What is the molar mass of calcium phosphate



- Slides: 16

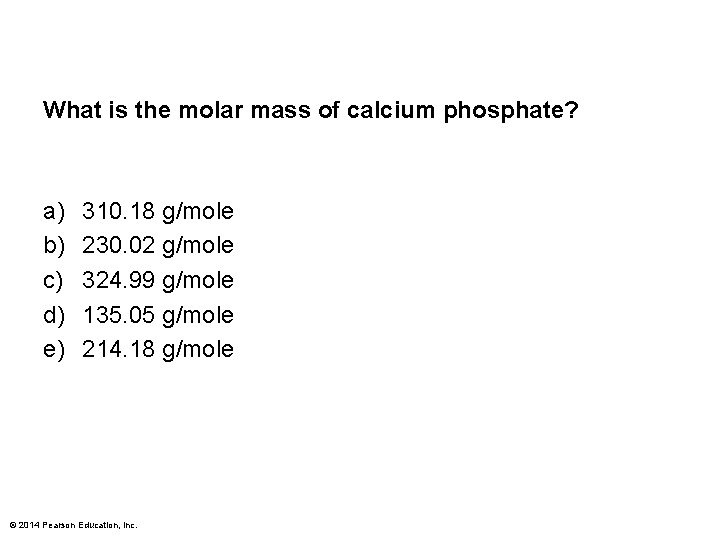
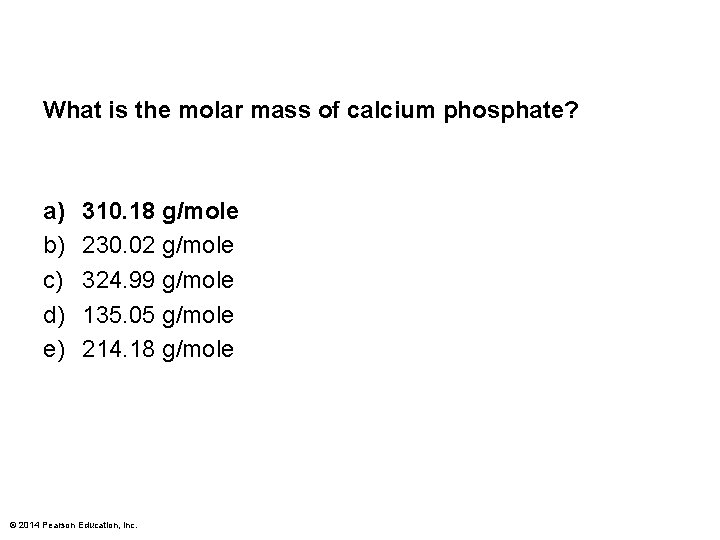
What is the molar mass of calcium phosphate? a) b) c) d) e) 310. 18 g/mole 230. 02 g/mole 324. 99 g/mole 135. 05 g/mole 214. 18 g/mole © 2014 Pearson Education, Inc.

What is the molar mass of calcium phosphate? a) b) c) d) e) 310. 18 g/mole 230. 02 g/mole 324. 99 g/mole 135. 05 g/mole 214. 18 g/mole © 2014 Pearson Education, Inc.

