Treatment of Severe 500 mgd L Hypertriglyceridemia in












- Slides: 42

Treatment of Severe (≥ 500 mg/d. L) Hypertriglyceridemia in Adult Patients LOVAZA® (omega-3 -acid ethyl esters)

LOVAZA: Indications and Usage • LOVAZA is indicated as an adjunct to diet to reduce triglyceride (TG) levels in adult patients with severe (≥ 500 mg/d. L) hypertriglyceridemia • Patients should be placed on an appropriate lipid-lowering diet before receiving LOVAZA and should continue this diet during treatment with LOVAZA • Laboratory studies should be done to ascertain that the lipid levels are consistently abnormal before instituting LOVAZA therapy Please see complete Prescribing Information for LOVAZA. 2

LOVAZA: Indications and Usage (continued) • Every attempt should be made to control serum lipids with appropriate diet, exercise, weight loss in obese patients, and control of any medical problems such as diabetes mellitus and hypothyroidism that are contributing to the lipid abnormalities • Medications known to exacerbate hypertriglyceridemia (such as beta blockers, thiazides, estrogens) should be discontinued or changed if possible prior to consideration of TG-lowering drug therapy • Limitations of use: The effect of LOVAZA on cardiovascular (CV) mortality and morbidity in patients with elevated TGs has not been determined Please see complete Prescribing Information for LOVAZA. 3

LOVAZA: Important Safety Information • LOVAZA is contraindicated in patients who exhibit hypersensitivity to any component of this medication • In patients with hepatic impairment, ALT and AST levels should be monitored periodically • In some patients, LOVAZA increases LDL-C and ALT levels (without a concurrent increase in AST) • TG and LDL-C levels should be monitored periodically during therapy with LOVAZA • LOVAZA should be used with caution in patients with known hypersensitivity or allergy to fish and/or shellfish ALT=alanine aminotransferase; AST=aspartate aminotransferase; LDL-C=low-density lipoprotein cholesterol. Please see complete Prescribing Information for LOVAZA. 4

LOVAZA: Important Safety Information (continued) • • • Some studies with omega-3 -acids demonstrated prolongation of bleeding time, which did not exceed normal limits and did not produce clinically significant bleeding episodes Clinical studies have not been done to thoroughly examine the effect of LOVAZA and concomitant anticoagulants. Patients receiving treatment with both LOVAZA and anticoagulants should be monitored periodically The most common adverse events reported were eructation (4. 0%), dyspepsia (3. 0%), and taste perversion (4. 0%) The effect of LOVAZA on cardiovascular mortality and morbidity in patients with elevated TG levels has not been determined How supplied: 1 -gram capsules Please see complete Prescribing Information for LOVAZA. 5

Presentation Objectives • This presentation will review the following: • Lipid and lipoprotein metabolism in patients with normal TG levels and severe hypertriglyceridemia • Proposed mechanism of action of LOVAZA • Efficacy and safety of LOVAZA 6

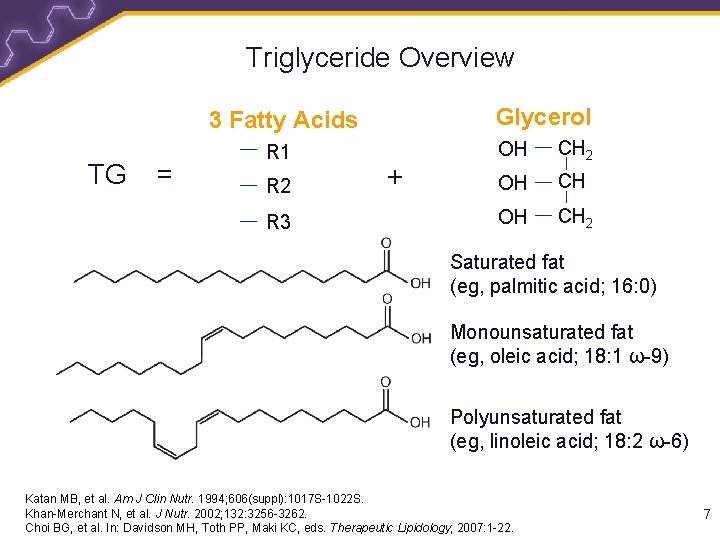
Triglyceride Overview Glycerol 3 Fatty Acids TG = R 1 R 2 R 3 + OH CH 2 Saturated fat (eg, palmitic acid; 16: 0) Monounsaturated fat (eg, oleic acid; 18: 1 ω-9) Polyunsaturated fat (eg, linoleic acid; 18: 2 ω-6) Katan MB, et al. Am J Clin Nutr. 1994; 606(suppl): 1017 S-1022 S. Khan-Merchant N, et al. J Nutr. 2002; 132: 3256 -3262. Choi BG, et al. In: Davidson MH, Toth PP, Maki KC, eds. Therapeutic Lipidology; 2007: 1 -22. 7

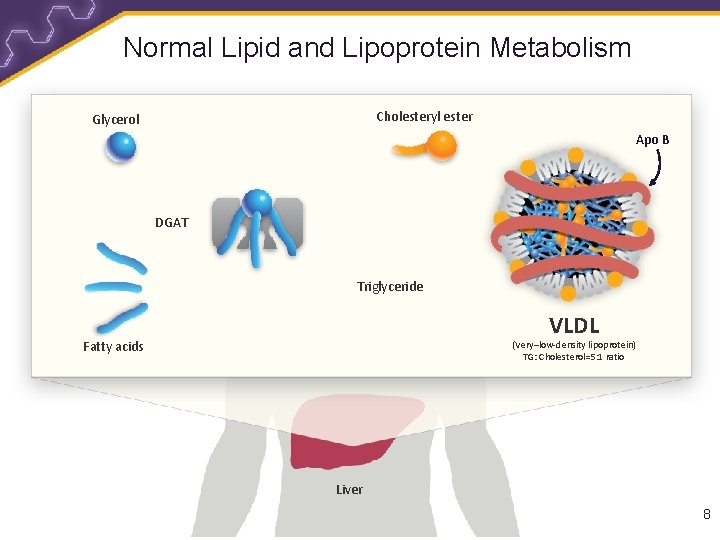
Normal Lipid and Lipoprotein Metabolism Cholesteryl ester Glycerol Apo B DGAT Triglyceride VLDL Fatty acids (Very–low-density lipoprotein) TG: Cholesterol=5: 1 ratio Liver 8

