Reduction of Revascularization in Patients with Hypertriglyceridemia with








- Slides: 38

Reduction of Revascularization in Patients with Hypertriglyceridemia with Icosapent Ethyl: Insights from REDUCE-IT REVASC Benjamin E. Peterson, M. D. , Deepak L. Bhatt, M. D. , M. P. H. , Ph. Gabriel Steg, M. D. , Michael Miller, M. D. , Eliot A. Brinton, M. D. , Terry A. Jacobson, M. D. , Steven B. Ketchum, Ph. D. , Rebecca A. Juliano, Ph. D. , Lixia Jiao, Ph. D. , Ralph T. Doyle, Jr. , B. A. , Craig Granowitz, M. D. , Ph. D. , C. Michael Gibson, M. D. , Duane Pinto, MD, Robert P. Giugliano, MD, Matthew J. Budoff, MD, Jean-Claude Tardif, M. D. , Subodh Verma, M. D. , Christie M. Ballantyne, M. D. , on Behalf of the REDUCE-IT Investigators

Disclosures Dr. Benjamin E. Peterson has no relevant disclosures. Dr. Deepak L. Bhatt discloses the following relationships - Advisory Board: Cardax, Cereno Scientific, Elsevier Practice Update Cardiology, Level. Ex, Medscape Cardiology, Phase. Bio, PLx Pharma, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care, Tobe. Soft; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute, for the PORTICO trial, funded by St. Jude Medical, now Abbott), Cleveland Clinic (including for the Ex. CEED trial, funded by Edwards), Duke Clinical Research Institute, Mayo Clinic, Mount Sinai School of Medicine (for the ENVISAGE trial, funded by Daiichi Sankyo), Population Health Research Institute; Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC. org; Vice-Chair, ACC Accreditation Committee), Baim Institute for Clinical Research (formerly Harvard Clinical Research Institute; RE-DUAL PCI clinical trial steering committee funded by Boehringer Ingelheim; AEGIS-II executive committee funded by CSL Behring), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees, including for the PRONOUNCE trial, funded by Ferring Pharmaceuticals), HMP Global (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Medtelligence/Reach. MD (CME steering committees), MJH Life Sciences, Population Health Research Institute (for the COMPASS operations committee, publications committee, steering committee, and USA national co-leader, funded by Bayer), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), Web. MD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDRACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Abbott, Afimmune, Amarin, Amgen, Astra. Zeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Cardax, Chiesi, CSL Behring, Eisai, Ethicon, Ferring Pharmaceuticals, Forest Laboratories, Fractyl, Idorsia, Ironwood, Ischemix, Lexicon, Lilly, Medtronic, Pfizer, Phase. Bio, PLx Pharma, Regeneron, Roche, Sanofi Aventis, Synaptic, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); Site Co-Investigator: Biotronik, Boston Scientific, CSI, St. Jude Medical (now Abbott), Svelte; Trustee: American College of Cardiology; Unfunded Research: Flow. Co, Merck, Novo Nordisk, Takeda. This presentation may include off-label and/or investigational uses of drugs. REDUCE-IT was sponsored by Amarin Pharma, Inc.

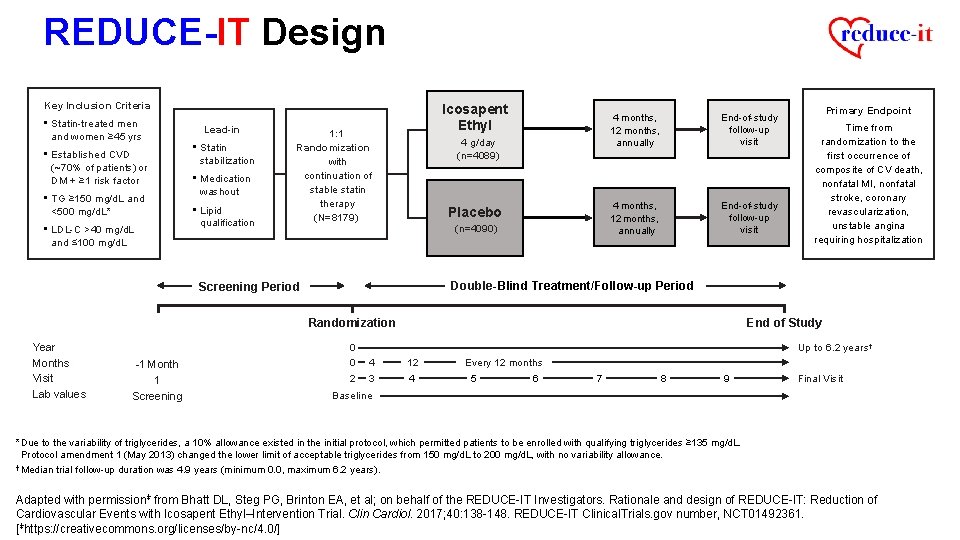
REDUCE-IT Design Key Inclusion Criteria • Statin-treated men and women ≥ 45 yrs • Established CVD (~70% of patients) or DM + ≥ 1 risk factor • TG ≥ 150 mg/d. L and <500 mg/d. L* • LDL-C >40 mg/d. L Lead-in • Statin stabilization • Medication washout • Lipid qualification Icosapent Ethyl 1: 1 Randomization with continuation of stable statin therapy (N=8179) 4 g/day (n=4089) Placebo (n=4090) 4 months, 12 months, annually End-of-study follow-up visit and ≤ 100 mg/d. L Randomization -1 Month 1 Screening Time from randomization to the first occurrence of composite of CV death, nonfatal MI, nonfatal stroke, coronary revascularization, unstable angina requiring hospitalization Double-Blind Treatment/Follow-up Period Screening Period Year Months Visit Lab values Primary Endpoint 0 0 2 End of Study Up to 6. 2 years† 4 3 12 4 Every 12 months 5 6 7 8 9 Final Visit Baseline *Due to the variability of triglycerides, a 10% allowance existed in the initial protocol, which permitted patients to be enrolled with qualifying triglycerides ≥ 135 mg/d. L. Protocol amendment 1 (May 2013) changed the lower limit of acceptable triglycerides from 150 mg/d. L to 200 mg/d. L, with no variability allowance. † Median trial follow-up duration was 4. 9 years (minimum 0. 0, maximum 6. 2 years). Adapted with permissionǂ from Bhatt DL, Steg PG, Brinton EA, et al; on behalf of the REDUCE-IT Investigators. Rationale and design of REDUCE-IT: Reduction of Cardiovascular Events with Icosapent Ethyl–Intervention Trial. Clin Cardiol. 2017; 40: 138 -148. REDUCE-IT Clinical. Trials. gov number, NCT 01492361. [ǂhttps: //creativecommons. org/licenses/by-nc/4. 0/]