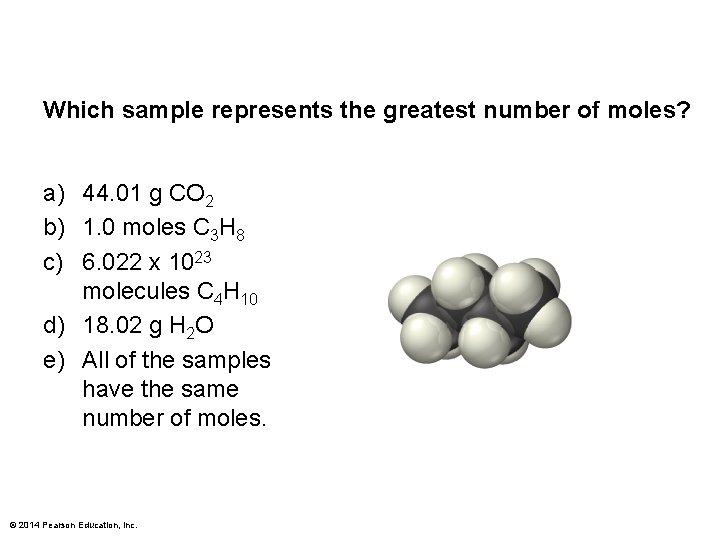
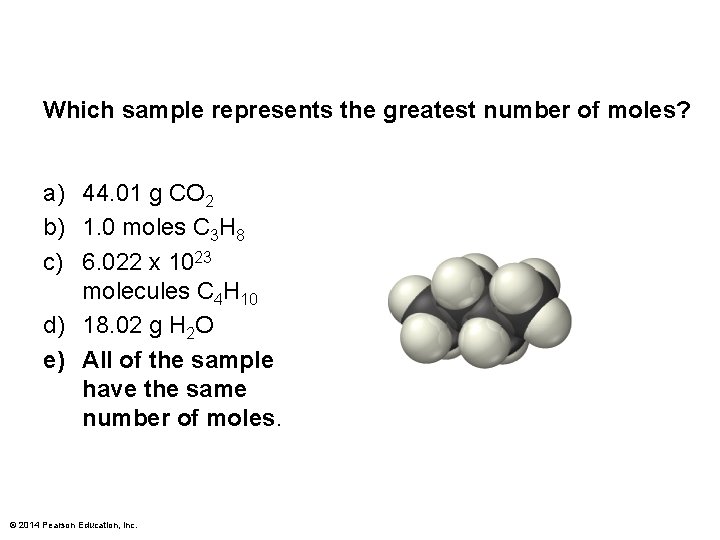
Which sample represents the greatest number of moles? a) 44. 01 g CO 2 b) 1. 0 moles C 3 H 8 c) 6. 022 x 1023 molecules C 4 H 10 d) 18. 02 g H 2 O e) All of the samples have the same number of moles. © 2014 Pearson Education, Inc.

Which sample represents the greatest number of moles? a) 44. 01 g CO 2 b) 1. 0 moles C 3 H 8 c) 6. 022 x 1023 molecules C 4 H 10 d) 18. 02 g H 2 O e) All of the sample have the same number of moles. © 2014 Pearson Education, Inc.

Which of the following contains the largest number of molecules? a) b) c) d) 10. 0 g CH 4 10. 0 g C 2 H 6 10. 0 g SO 2 10. 0 g Xe © 2014 Pearson Education, Inc.

Which of the following contains the largest number of molecules? a) b) c) d) 10. 0 g CH 4 10. 0 g C 2 H 6 10. 0 g SO 2 10. 0 g Xe © 2014 Pearson Education, Inc.