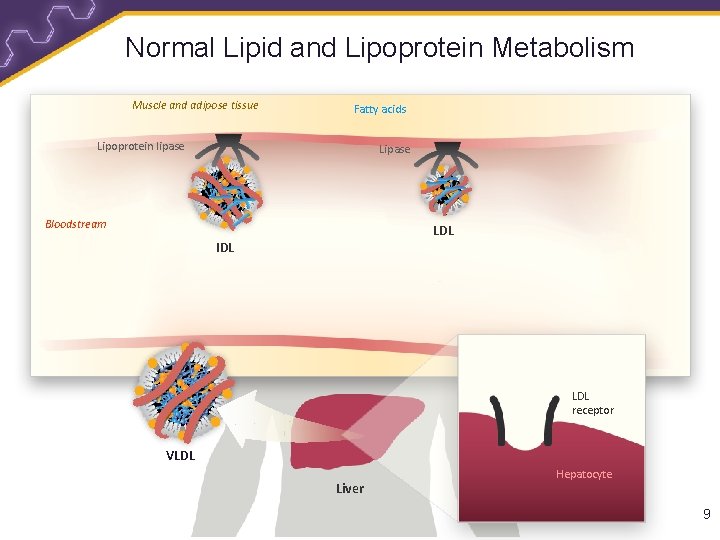
Normal Lipid and Lipoprotein Metabolism Muscle and adipose tissue Fatty acids Lipoprotein lipase Lipase Bloodstream LDL IDL LDL receptor VLDL Liver Hepatocyte 9

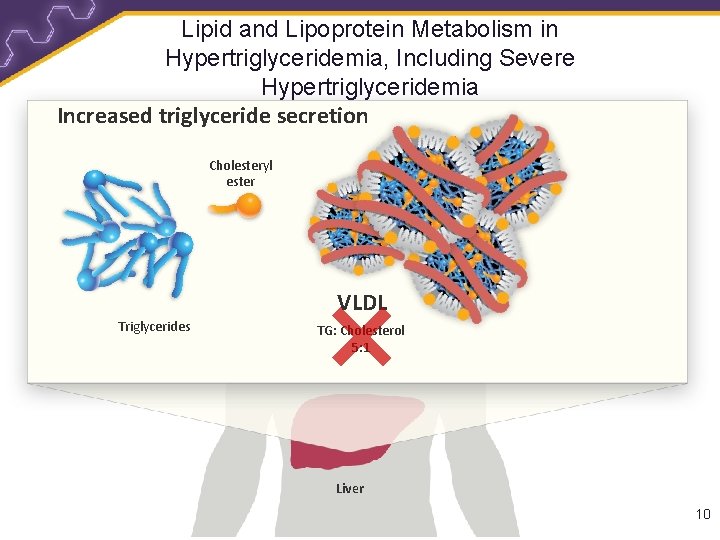
Lipid and Lipoprotein Metabolism in Hypertriglyceridemia, Including Severe Hypertriglyceridemia Increased triglyceride secretion Cholesteryl ester VLDL Triglycerides TG: Cholesterol 5: 1 Liver 10

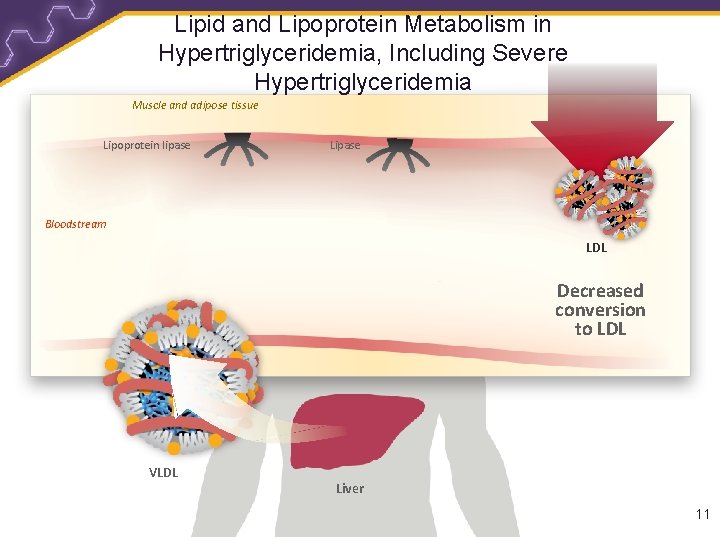
Lipid and Lipoprotein Metabolism in Hypertriglyceridemia, Including Severe Hypertriglyceridemia Muscle and adipose tissue Lipoprotein lipase Lipase Bloodstream LDL Decreased conversion to LDL VLDL Liver 11

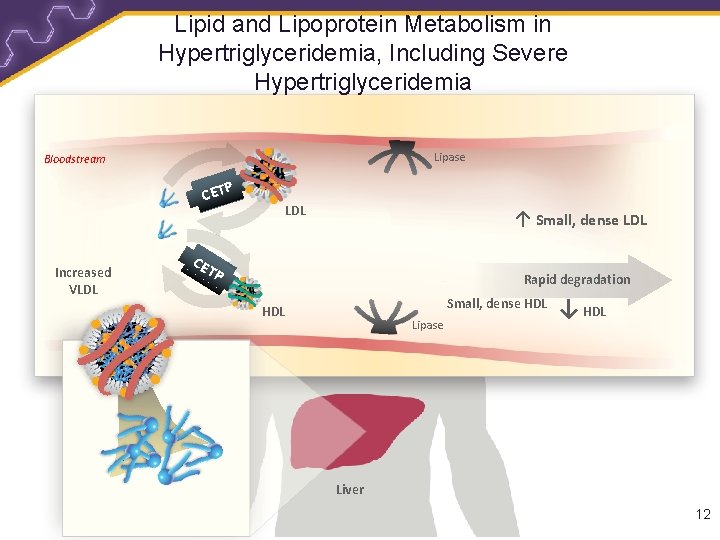
Lipid and Lipoprotein Metabolism in Hypertriglyceridemia, Including Severe Hypertriglyceridemia Lipase Bloodstream CETP Increased VLDL ↑ Small, dense LDL CE TP Rapid degradation Small, dense HDL Lipase ↓ HDL Liver 12

