent Polar Ionic Non-Polar

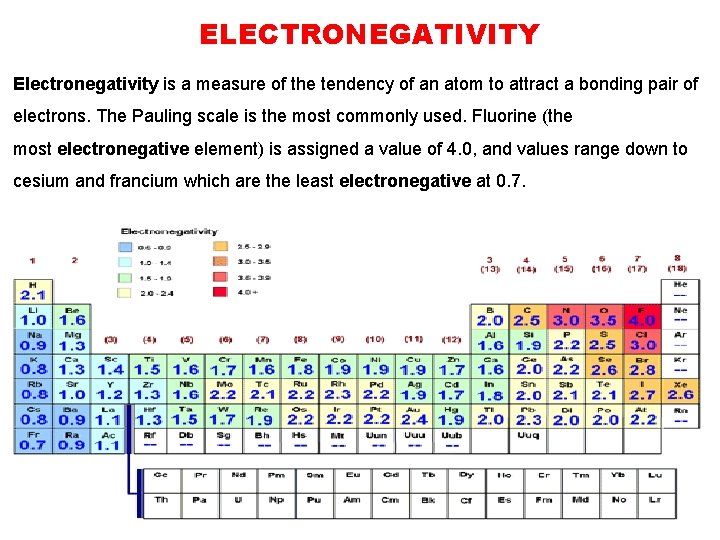
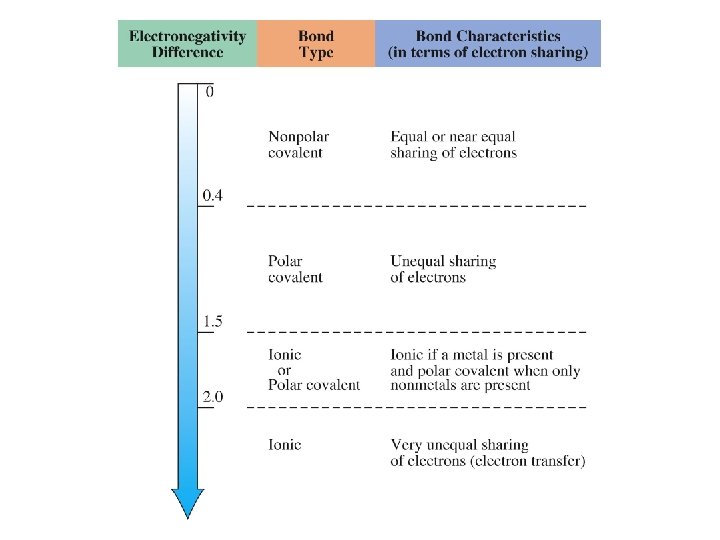
ELECTRONEGATIVITY Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. The Pauling scale is the most commonly used. Fluorine (the most electronegative element) is assigned a value of 4. 0, and values range down to cesium and francium which are the least electronegative at 0. 7.



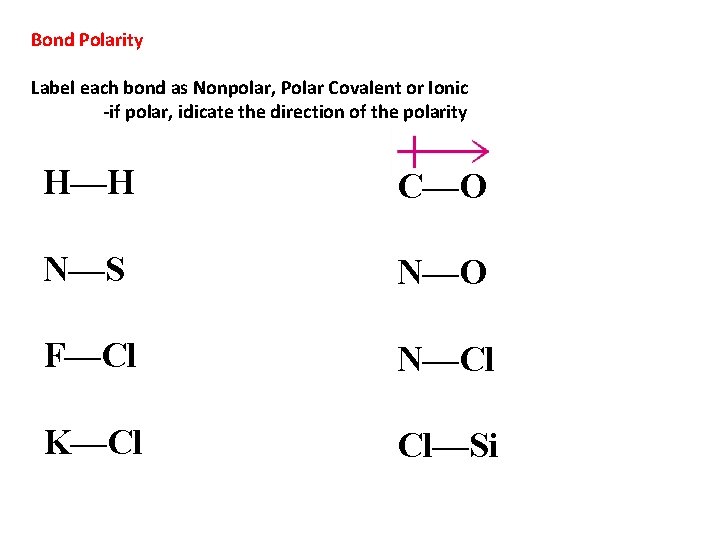
Bond Polarity Label each bond as Nonpolar, Polar Covalent or Ionic -if polar, idicate the direction of the polarity H—H C—O N—S N—O F—Cl N—Cl K—Cl Cl—Si