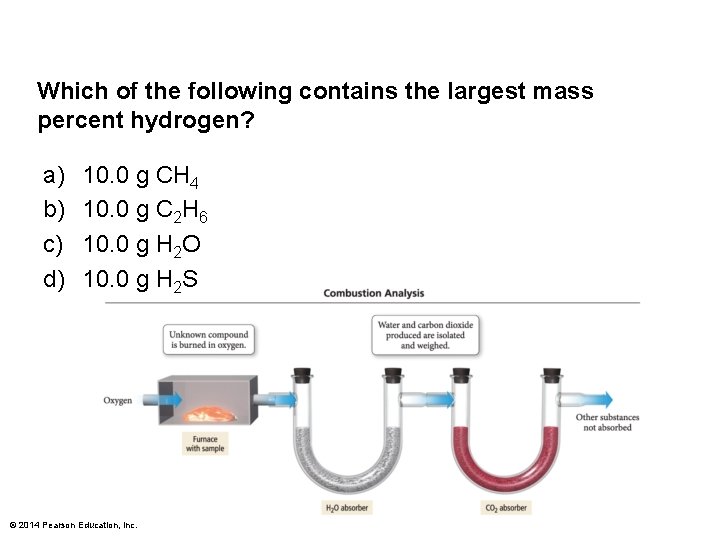
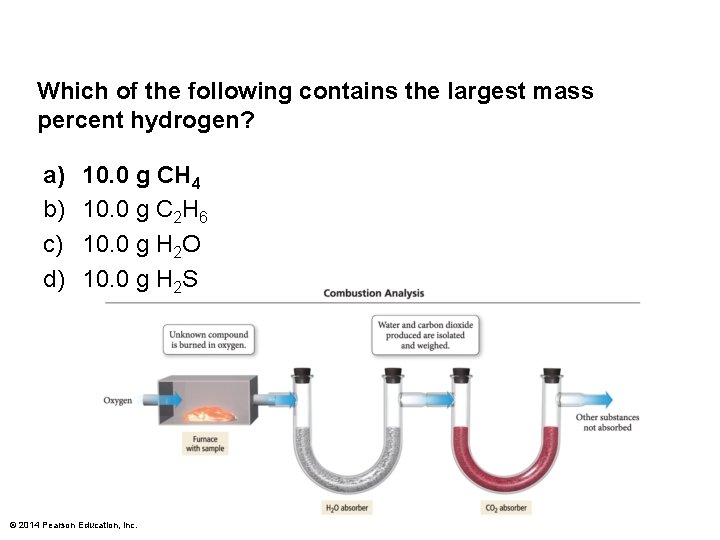
Which of the following contains the largest mass percent hydrogen? a) b) c) d) 10. 0 g CH 4 10. 0 g C 2 H 6 10. 0 g H 2 O 10. 0 g H 2 S © 2014 Pearson Education, Inc.

Which of the following contains the largest mass percent hydrogen? a) b) c) d) 10. 0 g CH 4 10. 0 g C 2 H 6 10. 0 g H 2 O 10. 0 g H 2 S © 2014 Pearson Education, Inc.

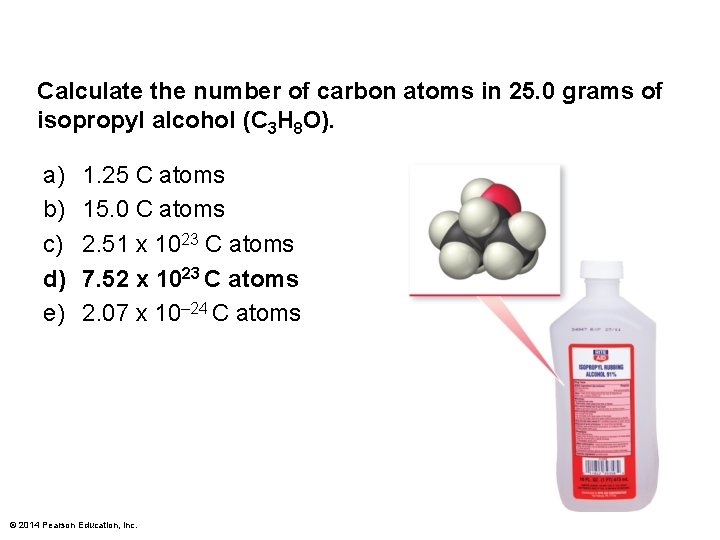
Calculate the number of carbon atoms in 25. 0 grams of isopropyl alcohol (C 3 H 8 O). a) b) c) d) e) 1. 25 C atoms 15. 0 C atoms 2. 51 x 1023 C atoms 7. 52 x 1023 C atoms 2. 07 x 10– 24 C atoms © 2014 Pearson Education, Inc.