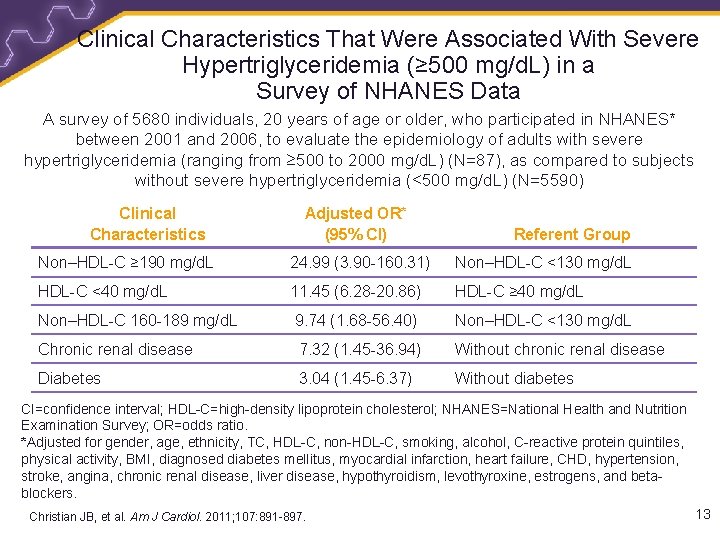
Clinical Characteristics That Were Associated With Severe Hypertriglyceridemia (≥ 500 mg/d. L) in a Survey of NHANES Data A survey of 5680 individuals, 20 years of age or older, who participated in NHANES* between 2001 and 2006, to evaluate the epidemiology of adults with severe hypertriglyceridemia (ranging from ≥ 500 to 2000 mg/d. L) (N=87), as compared to subjects without severe hypertriglyceridemia (<500 mg/d. L) (N=5590) Clinical Characteristics Adjusted OR* (95% CI) Referent Group Non–HDL-C ≥ 190 mg/d. L 24. 99 (3. 90 -160. 31) Non–HDL-C <130 mg/d. L HDL-C <40 mg/d. L 11. 45 (6. 28 -20. 86) HDL-C ≥ 40 mg/d. L Non–HDL-C 160 -189 mg/d. L 9. 74 (1. 68 -56. 40) Non–HDL-C <130 mg/d. L Chronic renal disease 7. 32 (1. 45 -36. 94) Without chronic renal disease Diabetes 3. 04 (1. 45 -6. 37) Without diabetes CI=confidence interval; HDL-C=high-density lipoprotein cholesterol; NHANES=National Health and Nutrition Examination Survey; OR=odds ratio. *Adjusted for gender, age, ethnicity, TC, HDL-C, non-HDL-C, smoking, alcohol, C-reactive protein quintiles, physical activity, BMI, diagnosed diabetes mellitus, myocardial infarction, heart failure, CHD, hypertension, stroke, angina, chronic renal disease, liver disease, hypothyroidism, levothyroxine, estrogens, and betablockers. Christian JB, et al. Am J Cardiol. 2011; 107: 891 -897. 13

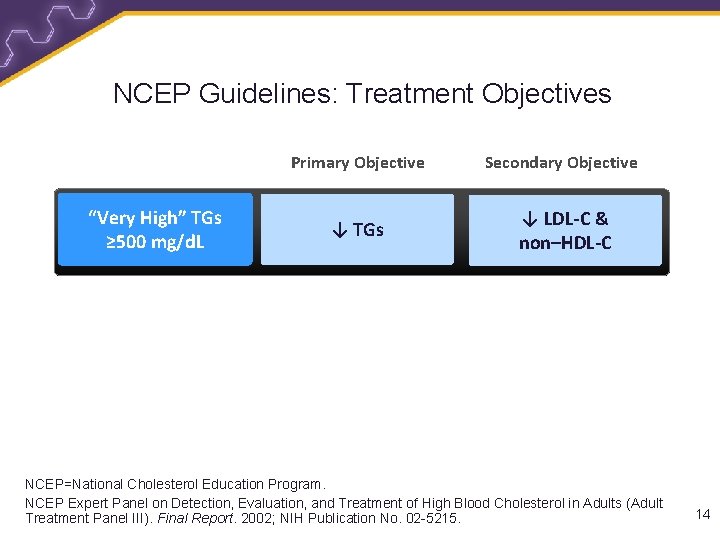
NCEP Guidelines: Treatment Objectives “Very High” TGs ≥ 500 mg/d. L Primary Objective Secondary Objective ↓ TGs ↓ LDL-C & non–HDL-C NCEP=National Cholesterol Education Program. NCEP Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Final Report. 2002; NIH Publication No. 02 -5215. 14

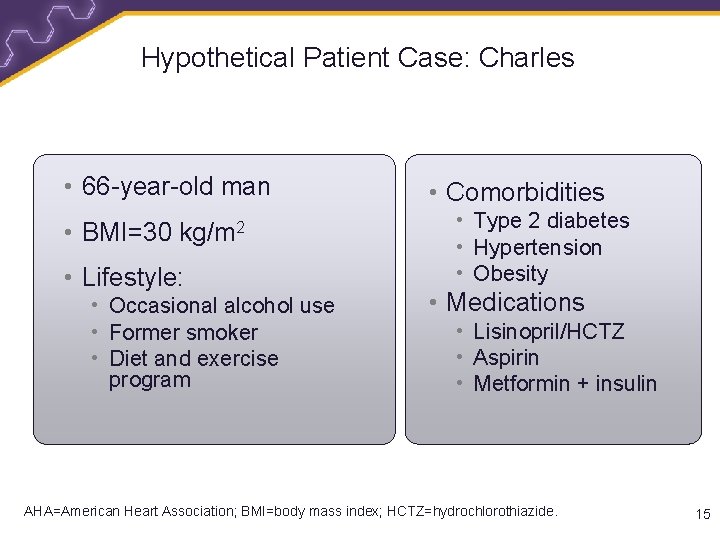
Hypothetical Patient Case: Charles • 66 -year-old man • BMI=30 kg/m 2 • Lifestyle: • Occasional alcohol use • Former smoker • Diet and exercise program • Comorbidities • Type 2 diabetes • Hypertension • Obesity • Medications • Lisinopril/HCTZ • Aspirin • Metformin + insulin AHA=American Heart Association; BMI=body mass index; HCTZ=hydrochlorothiazide. 15