Chemical Bond Formation • Chemical Bonds = the attractive forces that hold atoms together in complex unit Ionic Bonds 1. Transfer of valence electron 2. Electrostatic attraction of opposite charged ions 3. Usually between: Metal + Non-Metal (Example: Na. Cl, Ca. O) Metal + Polyanion (Example: Mg. SO 4, Na 2 CO 3 ) Polyanion + Non-Metal (Example: NH 4 Cl, (NH 4)3 PO 4) • Covalent Bonds 1. Sharing of valence electron 2. Electrostatic attraction of electron of one atom to nucleus of another atom 3. Usually between: Non-Metal + Non-Metal (Example: CO 2, H 2 O, CH 4)

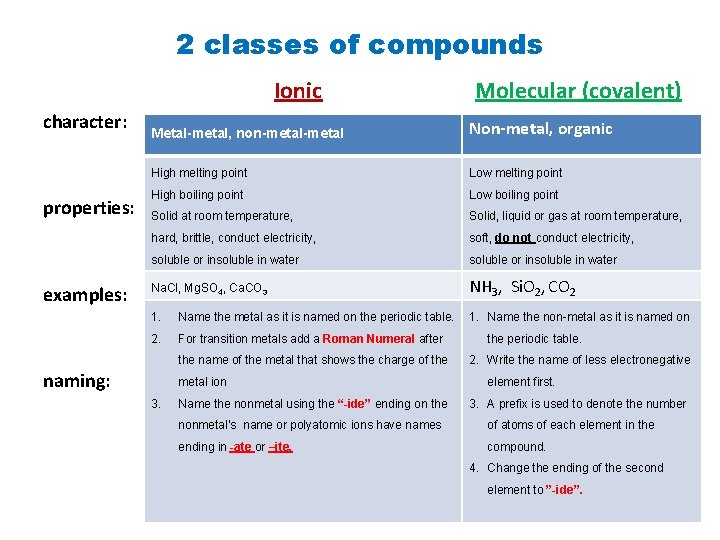
2 classes of compounds Ionic character: properties: examples: Molecular (covalent) Metal-metal, non-metal Non-metal, organic High melting point Low melting point High boiling point Low boiling point Solid at room temperature, Solid, liquid or gas at room temperature, hard, brittle, conduct electricity, soft, do not conduct electricity, soluble or insoluble in water Na. Cl, Mg. SO 4, Ca. CO 3 NH 3, Si. O 2, CO 2 1. Name the metal as it is named on the periodic table. 1. Name the non-metal as it is named on 2. For transition metals add a Roman Numeral after the name of the metal that shows the charge of the naming: metal ion 3. Name the nonmetal using the “-ide” ending on the periodic table. 2. Write the name of less electronegative element first. 3. A prefix is used to denote the number nonmetal’s name or polyatomic ions have names of atoms of each element in the ending in -ate or –ite. compound. 4. Change the ending of the second element to ”-ide”.

You must memorize the sheet of ions, including the formula, charge, and name of each ion, before you can name compounds successfully or write formulas for them. The best way to do this is make flash cards with the formula of the ion and its charge on one side and the name or names on the other side. This information will be needed for the rest of your chemistry course, so it is important to learn it now. http: //www. quia. com/quiz/1240133. html https: //quizlet. com/6143206/all-24 -polyanions-flash-cards/ http: //chemistry. about. com/od/testsquizzes/l/blionicnamesquiz. htm http: //chemistry. about. com/library/weekly/blcompnamequiz. htm http: //www. chem. purdue. edu/gchelp/nomenclature/simple_ionic. htm

