Chemical formulas Molar Mass Molar Volume Mr Shields








- Slides: 21

Chemical formulas, Molar Mass, Molar Volume & Mr. Shields Regents Chemistry U 04 L 02 1

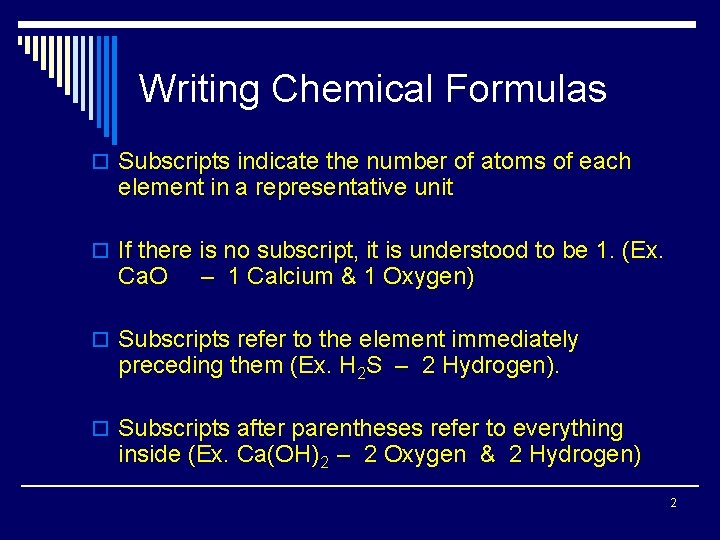
Writing Chemical Formulas o Subscripts indicate the number of atoms of each element in a representative unit o If there is no subscript, it is understood to be 1. (Ex. Ca. O – 1 Calcium & 1 Oxygen) o Subscripts refer to the element immediately preceding them (Ex. H 2 S – 2 Hydrogen). o Subscripts after parentheses refer to everything inside (Ex. Ca(OH)2 – 2 Oxygen & 2 Hydrogen) 2

Chemical Formulas o What atoms and how many of each are present in the following compounds? o CH 4 ? o Ba(CN)2 ? o Cr 2 O 3 ? o Cr(OH)3 ? 3

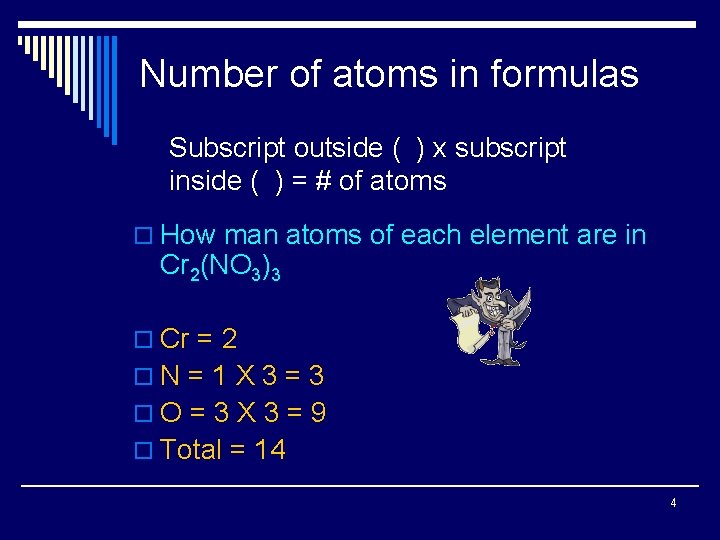
Number of atoms in formulas Subscript outside ( ) x subscript inside ( ) = # of atoms o How man atoms of each element are in Cr 2(NO 3)3 o Cr = 2 o. N = 1 X 3 = 3 o. O = 3 X 3 = 9 o Total = 14 4