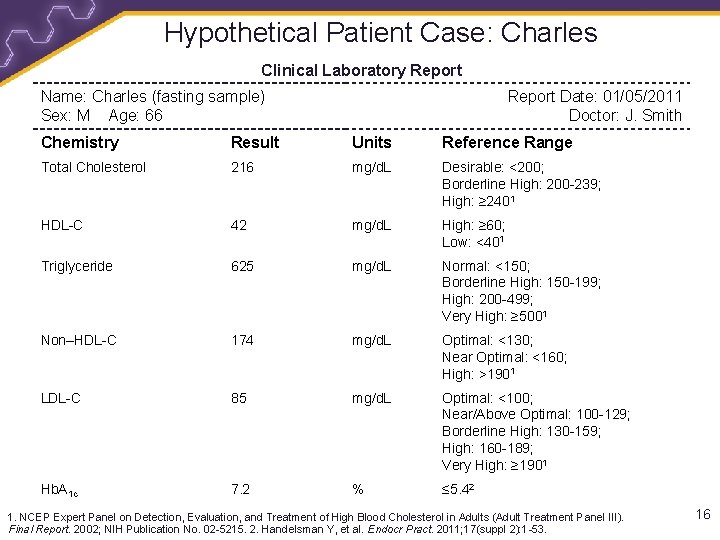
Hypothetical Patient Case: Charles Clinical Laboratory Report Name: Charles (fasting sample) Sex: M Age: 66 Report Date: 01/05/2011 Doctor: J. Smith Chemistry Result Units Reference Range Total Cholesterol 216 mg/d. L Desirable: <200; Borderline High: 200 -239; High: ≥ 2401 HDL-C 42 mg/d. L High: ≥ 60; Low: <401 Triglyceride 625 mg/d. L Normal: <150; Borderline High: 150 -199; High: 200 -499; Very High: ≥ 5001 Non–HDL-C 174 mg/d. L Optimal: <130; Near Optimal: <160; High: >1901 LDL-C 85 mg/d. L Optimal: <100; Near/Above Optimal: 100 -129; Borderline High: 130 -159; High: 160 -189; Very High: ≥ 1901 Hb. A 1 c 7. 2 % ≤ 5. 42 1. NCEP Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Final Report. 2002; NIH Publication No. 02 -5215. 2. Handelsman Y, et al. Endocr Pract. 2011; 17(suppl 2): 1 -53. 16

Hypothetical Patient Case: Charles • What is your clinical impression of Charles? • What are your management considerations? 17

LOVAZA: Proposed Mechanism of Action

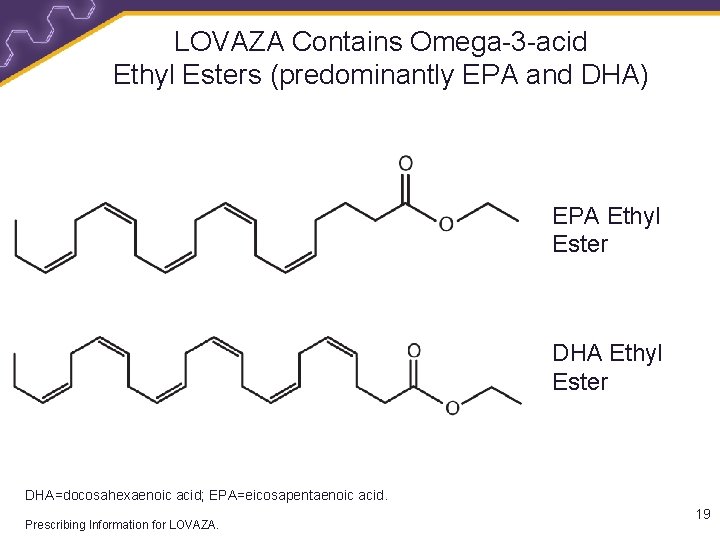
LOVAZA Contains Omega-3 -acid Ethyl Esters (predominantly EPA and DHA) EPA Ethyl Ester DHA=docosahexaenoic acid; EPA=eicosapentaenoic acid. Prescribing Information for LOVAZA. 19

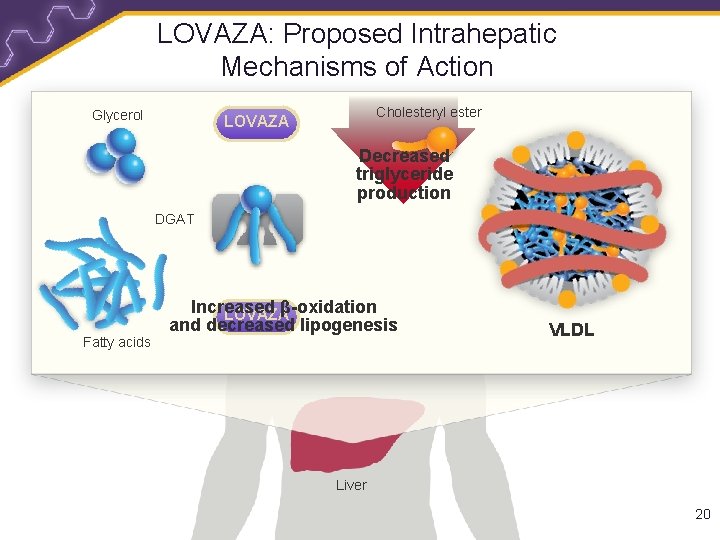
LOVAZA: Proposed Intrahepatic Mechanisms of Action Glycerol Cholesteryl ester LOVAZA Decreased triglyceride production DGAT Fatty acids Increased β-oxidation LOVAZA and decreased lipogenesis VLDL Liver 20

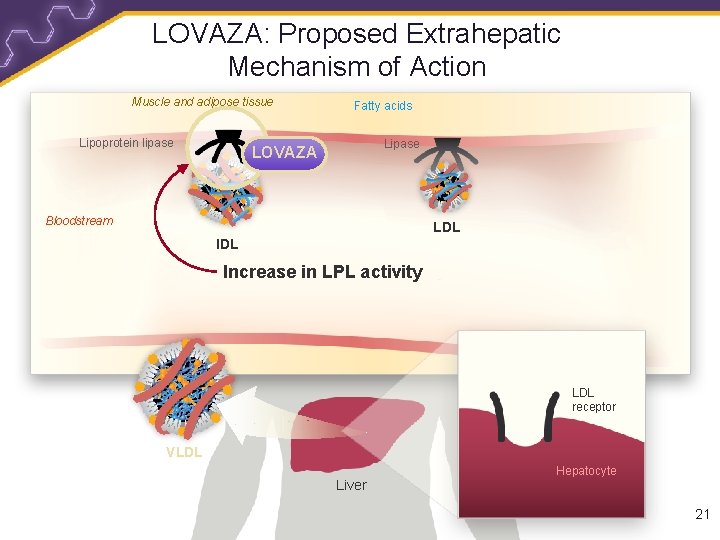
LOVAZA: Proposed Extrahepatic Mechanism of Action Muscle and adipose tissue Lipoprotein lipase Fatty acids Lipase LOVAZA Bloodstream LDL Increase in LPL activity LDL receptor VLDL Liver Hepatocyte 21