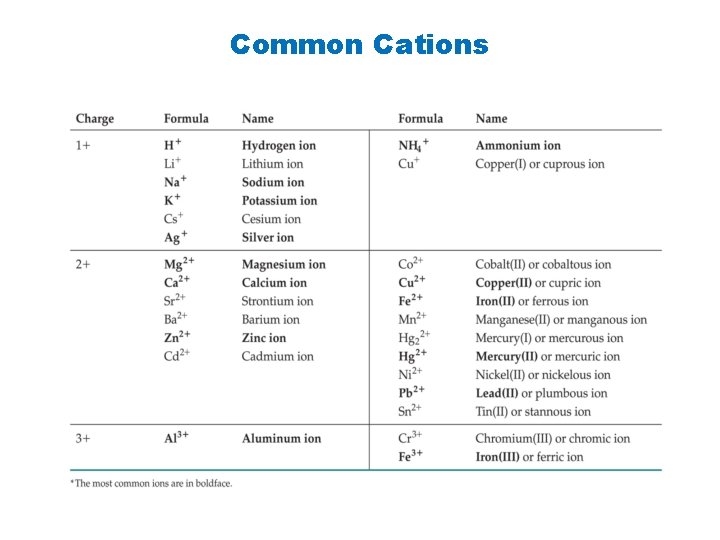
Common Cations

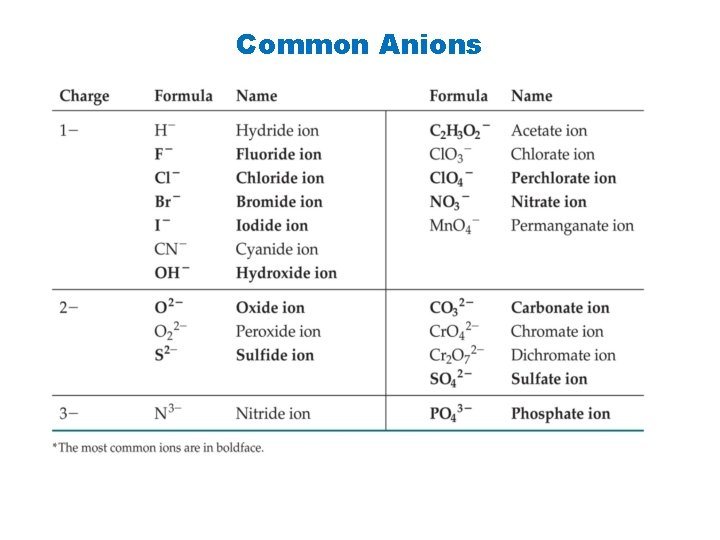
Common Anions

Nonmetals named with “ide” ending • • • carbon nitrogen oxygen fluorine phosphorous sulfur chlorine selenium bromine iodine • • • carbide nitride oxide fluoride phosphide sulfide chloride selenide bromide iodide

Steps for Naming Ionic Compounds • Binary Compounds – These compounds contain only two elements – Name the metal as it is named on the periodic table – Name the nonmetal using the “ide” ending on the nonmetal’s name Examples: Na. Cl sodium chloride Zn. I 2 zinc iodide Al 2 O 3 aluminum oxide Na 3 N sodium nitride KBr potassium bromide Al 2 O 3 aluminum oxide Mg. S magnesium sulfide

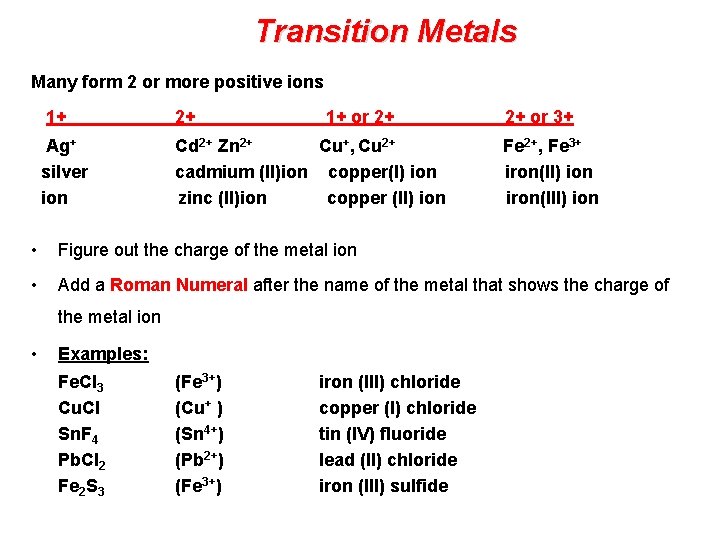
Transition Metals Many form 2 or more positive ions 1+ 2+ 1+ or 2+ 2+ or 3+ Ag+ Cd 2+ Zn 2+ Cu+, Cu 2+ Fe 2+, Fe 3+ silver cadmium (II)ion copper(I) ion iron(II) ion zinc (II)ion copper (II) ion iron(III) ion • Figure out the charge of the metal ion • Add a Roman Numeral after the name of the metal that shows the charge of the metal ion • Examples: Fe. Cl 3 Cu. Cl Sn. F 4 Pb. Cl 2 Fe 2 S 3 (Fe 3+) (Cu+ ) (Sn 4+) (Pb 2+) (Fe 3+) iron (III) chloride copper (I) chloride tin (IV) fluoride lead (II) chloride iron (III) sulfide