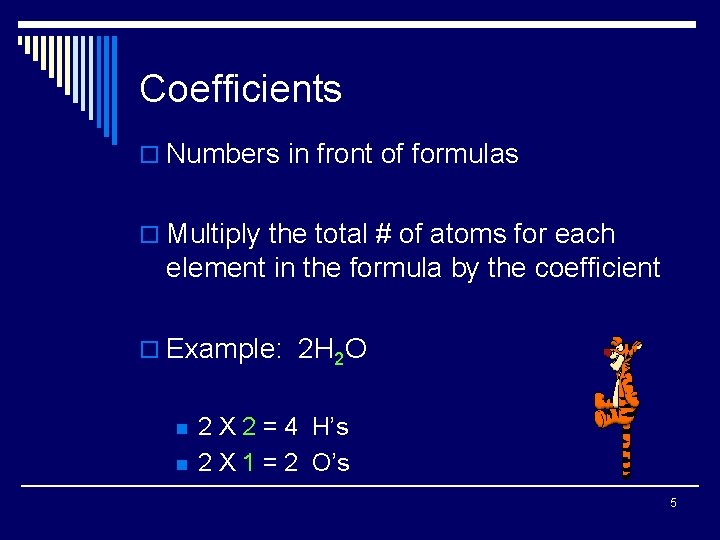
Coefficients o Numbers in front of formulas o Multiply the total # of atoms for each element in the formula by the coefficient o Example: 2 H 2 O n n 2 X 2 = 4 H’s 2 X 1 = 2 O’s 5

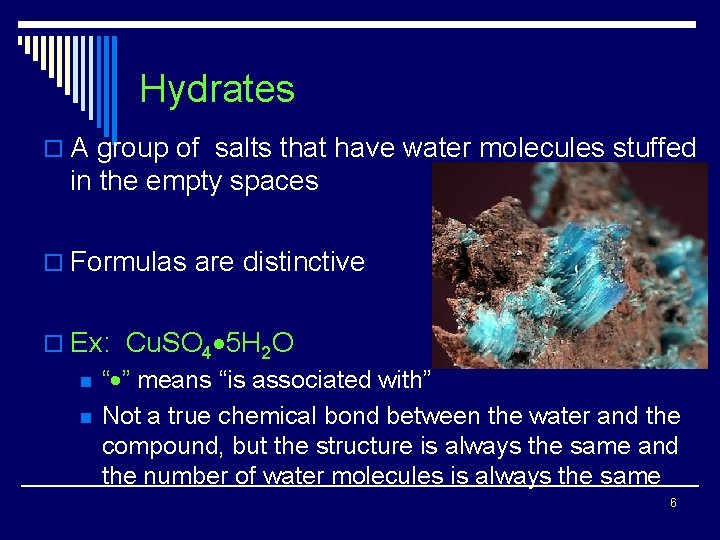
Hydrates o A group of salts that have water molecules stuffed in the empty spaces o Formulas are distinctive o Ex: Cu. SO 4 5 H 2 O n n “ ” means “is associated with” Not a true chemical bond between the water and the compound, but the structure is always the same and the number of water molecules is always the same 6

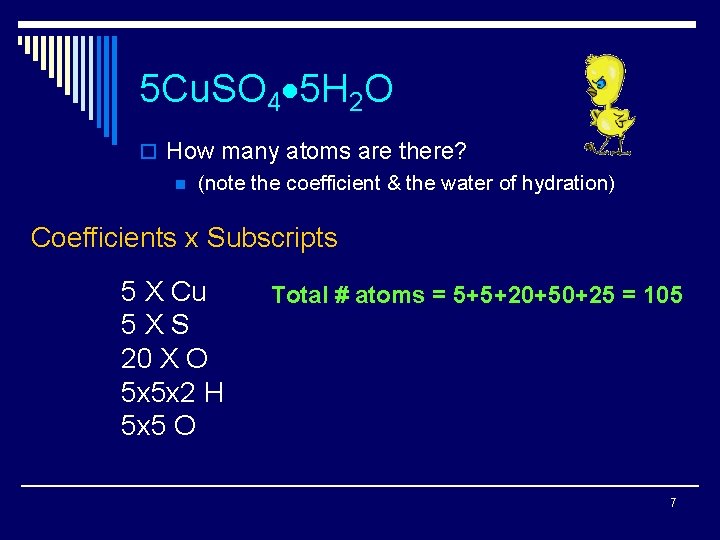
5 Cu. SO 4 5 H 2 O o How many atoms are there? n (note the coefficient & the water of hydration) Coefficients x Subscripts 5 X Cu 5 XS 20 X O 5 x 5 x 2 H 5 x 5 O Total # atoms = 5+5+20+50+25 = 105 7