LOVAZA: Efficacy

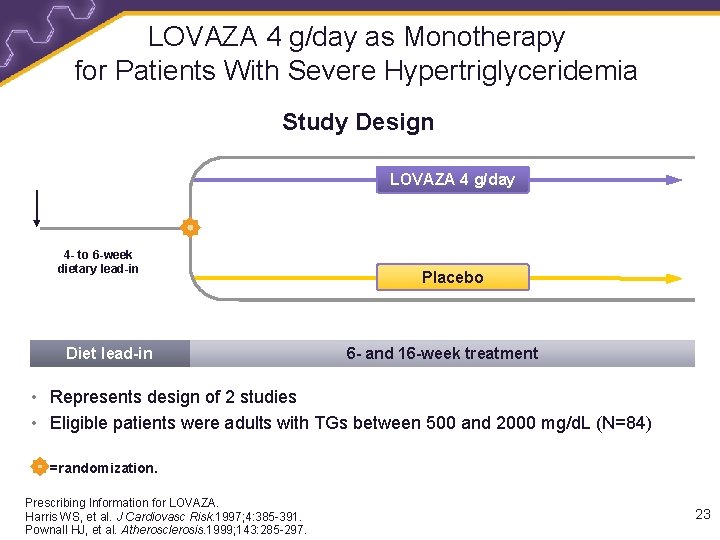
LOVAZA 4 g/day as Monotherapy for Patients With Severe Hypertriglyceridemia Study Design LOVAZA 4 g/day 4 - to 6 -week dietary lead-in Diet lead-in Placebo 6 - and 16 -week treatment • Represents design of 2 studies • Eligible patients were adults with TGs between 500 and 2000 mg/d. L (N=84) =randomization. Prescribing Information for LOVAZA. Harris WS, et al. J Cardiovasc Risk. 1997; 4: 385 -391. Pownall HJ, et al. Atherosclerosis. 1999; 143: 285 -297. 23

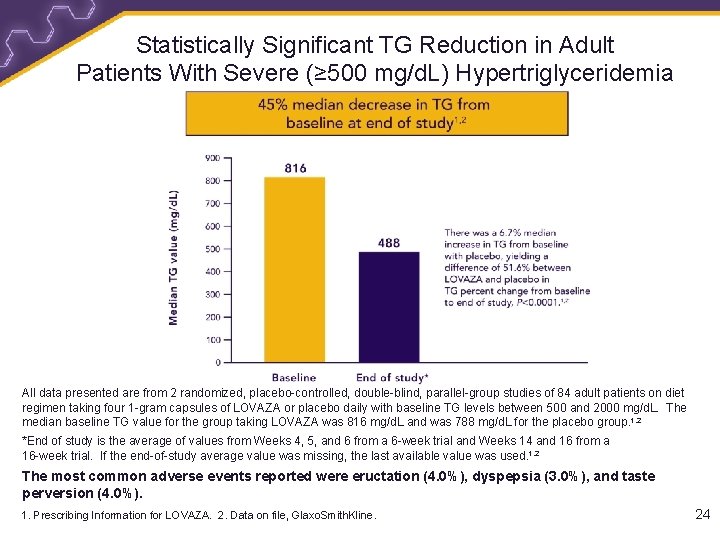
Statistically Significant TG Reduction in Adult Patients With Severe (≥ 500 mg/d. L) Hypertriglyceridemia All data presented are from 2 randomized, placebo-controlled, double-blind, parallel-group studies of 84 adult patients on diet regimen taking four 1 -gram capsules of LOVAZA or placebo daily with baseline TG levels between 500 and 2000 mg/d. L. The median baseline TG value for the group taking LOVAZA was 816 mg/d. L and was 788 mg/d. L for the placebo group. 1, 2 *End of study is the average of values from Weeks 4, 5, and 6 from a 6 -week trial and Weeks 14 and 16 from a 16 -week trial. If the end-of-study average value was missing, the last available value was used. 1, 2 The most common adverse events reported were eructation (4. 0%), dyspepsia (3. 0%), and taste perversion (4. 0%). 1. Prescribing Information for LOVAZA. 2. Data on file, Glaxo. Smith. Kline. 24

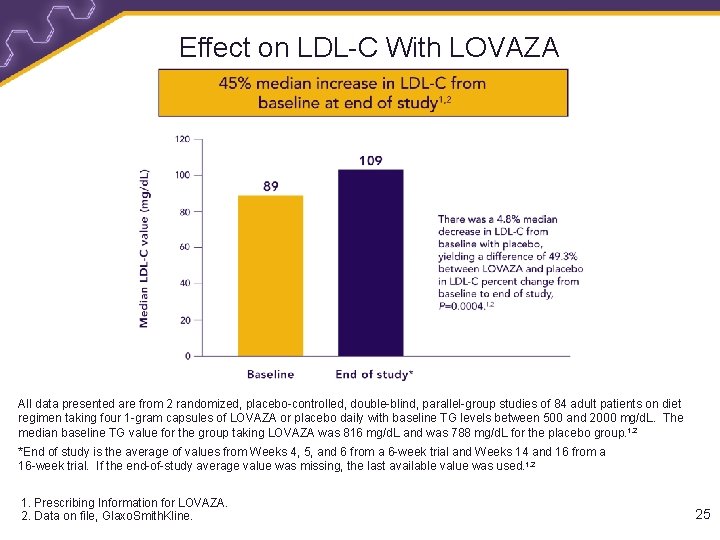
Effect on LDL-C With LOVAZA All data presented are from 2 randomized, placebo-controlled, double-blind, parallel-group studies of 84 adult patients on diet regimen taking four 1 -gram capsules of LOVAZA or placebo daily with baseline TG levels between 500 and 2000 mg/d. L. The median baseline TG value for the group taking LOVAZA was 816 mg/d. L and was 788 mg/d. L for the placebo group. 1, 2 *End of study is the average of values from Weeks 4, 5, and 6 from a 6 -week trial and Weeks 14 and 16 from a 16 -week trial. If the end-of-study average value was missing, the last available value was used. 1, 2 1. Prescribing Information for LOVAZA. 2. Data on file, Glaxo. Smith. Kline. 25