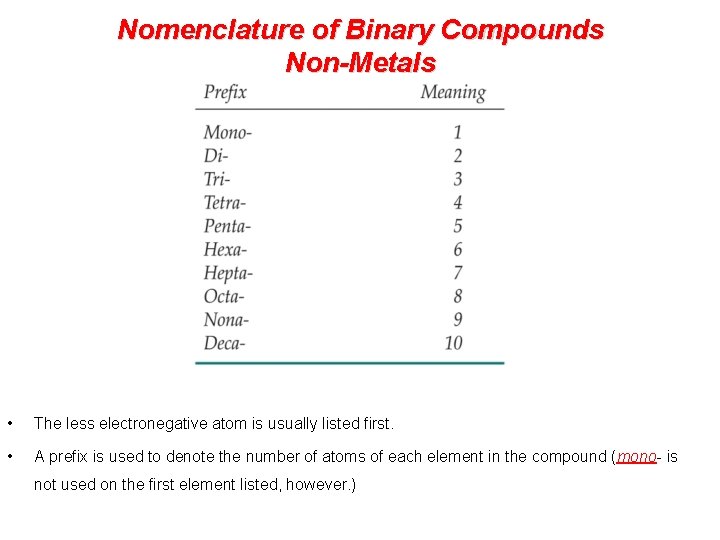
Nomenclature of Binary Compounds Non-Metals • The less electronegative atom is usually listed first. • A prefix is used to denote the number of atoms of each element in the compound (mono- is not used on the first element listed, however. )

Mixed Practice 1. 2. 3. 4. 5. 6. 7. 8. 9. N 2 O K 2 S Cu(NO 3)2 Cl 2 O 7 Cr 2(SO 4)3 Fe 2(SO 3)3 Ca. O Ba. CO 3 ICl 1. 2. 3. 4. 5. 6. 7. 8. 9. Dinitrogen monoxide Potassium sulfide Copper (II) nitrate Dichlorine heptoxide Chromium (III) sulfate Ferric sulfite Calcium oxide Barium carbonate Iodine monochloride

Mixed Practice 1. 2. 3. 4. 5. 6. 7. 8. 9. Ba. I 2 P 4 S 3 Ca(OH)2 Fe. CO 3 Na 2 Cr 2 O 7 I 2 O 5 Cu(Cl. O 4)2 CS 2 B 2 Cl 4 1. 2. 3. 4. 5. 6. 7. 8. 9. Barium iodide Tetraphosphorus trisulfide Calcium hydroxide Iron (II) carbonate Sodium dichromate Diiodine pentoxide Cupric perchlorate Carbon disulfide Diboron tetrachloride

Drawing Lewis Structures Follow Step by Step Method 1. Calculate total number of valence electrons in the molecule by adding together the valence electrons count for all atoms in the molecule. 2. Write the chemical symbols for the atoms in the molecule in the order in which are bonded to one another. 3. Guess skeleton structure and define a central atom. 4. Place a single covalent bond, involving two electrons between each pair of bonded atoms. 5. Complete octets of surrounding atoms. [H = 2 only, B=only 3] 6. Place leftover electrons in pairs on the central atom. 7. If there are not enough electrons to give the central atom an octet, look for multiple bonds by transferring electrons until each atom has eight electrons around it. 8. Count the total number of electrons in your completed Lewis structure to make sure it is equal to the total number of valence electrons available for bonding.