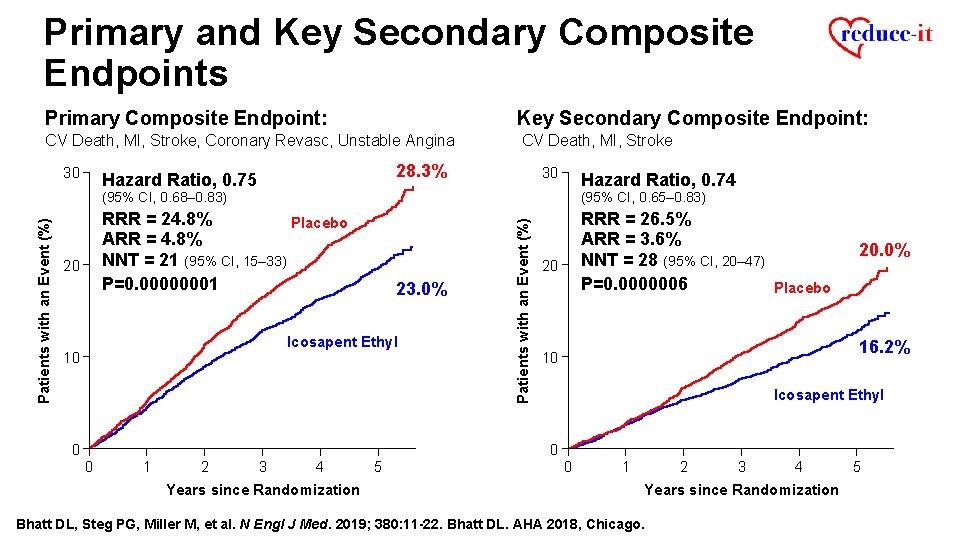
Primary and Key Secondary Composite Endpoints Primary Composite Endpoint: Key Secondary Composite Endpoint: CV Death, MI, Stroke, Coronary Revasc, Unstable Angina CV Death, MI, Stroke 28. 3% Hazard Ratio, 0. 75 20 30 Hazard Ratio, 0. 74 (95% CI, 0. 68– 0. 83) (95% CI, 0. 65– 0. 83) RRR = 24. 8% Placebo ARR = 4. 8% NNT = 21 (95% CI, 15– 33) P=0. 00000001 RRR = 26. 5% ARR = 3. 6% NNT = 28 (95% CI, 20– 47) P=0. 0000006 Placebo 23. 0% Icosapent Ethyl 10 0 Patients with an Event (%) 30 20 20. 0% 16. 2% 10 Icosapent Ethyl 0 0 1 2 3 4 Years since Randomization 5 0 1 2 3 4 Years since Randomization Bhatt DL, Steg PG, Miller M, et al. N Engl J Med. 2019; 380: 11 -22. Bhatt DL. AHA 2018, Chicago. 5

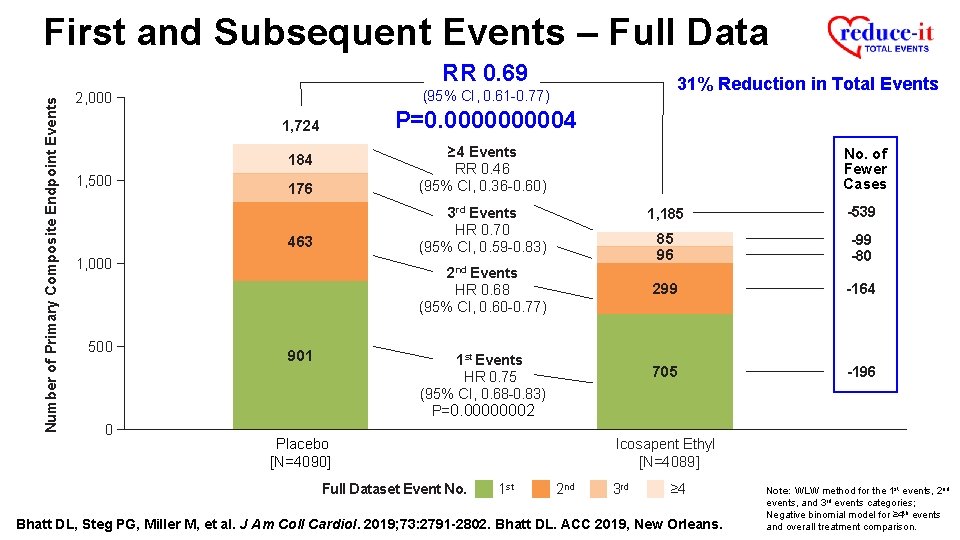
First and Subsequent Events – Full Data Number of Primary Composite Endpoint Events RR 0. 69 P=0. 000004 1, 724 176 ≥ 4 Events RR 0. 46 (95% CI, 0. 36 -0. 60) 463 3 rd Events HR 0. 70 (95% CI, 0. 59 -0. 83) 184 1, 500 1, 000 500 0 31% Reduction in Total Events (95% CI, 0. 61 -0. 77) 2, 000 No. of Fewer Cases 2 nd Events HR 0. 68 (95% CI, 0. 60 -0. 77) 901 1 st Events HR 0. 75 (95% CI, 0. 68 -0. 83) P=0. 00000002 -539 85 96 -99 -80 299 -164 705 -196 Icosapent Ethyl [N=4089] Placebo [N=4090] Full Dataset Event No. 1, 185 1 st 2 nd 3 rd ≥ 4 Bhatt DL, Steg PG, Miller M, et al. J Am Coll Cardiol. 2019; 73: 2791 -2802. Bhatt DL. ACC 2019, New Orleans. Note: WLW method for the 1 st events, 2 nd events, and 3 rd events categories; Negative binomial model for ≥ 4 th events and overall treatment comparison.