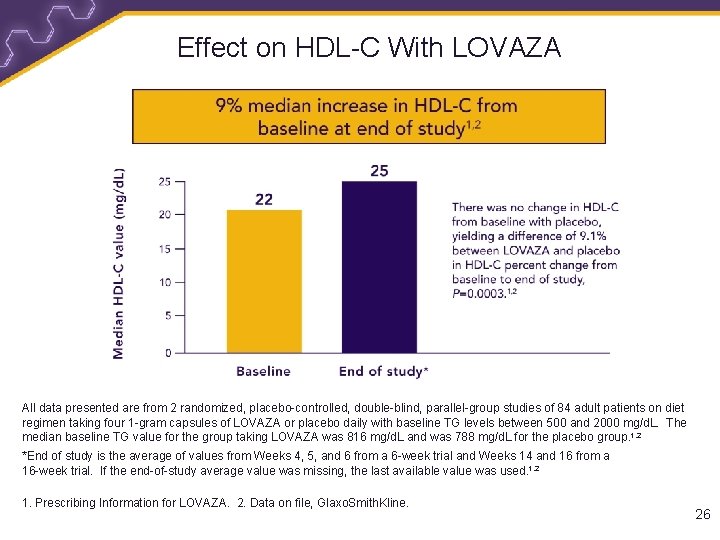
Effect on HDL-C With LOVAZA All data presented are from 2 randomized, placebo-controlled, double-blind, parallel-group studies of 84 adult patients on diet regimen taking four 1 -gram capsules of LOVAZA or placebo daily with baseline TG levels between 500 and 2000 mg/d. L. The median baseline TG value for the group taking LOVAZA was 816 mg/d. L and was 788 mg/d. L for the placebo group. 1, 2 *End of study is the average of values from Weeks 4, 5, and 6 from a 6 -week trial and Weeks 14 and 16 from a 16 -week trial. If the end-of-study average value was missing, the last available value was used. 1, 2 1. Prescribing Information for LOVAZA. 2. Data on file, Glaxo. Smith. Kline. 26

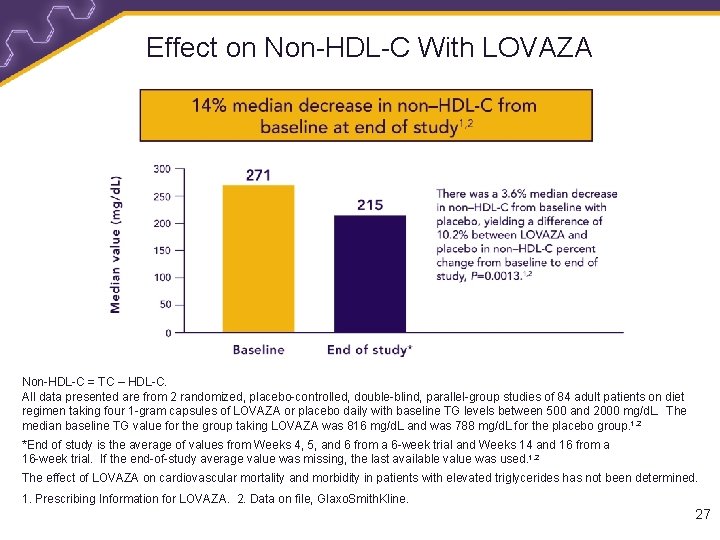
Effect on Non-HDL-C With LOVAZA Non-HDL-C = TC – HDL-C. All data presented are from 2 randomized, placebo-controlled, double-blind, parallel-group studies of 84 adult patients on diet regimen taking four 1 -gram capsules of LOVAZA or placebo daily with baseline TG levels between 500 and 2000 mg/d. L. The median baseline TG value for the group taking LOVAZA was 816 mg/d. L and was 788 mg/d. L for the placebo group. 1, 2 *End of study is the average of values from Weeks 4, 5, and 6 from a 6 -week trial and Weeks 14 and 16 from a 16 -week trial. If the end-of-study average value was missing, the last available value was used. 1, 2 The effect of LOVAZA on cardiovascular mortality and morbidity in patients with elevated triglycerides has not been determined. 1. Prescribing Information for LOVAZA. 2. Data on file, Glaxo. Smith. Kline. 27

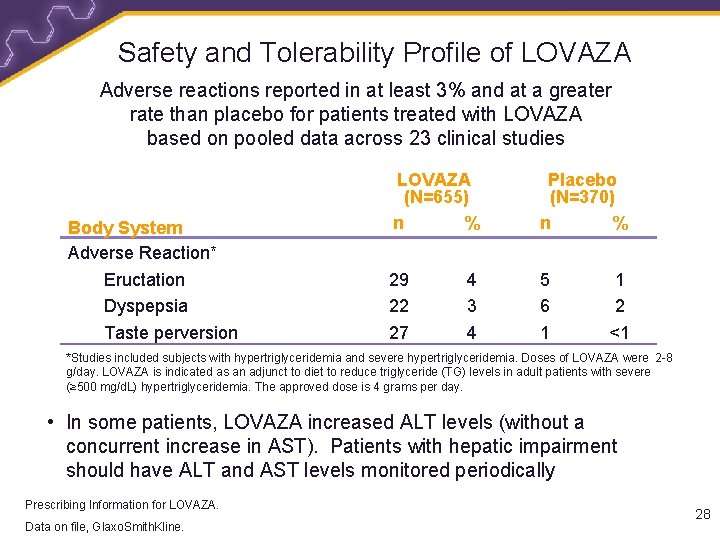
Safety and Tolerability Profile of LOVAZA Adverse reactions reported in at least 3% and at a greater rate than placebo for patients treated with LOVAZA based on pooled data across 23 clinical studies Body System Adverse Reaction* Eructation Dyspepsia Taste perversion LOVAZA (N=655) n % Placebo (N=370) n % 29 22 27 5 6 1 4 3 4 1 2 <1 *Studies included subjects with hypertriglyceridemia and severe hypertriglyceridemia. Doses of LOVAZA were 2 -8 g/day. LOVAZA is indicated as an adjunct to diet to reduce triglyceride (TG) levels in adult patients with severe (≥ 500 mg/d. L) hypertriglyceridemia. The approved dose is 4 grams per day. • In some patients, LOVAZA increased ALT levels (without a concurrent increase in AST). Patients with hepatic impairment should have ALT and AST levels monitored periodically Prescribing Information for LOVAZA. Data on file, Information Glaxo. Smith. Kline. Prescribing for LOVAZA. 28