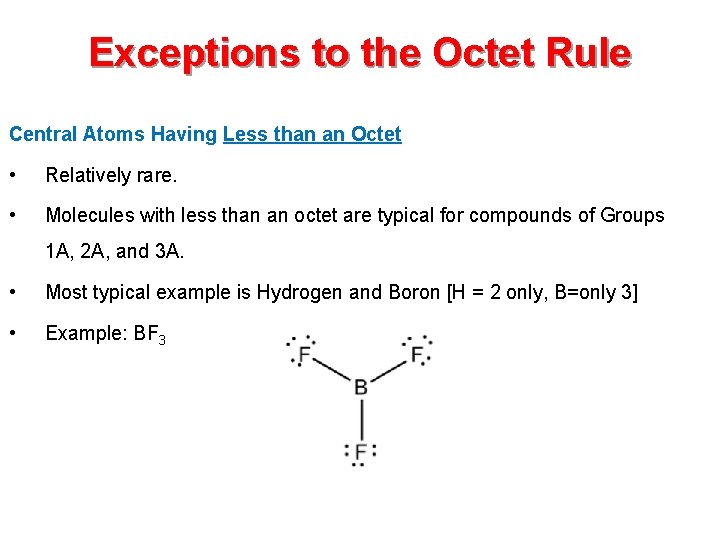
Exceptions to the Octet Rule Central Atoms Having Less than an Octet • Relatively rare. • Molecules with less than an octet are typical for compounds of Groups 1 A, 2 A, and 3 A. • Most typical example is Hydrogen and Boron [H = 2 only, B=only 3] • Example: BF 3

electron dot electron pairs/ Formula valence e structure lone pair shape CO 2 NH 4+ SO 3 CO 2 CCl 4 BF 3 NO 3 - angles polar or nonpolar

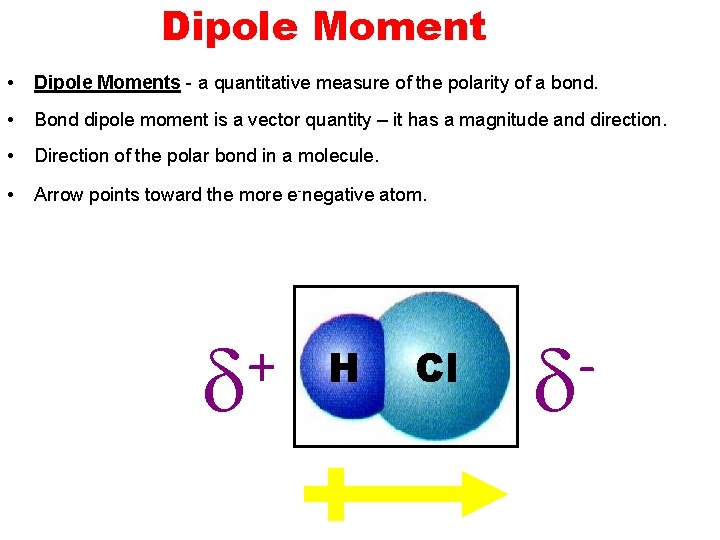
Dipole Moment • Dipole Moments - a quantitative measure of the polarity of a bond. • Bond dipole moment is a vector quantity – it has a magnitude and direction. • Direction of the polar bond in a molecule. • Arrow points toward the more e-negative atom. + H Cl

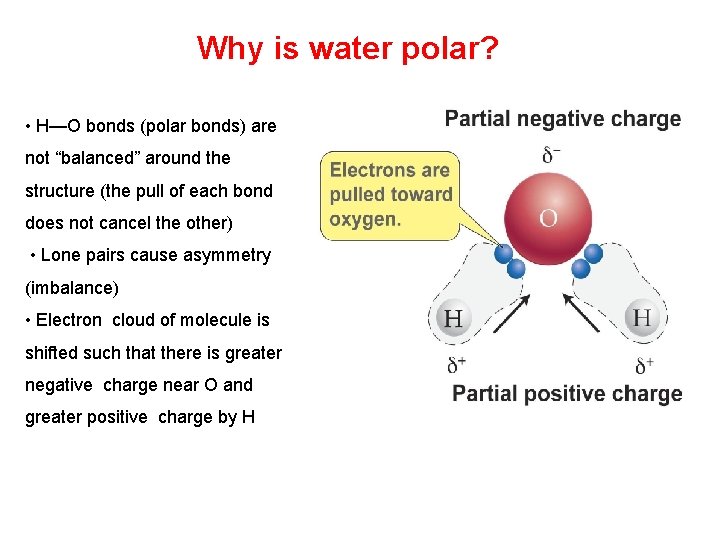
Why is water polar? • H—O bonds (polar bonds) are not “balanced” around the structure (the pull of each bond does not cancel the other) • Lone pairs cause asymmetry (imbalance) • Electron cloud of molecule is shifted such that there is greater negative charge near O and greater positive charge by H