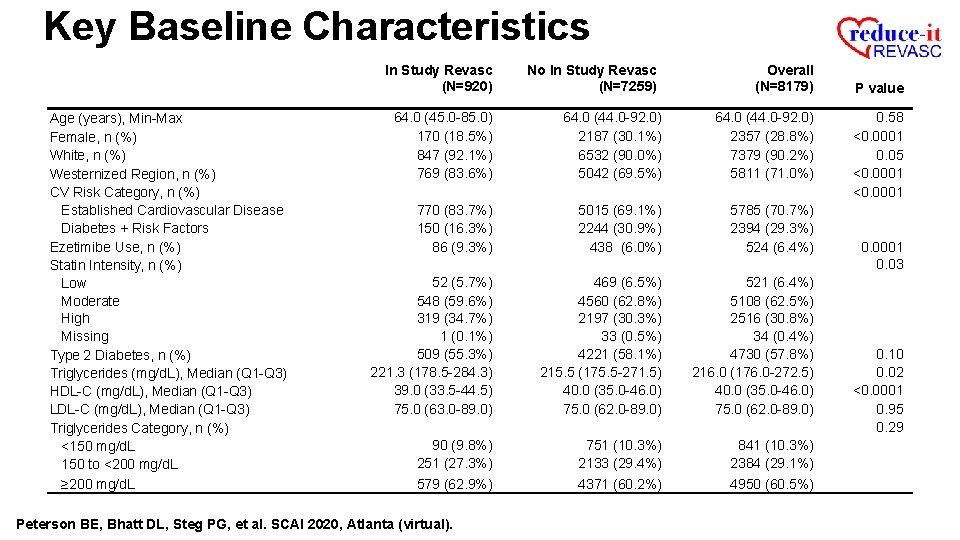
Key Baseline Characteristics In Study Revasc (N=920) Age (years), Min-Max Female, n (%) White, n (%) Westernized Region, n (%) CV Risk Category, n (%) Established Cardiovascular Disease Diabetes + Risk Factors Ezetimibe Use, n (%) Statin Intensity, n (%) Low Moderate High Missing Type 2 Diabetes, n (%) Triglycerides (mg/d. L), Median (Q 1 -Q 3) HDL-C (mg/d. L), Median (Q 1 -Q 3) LDL-C (mg/d. L), Median (Q 1 -Q 3) Triglycerides Category, n (%) <150 mg/d. L 150 to <200 mg/d. L ≥ 200 mg/d. L No In Study Revasc (N=7259) Overall (N=8179) 64. 0 (45. 0 -85. 0) 170 (18. 5%) 847 (92. 1%) 769 (83. 6%) 64. 0 (44. 0 -92. 0) 2187 (30. 1%) 6532 (90. 0%) 5042 (69. 5%) 64. 0 (44. 0 -92. 0) 2357 (28. 8%) 7379 (90. 2%) 5811 (71. 0%) 770 (83. 7%) 150 (16. 3%) 86 (9. 3%) 5015 (69. 1%) 2244 (30. 9%) 438 (6. 0%) 5785 (70. 7%) 2394 (29. 3%) 524 (6. 4%) 52 (5. 7%) 548 (59. 6%) 319 (34. 7%) 1 (0. 1%) 509 (55. 3%) 221. 3 (178. 5 -284. 3) 39. 0 (33. 5 -44. 5) 75. 0 (63. 0 -89. 0) 469 (6. 5%) 4560 (62. 8%) 2197 (30. 3%) 33 (0. 5%) 4221 (58. 1%) 215. 5 (175. 5 -271. 5) 40. 0 (35. 0 -46. 0) 75. 0 (62. 0 -89. 0) 521 (6. 4%) 5108 (62. 5%) 2516 (30. 8%) 34 (0. 4%) 4730 (57. 8%) 216. 0 (176. 0 -272. 5) 40. 0 (35. 0 -46. 0) 75. 0 (62. 0 -89. 0) 90 (9. 8%) 251 (27. 3%) 579 (62. 9%) 751 (10. 3%) 2133 (29. 4%) 4371 (60. 2%) 841 (10. 3%) 2384 (29. 1%) 4950 (60. 5%) Peterson BE, Bhatt DL, Steg PG, et al. SCAI 2020, Atlanta (virtual). P value 0. 58 <0. 0001 0. 05 <0. 0001 0. 03 0. 10 0. 02 <0. 0001 0. 95 0. 29

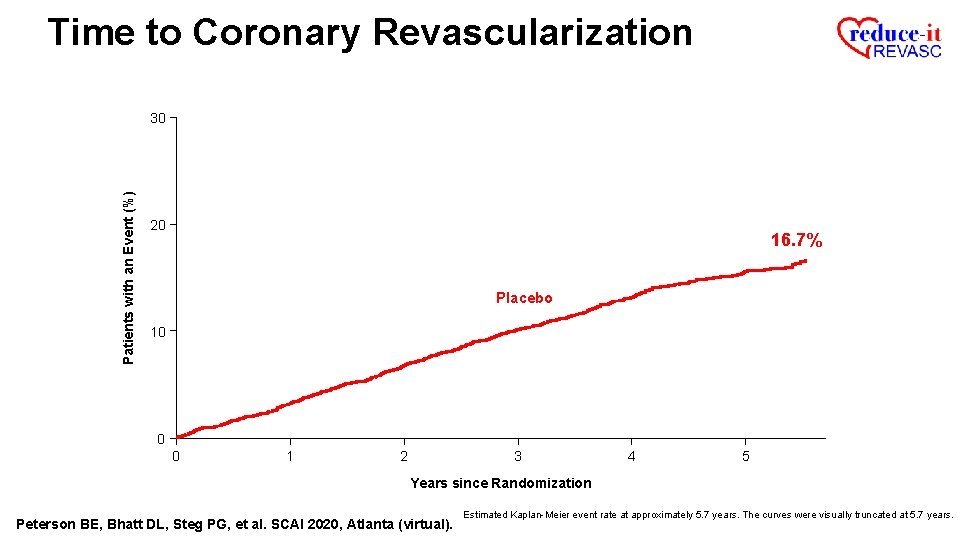
Time to Coronary Revascularization Patients with an Event (%) 30 20 16. 7% Placebo 10 0 0 1 2 3 4 5 Years since Randomization Peterson BE, Bhatt DL, Steg PG, et al. SCAI 2020, Atlanta (virtual). Estimated Kaplan-Meier event rate at approximately 5. 7 years. The curves were visually truncated at 5. 7 years.