Drug Interactions • Drug interactions • Patients receiving treatment with both LOVAZA and an anticoagulant or other drug affecting coagulation should be monitored periodically (eg, aspirin, NSAIDS, warfarin, coumarin) • Pharmacokinetic Drug-Drug Interactions • In vitro studies using human liver microsomes indicated that clinically significant cytochrome P 450 mediated inhibition by EPA/DHA combinations are not expected in humans NSAIDS=nonsteroidal anti-inflammatory drugs. Prescribing Information for LOVAZA. 29

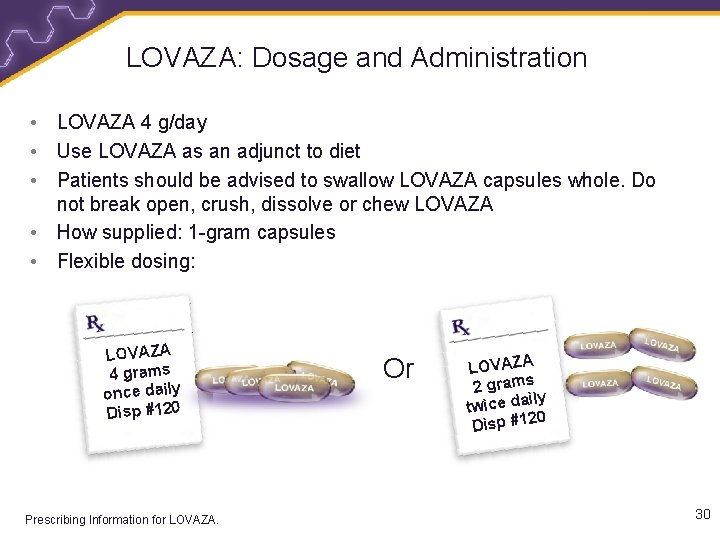
LOVAZA: Dosage and Administration • LOVAZA 4 g/day • Use LOVAZA as an adjunct to diet • Patients should be advised to swallow LOVAZA capsules whole. Do not break open, crush, dissolve or chew LOVAZA • How supplied: 1 -gram capsules • Flexible dosing: LOVAZA 4 grams once daily Disp #120 Prescribing Information for LOVAZA. Or LOVAZA 2 grams ily twice da 0 Disp #12 30

LOVAZA: Purification

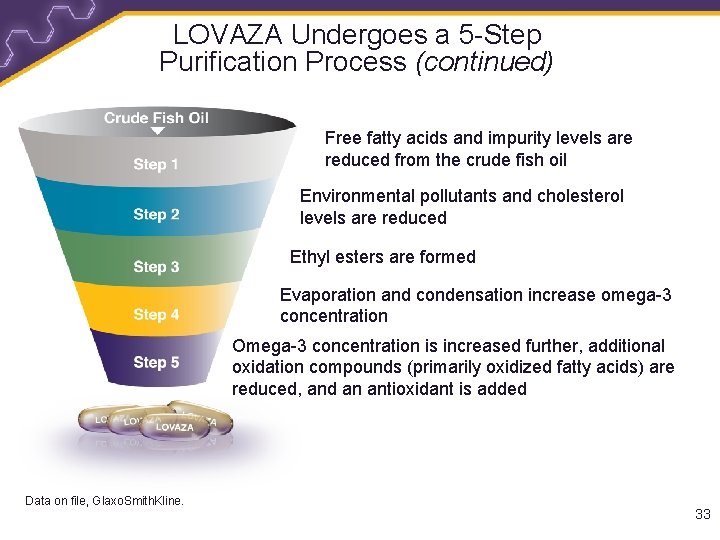
LOVAZA Undergoes a 5 -Step Purification Process • LOVAZA is derived from anchovies, herring, salmon, mackerel, smelt, and jacks/scads/trevally 1 • LOVAZA undergoes a 5 -step purification process to help reduce mercury and other impurities that can be present in fish oil 1 • LOVAZA should be used with caution in patients with known sensitivity or allergy to fish and/or shellfish 2 1. Data on file, Glaxo. Smith. Kline. 2. Prescribing Information for LOVAZA. 32

LOVAZA Undergoes a 5 -Step Purification Process (continued) Free fatty acids and impurity levels are reduced from the crude fish oil Environmental pollutants and cholesterol levels are reduced Ethyl esters are formed Evaporation and condensation increase omega-3 concentration Omega-3 concentration is increased further, additional oxidation compounds (primarily oxidized fatty acids) are reduced, and an antioxidant is added Data on file, Glaxo. Smith. Kline. 33

LOVAZA: Delivers Reliable Concentrations • LOVAZA contains concentrated omega-3 -acid ethyl esters (eicosapentaenoic acid [EPA] and docosahexaenoic acid [DHA])1 • Each 1 -g capsule of LOVAZA contains 1: • Approximately 465 mg of EPA and 375 mg of DHA • At least 900 mg of omega-3 -acid ethyl esters • 4 mg α-tocopherol • LOVAZA also contains these inactive ingredients: gelatin, glycerol, and purified water (components of the capsule shell) • Each 1 -g capsule contains ~11 calories 2 1. Prescribing Information for LOVAZA. 2. Data on file, Glaxo. Smith. Kline. 34

LOVAZA 4 g/day: Summary Proven in clinical trials, as an adjunct to diet, to significantly reduce TG levels in adult patients with TG levels ≥ 500 mg/d. L, but has not been proven to prevent heart attacks or strokes 1 Demonstrated safety and tolerability profile. The most common adverse events reported were eructation, dyspepsia, and taste perversion 1 The daily dose of LOVAZA is 4 g/day 1 Undergoes a 5 -step purification process 2 The only FDA-approved medication made from omega-3 fatty acids for the treatment of severe (≥ 500 mg/d. L) hypertriglyceridemia as an adjunct to diet 1 Please see complete Prescribing Information for LOVAZA. 1. Prescribing Information for LOVAZA. 2. Data on file, Glaxo. Smith. Kline. 35