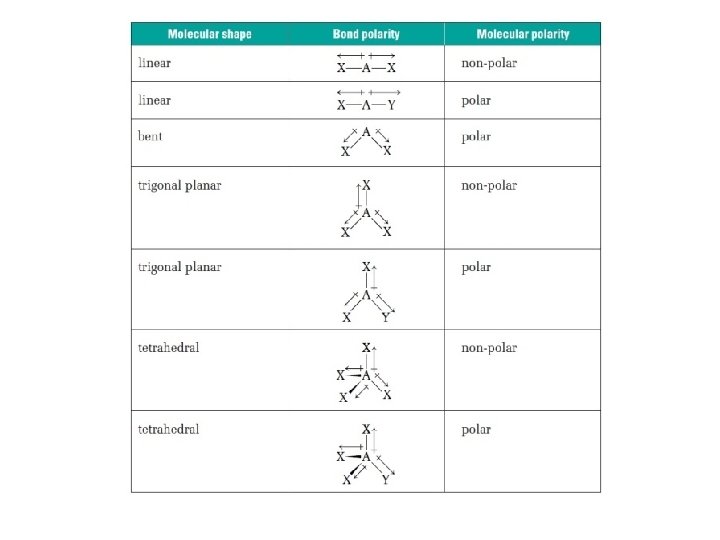
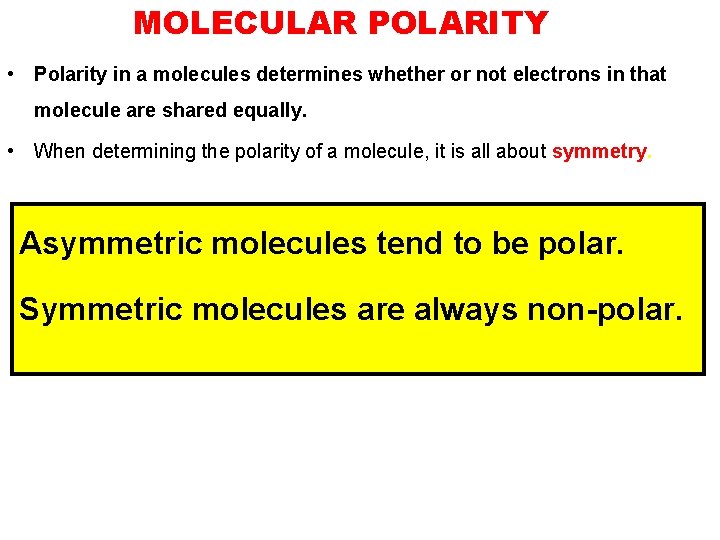
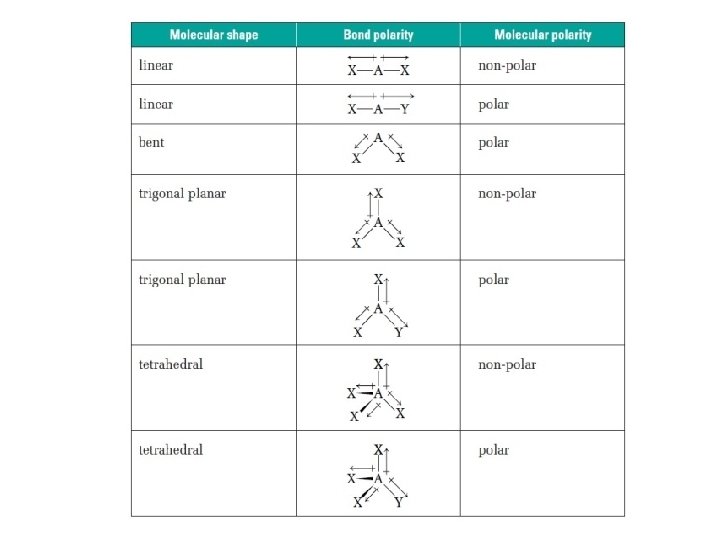
MOLECULAR POLARITY • Polarity in a molecules determines whether or not electrons in that molecule are shared equally. • When determining the polarity of a molecule, it is all about symmetry. Asymmetric molecules tend to be polar. Symmetric molecules are always non-polar.