Calculate the number of carbon atoms in 25. 0 grams of isopropyl alcohol (C 3 H 8 O). a) b) c) d) e) 1. 25 C atoms 15. 0 C atoms 2. 51 x 1023 C atoms 7. 52 x 1023 C atoms 2. 07 x 10– 24 C atoms © 2014 Pearson Education, Inc.

An unknown compound contains the following percents by mass: C: 60. 86%, H: 5. 83%, O: 23. 16%, and N: 10. 14%. Find the empirical formula. a) b) c) d) e) C 6 H 8 O 2 N 2 C 7 H 8 O 2 N C 6 H 8 O 2 N C 8 H 8 ON © 2014 Pearson Education, Inc.

An unknown compound contains the following percents by mass: C: 60. 86%, H: 5. 83%, O: 23. 16%, and N: 10. 14%. Find the empirical formula. a) b) c) d) e) C 6 H 8 O 2 N 2 C 7 H 8 O 2 N C 6 H 8 O 2 N C 8 H 8 ON © 2014 Pearson Education, Inc.

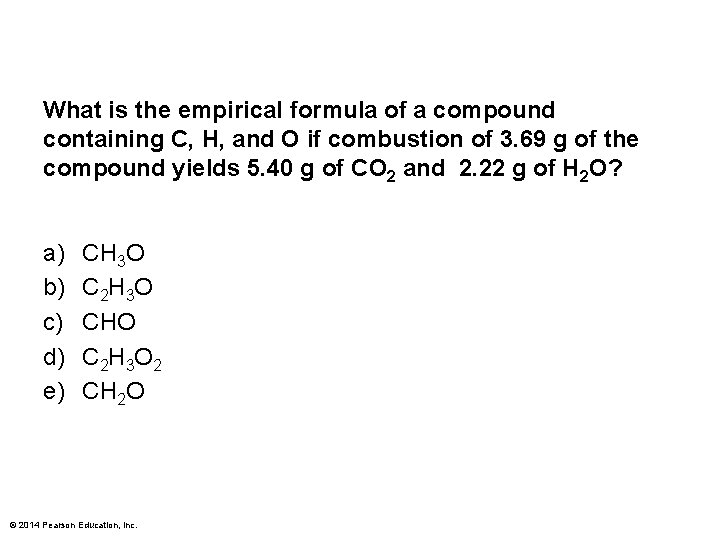
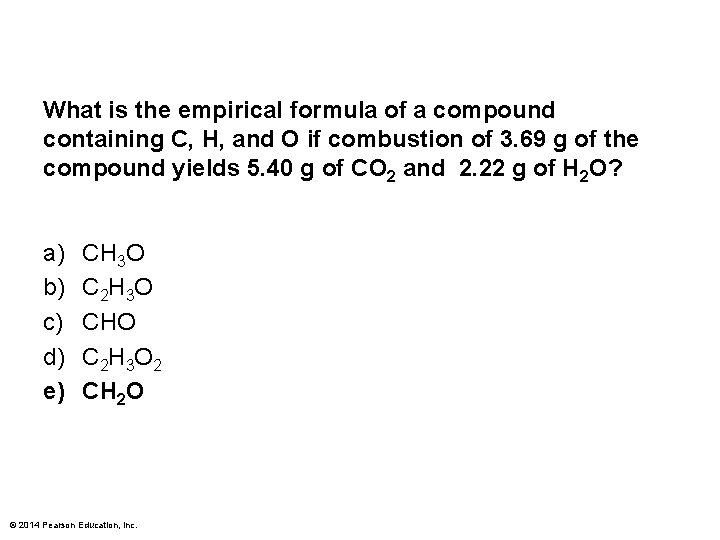
What is the empirical formula of a compound containing C, H, and O if combustion of 3. 69 g of the compound yields 5. 40 g of CO 2 and 2. 22 g of H 2 O? a) b) c) d) e) CH 3 O C 2 H 3 O CHO C 2 H 3 O 2 CH 2 O © 2014 Pearson Education, Inc.