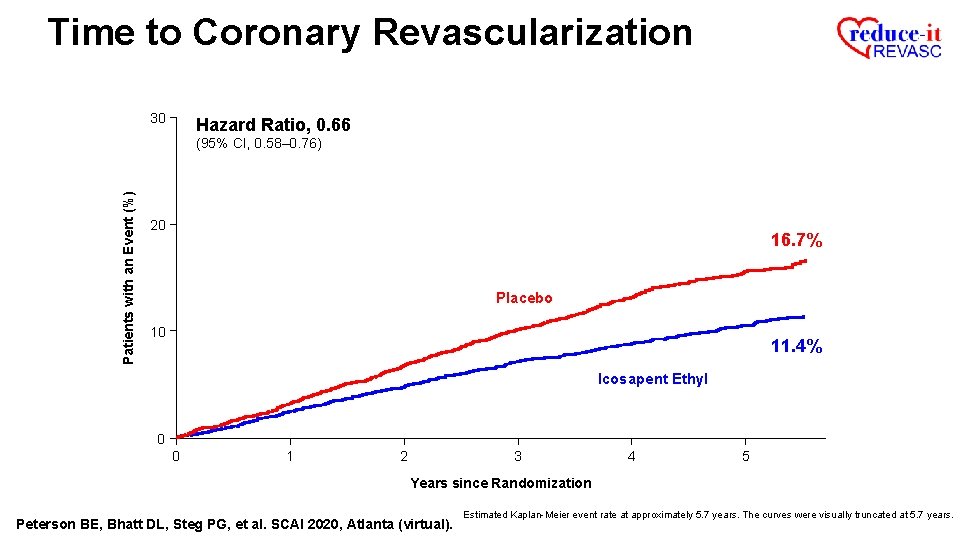
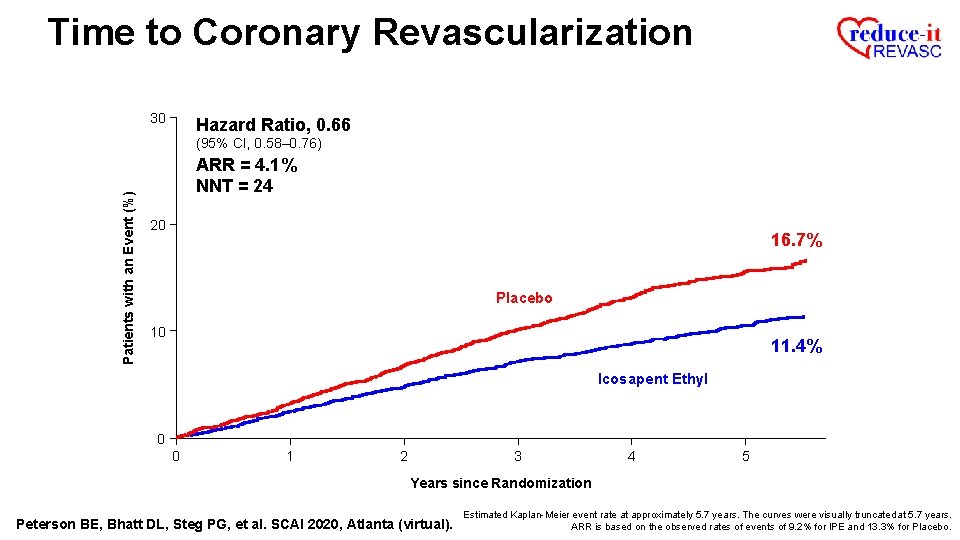
Time to Coronary Revascularization 30 Hazard Ratio, 0. 66 Patients with an Event (%) (95% CI, 0. 58– 0. 76) 20 16. 7% Placebo 10 11. 4% Icosapent Ethyl 0 0 1 2 3 4 5 Years since Randomization Peterson BE, Bhatt DL, Steg PG, et al. SCAI 2020, Atlanta (virtual). Estimated Kaplan-Meier event rate at approximately 5. 7 years. The curves were visually truncated at 5. 7 years.

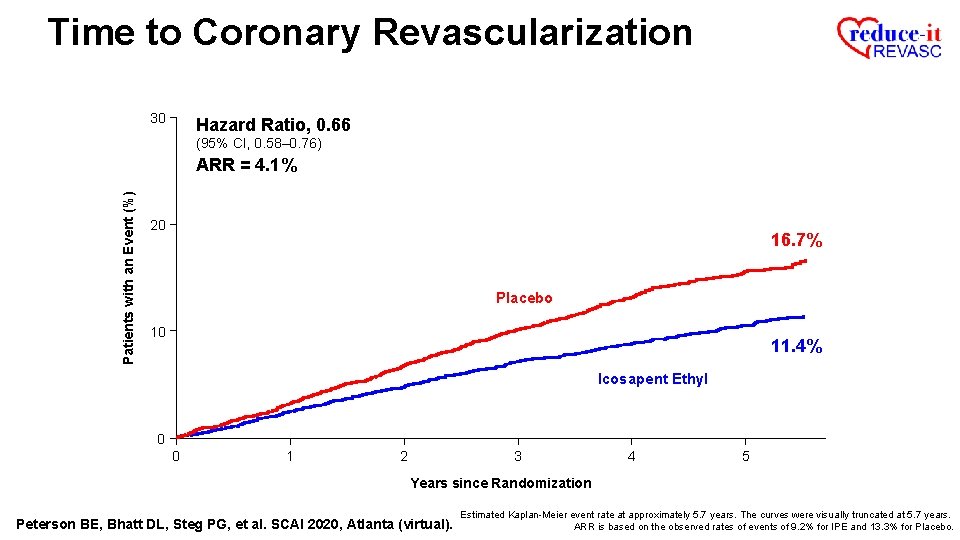
Time to Coronary Revascularization 30 Hazard Ratio, 0. 66 (95% CI, 0. 58– 0. 76) Patients with an Event (%) ARR = 4. 1% 20 16. 7% Placebo 10 11. 4% Icosapent Ethyl 0 0 1 2 3 4 5 Years since Randomization Peterson BE, Bhatt DL, Steg PG, et al. SCAI 2020, Atlanta (virtual). Estimated Kaplan-Meier event rate at approximately 5. 7 years. The curves were visually truncated at 5. 7 years. ARR is based on the observed rates of events of 9. 2% for IPE and 13. 3% for Placebo.