GSK’s Commitment to Patient Assistance • The Naturally Simple™ Program is a free support program from GSK specifically for patients on LOVAZA • Enrolled patients receive education, resources, and savings on LOVAZA (subject to eligibility*) to help them manage severe (≥ 500 mg/d. L) hypertriglyceridemia • Two easy ways to enroll: by Web (www. LOVAZASupport. com) or by phone (1 -877 -LOVAZA 1) *Some restrictions may apply. Please see complete details at http: //www. lovaza. com/eligibility. html. 36

GSK’s Commitment to Patient Assistance (continued) • Other Assistance Programs offered by GSK: • GSK for You (www. gskforyou. com or 1 -866 -GSK-FOR-U): helps patients without prescription drug coverage afford the medicines they need • Bridges to Access (www. bridgestoaccess. com or 1866 -PATIENT): provides GSK prescription drugs to eligible low -income patients who do not have prescription coverage • GSK Access (www. gsk-access. com): provides GSK prescription medications at no cost to Medicare Part D Prescription Drug Plan enrollees who meet eligibility requirements • Together Rx Access (www. togetherrxaccess. com): a prescription savings program sponsored by GSK and several other leading pharmaceutical companies 37

LOVAZA: Indications and Usage • LOVAZA is indicated as an adjunct to diet to reduce triglyceride (TG) levels in adult patients with severe (≥ 500 mg/d. L) hypertriglyceridemia • Patients should be placed on an appropriate lipid-lowering diet before receiving LOVAZA and should continue this diet during treatment with LOVAZA • Laboratory studies should be done to ascertain that the lipid levels are consistently abnormal before instituting LOVAZA therapy Please see complete Prescribing Information for LOVAZA. 38

LOVAZA: Indications and Usage (continued) • Every attempt should be made to control serum lipids with appropriate diet, exercise, weight loss in obese patients, and control of any medical problems such as diabetes mellitus and hypothyroidism that are contributing to the lipid abnormalities • Medications known to exacerbate hypertriglyceridemia (such as beta blockers, thiazides, estrogens) should be discontinued or changed if possible prior to consideration of TG-lowering drug therapy • Limitations of use: The effect of LOVAZA on cardiovascular (CV) mortality and morbidity in patients with elevated TGs has not been determined Please see complete Prescribing Information for LOVAZA. 39

LOVAZA: Important Safety Information • LOVAZA is contraindicated in patients who exhibit hypersensitivity to any component of this medication • In patients with hepatic impairment, ALT and AST levels should be monitored periodically • In some patients, LOVAZA increases LDL-C and ALT levels (without a concurrent increase in AST) • TG and LDL-C levels should be monitored periodically during therapy with LOVAZA • LOVAZA should be used with caution in patients with known hypersensitivity or allergy to fish and/or shellfish Please see complete Prescribing Information for LOVAZA. 40

LOVAZA: Important Safety Information (continued) • • • Some studies with omega-3 -acids demonstrated prolongation of bleeding time, which did not exceed normal limits and did not produce clinically significant bleeding episodes Clinical studies have not been done to thoroughly examine the effect of LOVAZA and concomitant anticoagulants. Patients receiving treatment with both LOVAZA and anticoagulants should be monitored periodically The most common adverse events reported were eructation (4. 0%), dyspepsia (3. 0%), and taste perversion (4. 0%) The effect of LOVAZA on cardiovascular mortality and morbidity in patients with elevated TG levels has not been determined How supplied: 1 -gram capsules Please see complete Prescribing Information for LOVAZA. 41

LOVAZA is a registered trademark of Glaxo. Smith. Kline. Distributed by Glaxo. Smith. Kline, Research Triangle Park, NC 27709 © 2011 The Glaxo. Smith. Kline Group of Companies All rights reserved. LVZ 789 R 0 November 2011
 Best treatment for hypertriglyceridemia
Best treatment for hypertriglyceridemia Hemoglobinuria vs hematuria
Hemoglobinuria vs hematuria Familial hypertriglyceridemia
Familial hypertriglyceridemia Mgd stellenbosch
Mgd stellenbosch Serum osmolality
Serum osmolality Severe asthma treatment
Severe asthma treatment 3,500/500
3,500/500 Severe obesity
Severe obesity Vce stress
Vce stress Severe weather safety precautions worksheet
Severe weather safety precautions worksheet Pong game cards
Pong game cards Severe weather graphic organizer
Severe weather graphic organizer S2 os gap
S2 os gap Bowl in medical term
Bowl in medical term Hypertonic dehydration
Hypertonic dehydration Kcl dose calculation
Kcl dose calculation Severe anxiety
Severe anxiety Mild moderate severe dehydration
Mild moderate severe dehydration Unmet needs in severe asthma
Unmet needs in severe asthma Dka criteria
Dka criteria Two main whmis 2015 hazard groups are
Two main whmis 2015 hazard groups are Pef in asthma
Pef in asthma Correcting hypernatremia
Correcting hypernatremia Chapter 7 outsiders vocabulary
Chapter 7 outsiders vocabulary Katrine zhiroff
Katrine zhiroff Causes of cotton wool spots
Causes of cotton wool spots Formula de adrogue calculator
Formula de adrogue calculator Asthma clinical pathway
Asthma clinical pathway Chapter 20 weather patterns and severe storms
Chapter 20 weather patterns and severe storms Diarrhea plan a
Diarrhea plan a How to make pong on scratch
How to make pong on scratch Wmo severe weather
Wmo severe weather Fluid of choice in severe dehydration
Fluid of choice in severe dehydration Chapter 20 weather patterns and severe storms
Chapter 20 weather patterns and severe storms Emt chapter 24 trauma overview
Emt chapter 24 trauma overview Hengameh raissy
Hengameh raissy Laxative effect
Laxative effect Severe dehydration
Severe dehydration Severe hypocalcemia
Severe hypocalcemia Carbonic anhydrase use
Carbonic anhydrase use What cause metabolic acidosis
What cause metabolic acidosis Malnutrition case study
Malnutrition case study Nude
Nude