What is the empirical formula of a compound containing C, H, and O if combustion of 3. 69 g of the compound yields 5. 40 g of CO 2 and 2. 22 g of H 2 O? a) b) c) d) e) CH 3 O C 2 H 3 O CHO C 2 H 3 O 2 CH 2 O © 2014 Pearson Education, Inc.

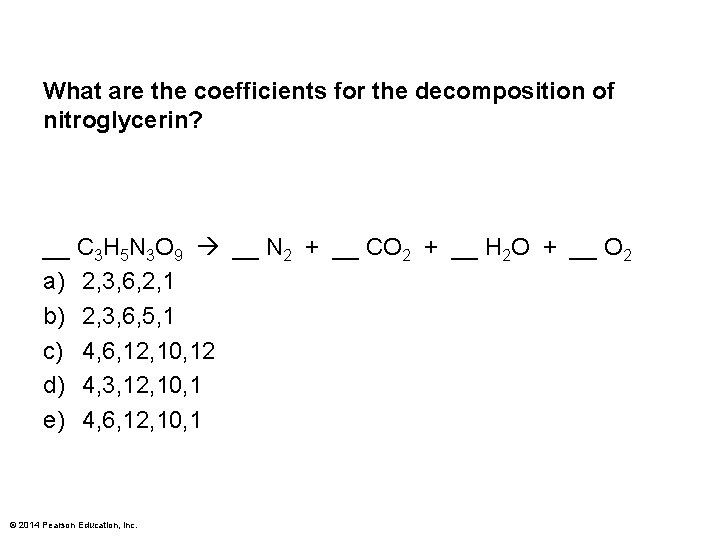
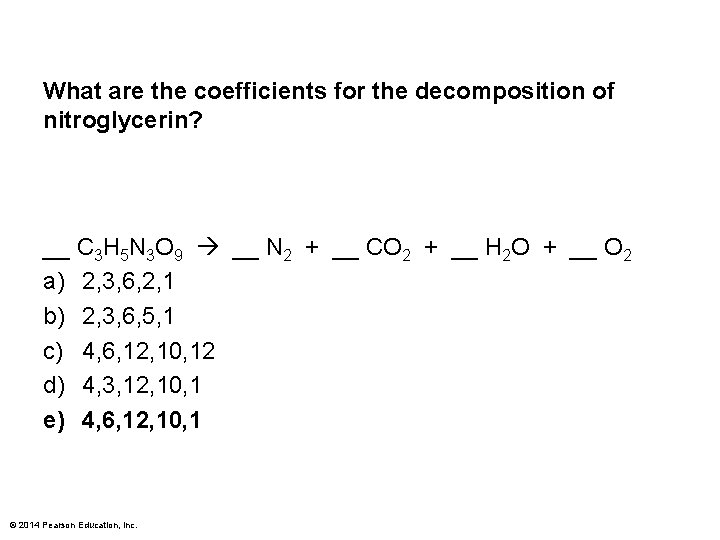
What are the coefficients for the decomposition of nitroglycerin? __ C 3 H 5 N 3 O 9 __ N 2 + __ CO 2 + __ H 2 O + __ O 2 a) 2, 3, 6, 2, 1 b) 2, 3, 6, 5, 1 c) 4, 6, 12, 10, 12 d) 4, 3, 12, 10, 1 e) 4, 6, 12, 10, 1 © 2014 Pearson Education, Inc.

What are the coefficients for the decomposition of nitroglycerin? __ C 3 H 5 N 3 O 9 __ N 2 + __ CO 2 + __ H 2 O + __ O 2 a) 2, 3, 6, 2, 1 b) 2, 3, 6, 5, 1 c) 4, 6, 12, 10, 12 d) 4, 3, 12, 10, 1 e) 4, 6, 12, 10, 1 © 2014 Pearson Education, Inc.