Time to Coronary Revascularization 30 Hazard Ratio, 0. 66 Patients with an Event (%) (95% CI, 0. 58– 0. 76) ARR = 4. 1% NNT = 24 20 16. 7% Placebo 10 11. 4% Icosapent Ethyl 0 0 1 2 3 4 5 Years since Randomization Peterson BE, Bhatt DL, Steg PG, et al. SCAI 2020, Atlanta (virtual). Estimated Kaplan-Meier event rate at approximately 5. 7 years. The curves were visually truncated at 5. 7 years. ARR is based on the observed rates of events of 9. 2% for IPE and 13. 3% for Placebo.

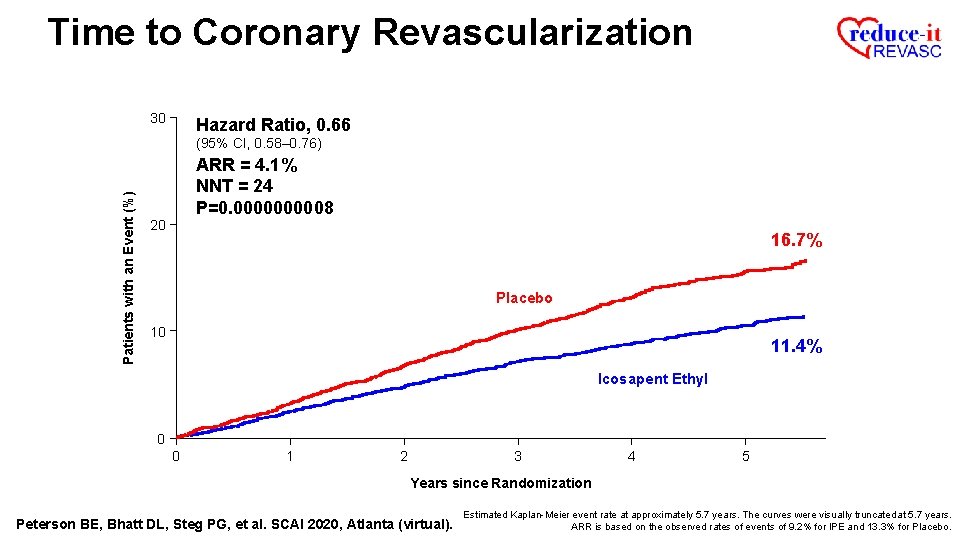
Time to Coronary Revascularization 30 Hazard Ratio, 0. 66 Patients with an Event (%) (95% CI, 0. 58– 0. 76) ARR = 4. 1% NNT = 24 P=0. 000008 20 16. 7% Placebo 10 11. 4% Icosapent Ethyl 0 0 1 2 3 4 5 Years since Randomization Peterson BE, Bhatt DL, Steg PG, et al. SCAI 2020, Atlanta (virtual). Estimated Kaplan-Meier event rate at approximately 5. 7 years. The curves were visually truncated at 5. 7 years. ARR is based on the observed rates of events of 9. 2% for IPE and 13. 3% for Placebo.

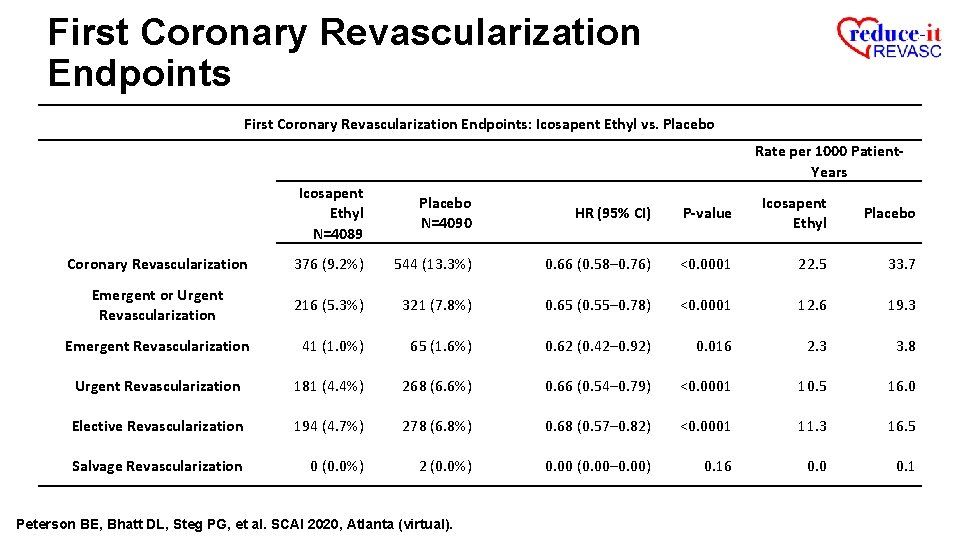
First Coronary Revascularization Endpoints: Icosapent Ethyl vs. Placebo Rate per 1000 Patient. Years Icosapent Ethyl N=4089 Placebo N=4090 HR (95% CI) P-value Icosapent Ethyl Placebo Coronary Revascularization 376 (9. 2%) 544 (13. 3%) 0. 66 (0. 58– 0. 76) <0. 0001 22. 5 33. 7 Emergent or Urgent Revascularization 216 (5. 3%) 321 (7. 8%) 0. 65 (0. 55– 0. 78) <0. 0001 12. 6 19. 3 Emergent Revascularization 41 (1. 0%) 65 (1. 6%) 0. 62 (0. 42– 0. 92) 0. 016 2. 3 3. 8 Urgent Revascularization 181 (4. 4%) 268 (6. 6%) 0. 66 (0. 54– 0. 79) <0. 0001 10. 5 16. 0 Elective Revascularization 194 (4. 7%) 278 (6. 8%) 0. 68 (0. 57– 0. 82) <0. 0001 11. 3 16. 5 Salvage Revascularization 0 (0. 0%) 2 (0. 0%) 0. 00 (0. 00– 0. 00) 0. 16 0. 0 0. 1 Peterson BE, Bhatt DL, Steg PG, et al. SCAI 2020, Atlanta (virtual).