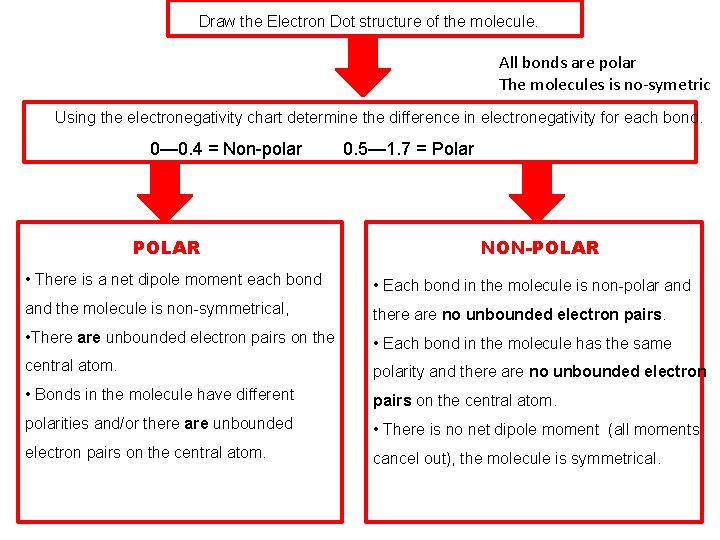
Draw the Electron Dot structure of the molecule. All bonds are polar The molecules is no-symetric Using the electronegativity chart determine the difference in electronegativity for each bond. 0— 0. 4 = Non-polar POLAR 0. 5— 1. 7 = Polar NON-POLAR • There is a net dipole moment each bond • Each bond in the molecule is non-polar and the molecule is non-symmetrical, there are no unbounded electron pairs. • There are unbounded electron pairs on the • Each bond in the molecule has the same central atom. polarity and there are no unbounded electron • Bonds in the molecule have different pairs on the central atom. polarities and/or there are unbounded • There is no net dipole moment (all moments electron pairs on the central atom. cancel out), the molecule is symmetrical.

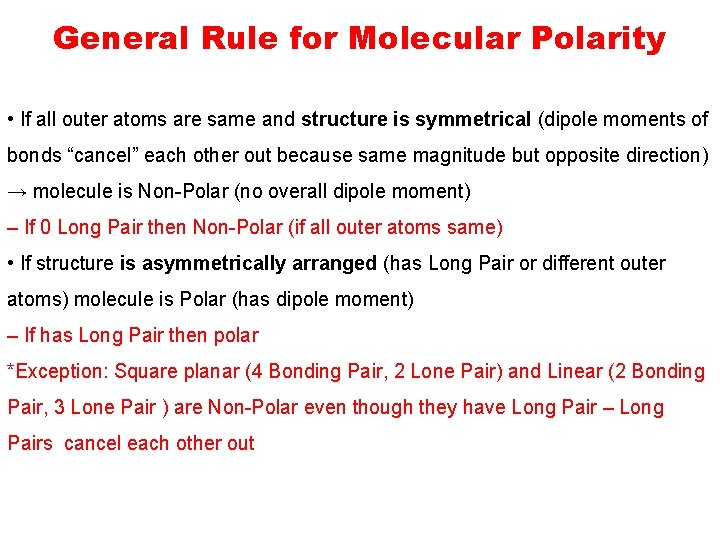
General Rule for Molecular Polarity • If all outer atoms are same and structure is symmetrical (dipole moments of bonds “cancel” each other out because same magnitude but opposite direction) → molecule is Non-Polar (no overall dipole moment) – If 0 Long Pair then Non-Polar (if all outer atoms same) • If structure is asymmetrically arranged (has Long Pair or different outer atoms) molecule is Polar (has dipole moment) – If has Long Pair then polar *Exception: Square planar (4 Bonding Pair, 2 Lone Pair) and Linear (2 Bonding Pair, 3 Lone Pair ) are Non-Polar even though they have Long Pair – Long Pairs cancel each other out