Cu. SO 4 5 H 2 O o What is the % water in Cu. SO 4 5 H 20? First calculate the formula mass: 1 X Cu = 1 XS = 4 XO = 5 x 2 H = 5 x 1 O = 63. 546 32. 066 15. 999 x 4 1. 008 x 10 15. 999 x 5 Second find the % water (90. 075 / 249. 687) x 100 = 36. 08% water formula mass = 249. 687 amu 8

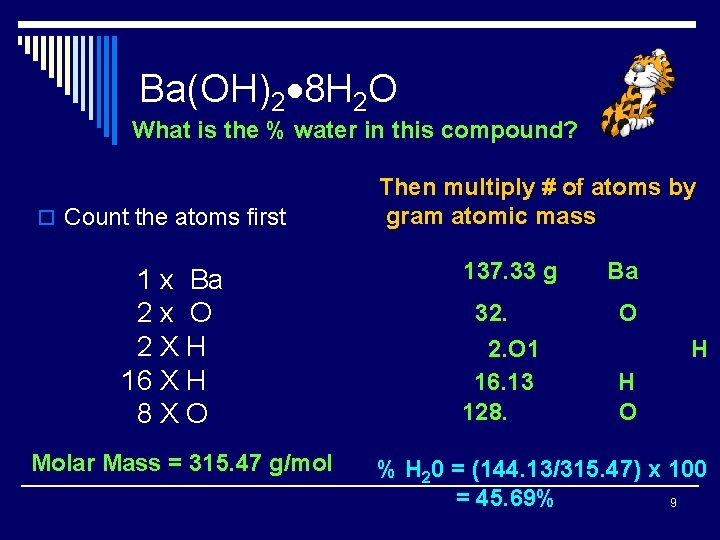
Ba(OH)2 8 H 2 O What is the % water in this compound? o Count the atoms first 1 x Ba 2 x O 2 XH 16 X H 8 XO Molar Mass = 315. 47 g/mol Then multiply # of atoms by gram atomic mass 137. 33 g 32. 2. O 1 16. 13 128. Ba O H H O % H 20 = (144. 13/315. 47) x 100 = 45. 69% 9

10

A Real Mole No, we are not Talking about This kind of mole! 11

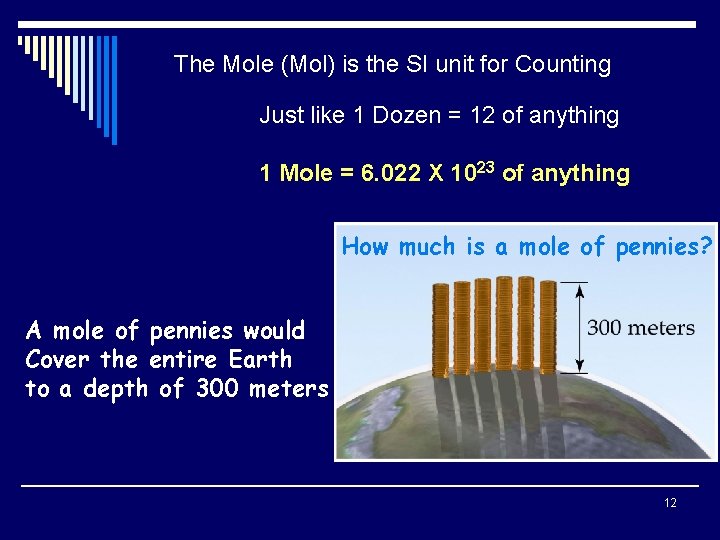
The Mole (Mol) is the SI unit for Counting Just like 1 Dozen = 12 of anything 1 Mole = 6. 022 X 1023 of anything How much is a mole of pennies? A mole of pennies would Cover the entire Earth to a depth of 300 meters 12