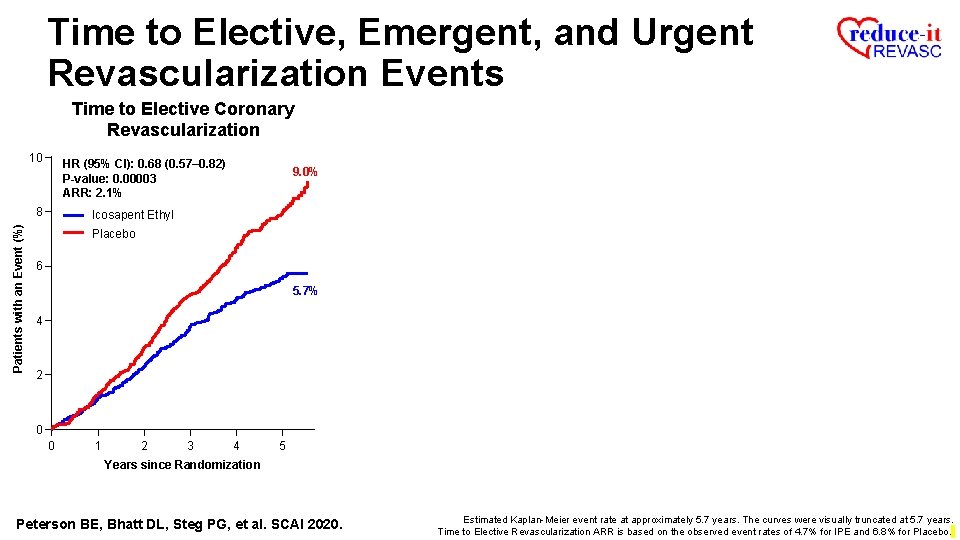
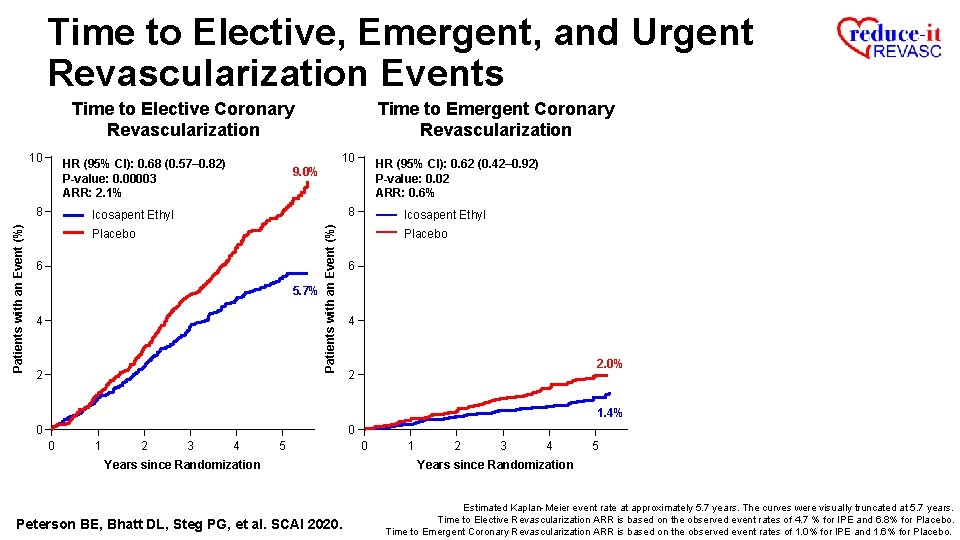
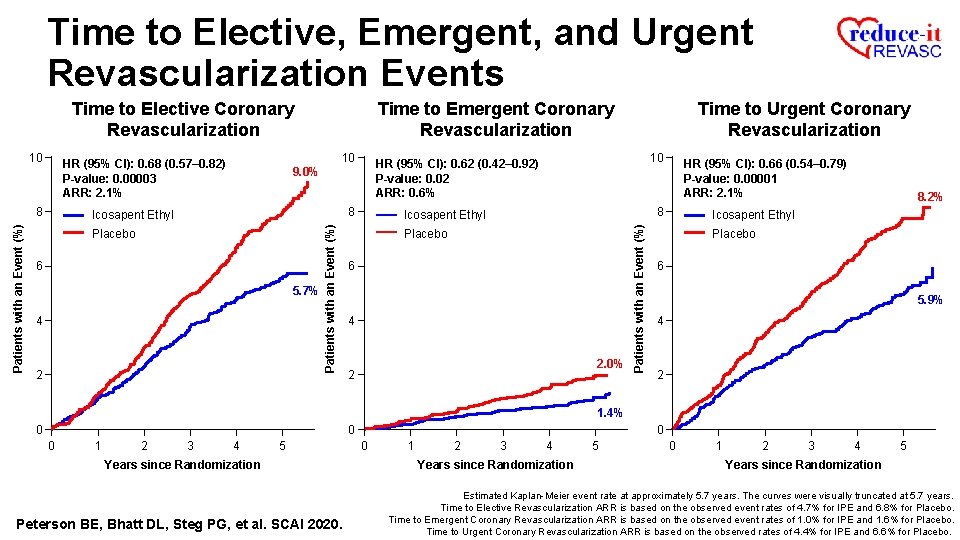
Time to Elective, Emergent, and Urgent Revascularization Events Time to Elective Coronary Revascularization 10 HR (95% CI): 0. 68 (0. 57– 0. 82) P-value: 0. 00003 ARR: 2. 1% Patients with an Event (%) 8 9. 0% Icosapent Ethyl Placebo 6 5. 7% 4 2 0 0 1 2 3 4 5 Years since Randomization Peterson BE, Bhatt DL, Steg PG, et al. SCAI 2020. Estimated Kaplan-Meier event rate at approximately 5. 7 years. The curves were visually truncated at 5. 7 years. Time to Elective Revascularization ARR is based on the observed event rates of 4. 7% for IPE and 6. 8% for Placebo.

Time to Elective, Emergent, and Urgent Revascularization Events Time to Elective Coronary Revascularization 10 HR (95% CI): 0. 62 (0. 42– 0. 92) P-value: 0. 02 ARR: 0. 6% 9. 0% 8 Icosapent Ethyl Placebo 6 5. 7% 4 2 Patients with an Event (%) 10 HR (95% CI): 0. 68 (0. 57– 0. 82) P-value: 0. 00003 ARR: 2. 1% 8 Time to Emergent Coronary Revascularization Icosapent Ethyl Placebo 6 4 2. 0% 2 1. 4% 0 0 0 1 2 3 4 5 Years since Randomization Peterson BE, Bhatt DL, Steg PG, et al. SCAI 2020. 0 1 2 3 4 5 Years since Randomization Estimated Kaplan-Meier event rate at approximately 5. 7 years. The curves were visually truncated at 5. 7 years. Time to Elective Revascularization ARR is based on the observed event rates of 4. 7 % for IPE and 6. 8% for Placebo. Time to Emergent Coronary Revascularization ARR is based on the observed event rates of 1. 0% for IPE and 1. 6% for Placebo.