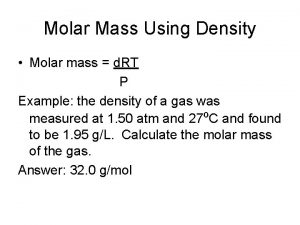
 Calculating molar mass using colligative properties
Calculating molar mass using colligative properties Calculating molar mass from freezing point depression
Calculating molar mass from freezing point depression Is concentration and molarity the same
Is concentration and molarity the same Atomic mass vs molar mass
Atomic mass vs molar mass Mass to moles
Mass to moles Grams to mol
Grams to mol Mol from mass
Mol from mass How do you go from molecules to moles
How do you go from molecules to moles Formula mass vs molar mass
Formula mass vs molar mass Molar mass def
Molar mass def Boron amu
Boron amu Molar mass of potassium permanganate
Molar mass of potassium permanganate How to calculate percent composition by mass
How to calculate percent composition by mass How to find the empirical formula from percent composition
How to find the empirical formula from percent composition Isotope abundance formula
Isotope abundance formula Percentage composition of acetic acid
Percentage composition of acetic acid Molecular mass of sodium hydroxide
Molecular mass of sodium hydroxide Cl- molar mass
Cl- molar mass Chapter 10 chemical quantities
Chapter 10 chemical quantities Allyl sulfide molar mass
Allyl sulfide molar mass Molar mass of butane
Molar mass of butane Stp vs satp
Stp vs satp What are the units for molar mass
What are the units for molar mass Ibuprofen percent composition
Ibuprofen percent composition Molar mass equation
Molar mass equation Molar mass of potassium dichromate
Molar mass of potassium dichromate How to calculate molar mass
How to calculate molar mass Define atomic mass unit
Define atomic mass unit Molar mass
Molar mass N/v=p/rt
N/v=p/rt Allyl sulfide molar mass
Allyl sulfide molar mass Molar mass of chocolate chips
Molar mass of chocolate chips Nickel(iii) carbonate
Nickel(iii) carbonate Mol road map
Mol road map Periodic table molar mass
Periodic table molar mass Molar mass cartoon
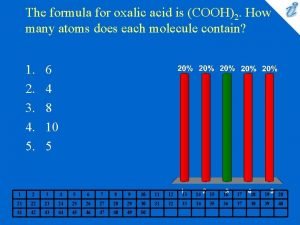
Molar mass cartoon Cooh2
Cooh2 Barium phosphite
Barium phosphite Mol per liter
Mol per liter Molar mass of calcium
Molar mass of calcium Potassium formula mass
Potassium formula mass Conversions examples
Conversions examples Unit of molar mass
Unit of molar mass Luminol molar mass
Luminol molar mass How to calculate grams to moles
How to calculate grams to moles Formula mass vs molecular mass
Formula mass vs molecular mass Molecular mass
Molecular mass Atomic mass unit
Atomic mass unit Ag molar mass
Ag molar mass Theoretical molar mass of butane
Theoretical molar mass of butane Stoichiometry molar mass
Stoichiometry molar mass Periodic table molar mass
Periodic table molar mass Formula mass
Formula mass Mass percent formula
Mass percent formula Stoichiometry island
Stoichiometry island Molar mass examples
Molar mass examples Molar mass label
Molar mass label How to find molar mass
How to find molar mass Molar mass units
Molar mass units Allyl sulfide molar mass
Allyl sulfide molar mass Molar mass of fructose
Molar mass of fructose Eric savory
Eric savory Formula mass
Formula mass Cl- molar mass
Cl- molar mass Percent composition of magnesium carbonate
Percent composition of magnesium carbonate Insulin molecular formula
Insulin molecular formula