Remember… 1 mole = 6. 02 x 1023 atoms = an elements Gram atomic mass 13

The gram molecular mass is obtained by Multiplying the number of atoms of each element By it’s gram atomic mass. Let’s consider hexane… For example the Gram molecular mass of Hexane (C 6 H 14) is 6 C + 14 H = 6(12. 011) + 14(1. 008) = 86. 178 g So … 1 mole = 86. 178 g = 3 moles = 258. 534 g = And 1/3 mole = ? 6. 022 x 1023 molecules 1. 807 x 1024 molecules 14

MOLAR MASS Definition: Molar mass is the mass in grams of one mole (or 6. 02 x 1023 molecules) of any element or chemical compound Therefore, the molar mass = gram molecular mass or gram formula mass or gram atomic mass of the element or compound What is the molar mass of Sr(C 2 H 3 O 2)2 ? 87. 62 + (4*12. 01) + (6*1. 01) + (4*16) = 205. 72 g/mol 15

MOLAR MASS Problem: If the molar mass of Sr(C 2 H 3 O 2)2 is 205. 72 g/mol then how many moles are in 134. 75 g? 134. 75 g / 205. 72 g/mol = 0. 66 moles 16

17

Molar Volume # N 2 100 L = # CO 2 100 L Back to Avogadro … Avogadro proposed that equal volumes of gases at the same temp and pressure contained equal numbers of particles. This was known as AVOGADRO’S HYPOTHESIS For example: 100 liters of N 2 at the same temperature and pressure has the same # of molecules as 100 liters of CO 2 18

Molar Volume 22. 4 L So 1 mole of any gas occupies the same volume as 1 mole of any other gas at the same temp and pressure Which means 6. 023 x 1023 molecules of any gas occupies the same volume for any gas The volume occupied by one Mole of any gas = 22. 4 Liters This is called the MOLAR VOLUME (Abbreviated “ Vm “) 19

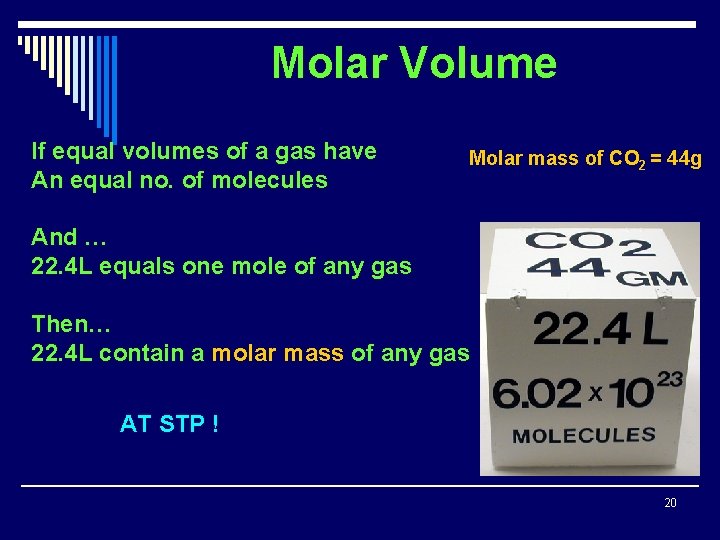
Molar Volume If equal volumes of a gas have An equal no. of molecules Molar mass of CO 2 = 44 g And … 22. 4 L equals one mole of any gas Then… 22. 4 L contain a molar mass of any gas AT STP ! 20

Molar Volume Problems PROBLEM 1: If one mole of a gas at STP occupies 22. 4 L, how many liters will two moles occupy? 22. 4 L x 2 Moles = no. of liters = 44. 8 Liters 1 mole Problem 2: How many moles are there in 14 L of O 2 at STP? 14 Liters / 22. 4 L 1 mole = # mole = 0. 625 mol PROBLEM 3: What is the mass of 1 L of O 2? (1 L / 22. 4 L) x 32 = 1. 43 g 21