Time to Elective, Emergent, and Urgent Revascularization Events Time to Elective Coronary Revascularization 8 8 Icosapent Ethyl Placebo 6 5. 7% 4 2 10 HR (95% CI): 0. 62 (0. 42– 0. 92) P-value: 0. 02 ARR: 0. 6% 9. 0% Patients with an Event (%) 10 HR (95% CI): 0. 68 (0. 57– 0. 82) P-value: 0. 00003 ARR: 2. 1% Time to Urgent Coronary Revascularization HR (95% CI): 0. 66 (0. 54– 0. 79) P-value: 0. 00001 ARR: 2. 1% 8 Icosapent Ethyl Placebo 6 4 2. 0% 2 Patients with an Event (%) 10 Time to Emergent Coronary Revascularization 8. 2% Icosapent Ethyl Placebo 6 5. 9% 4 2 1. 4% 0 0 0 1 2 3 4 5 Years since Randomization Peterson BE, Bhatt DL, Steg PG, et al. SCAI 2020. 0 0 1 2 3 4 Years since Randomization 5 0 1 2 3 4 5 Years since Randomization Estimated Kaplan-Meier event rate at approximately 5. 7 years. The curves were visually truncated at 5. 7 years. Time to Elective Revascularization ARR is based on the observed event rates of 4. 7% for IPE and 6. 8% for Placebo. Time to Emergent Coronary Revascularization ARR is based on the observed event rates of 1. 0% for IPE and 1. 6% for Placebo. Time to Urgent Coronary Revascularization ARR is based on the observed rates of 4. 4% for IPE and 6. 6% for Placebo.

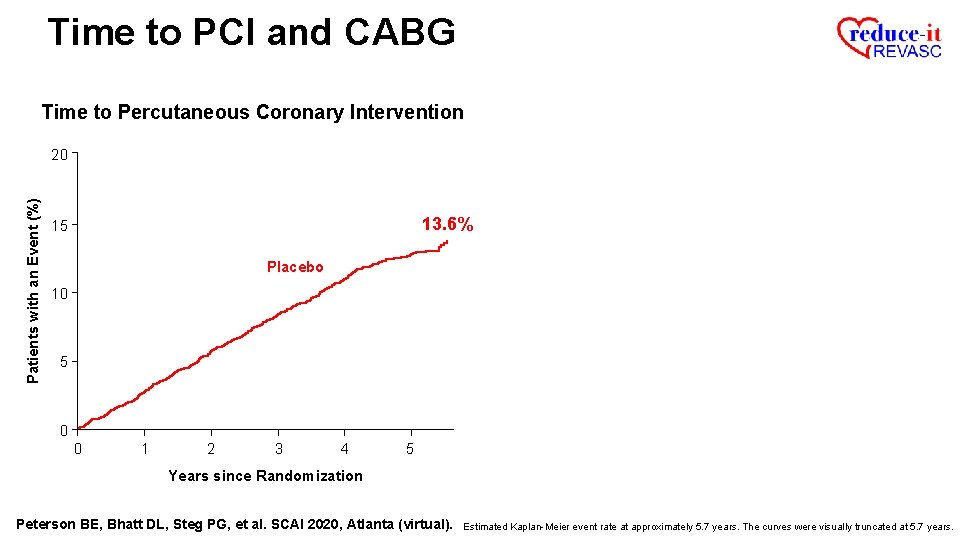
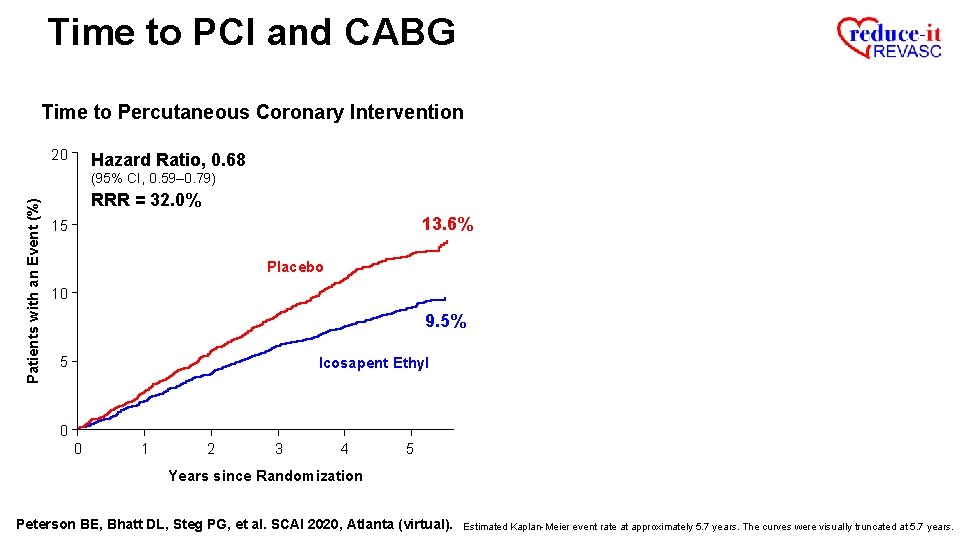
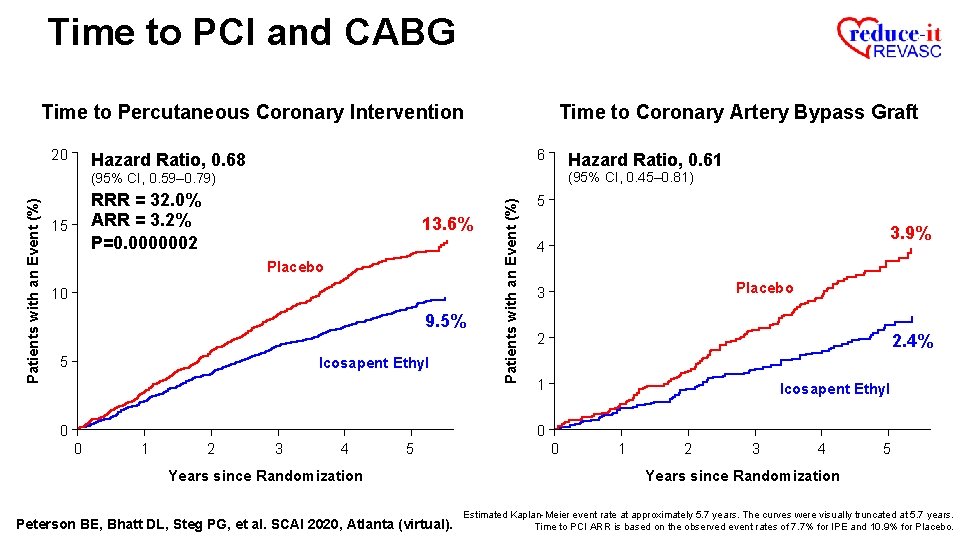
Time to PCI and CABG Time to Percutaneous Coronary Intervention Patients with an Event (%) 20 13. 6% 15 Placebo 10 5 0 0 1 2 3 4 5 Years since Randomization Peterson BE, Bhatt DL, Steg PG, et al. SCAI 2020, Atlanta (virtual). Estimated Kaplan-Meier event rate at approximately 5. 7 years. The curves were visually truncated at 5. 7 years.

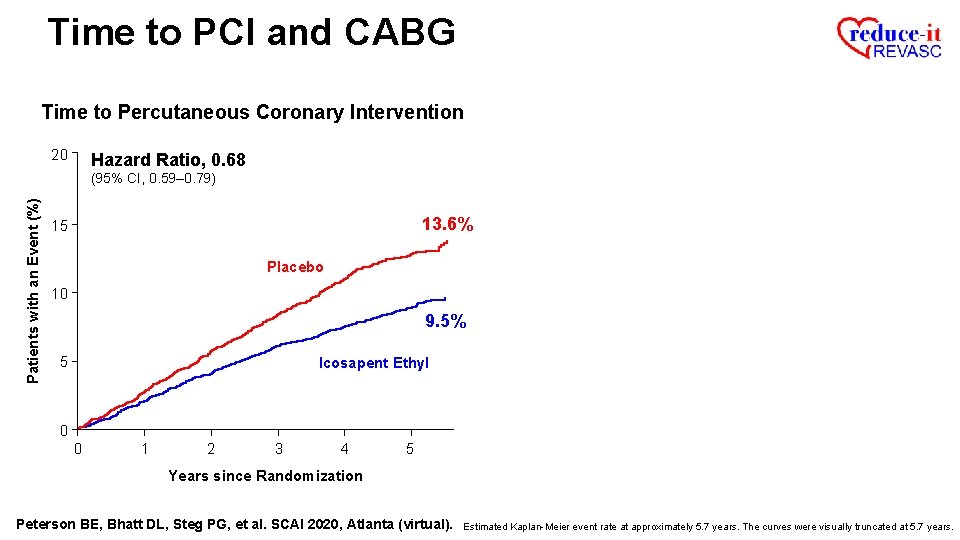
Time to PCI and CABG Time to Percutaneous Coronary Intervention 20 Hazard Ratio, 0. 68 Patients with an Event (%) (95% CI, 0. 59– 0. 79) 13. 6% 15 Placebo 10 9. 5% 5 Icosapent Ethyl 0 0 1 2 3 4 5 Years since Randomization Peterson BE, Bhatt DL, Steg PG, et al. SCAI 2020, Atlanta (virtual). Estimated Kaplan-Meier event rate at approximately 5. 7 years. The curves were visually truncated at 5. 7 years.

Time to PCI and CABG Time to Percutaneous Coronary Intervention 20 Hazard Ratio, 0. 68 Patients with an Event (%) (95% CI, 0. 59– 0. 79) RRR = 32. 0% 13. 6% 15 Placebo 10 9. 5% 5 Icosapent Ethyl 0 0 1 2 3 4 5 Years since Randomization Peterson BE, Bhatt DL, Steg PG, et al. SCAI 2020, Atlanta (virtual). Estimated Kaplan-Meier event rate at approximately 5. 7 years. The curves were visually truncated at 5. 7 years.

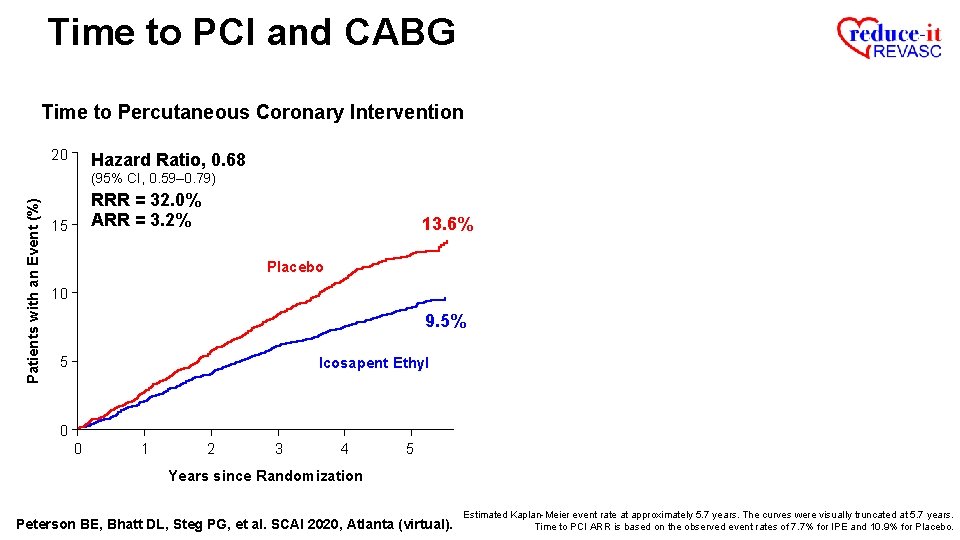
Time to PCI and CABG Time to Percutaneous Coronary Intervention 20 Hazard Ratio, 0. 68 Patients with an Event (%) (95% CI, 0. 59– 0. 79) RRR = 32. 0% ARR = 3. 2% 15 13. 6% Placebo 10 9. 5% 5 Icosapent Ethyl 0 0 1 2 3 4 5 Years since Randomization Peterson BE, Bhatt DL, Steg PG, et al. SCAI 2020, Atlanta (virtual). Estimated Kaplan-Meier event rate at approximately 5. 7 years. The curves were visually truncated at 5. 7 years. Time to PCI ARR is based on the observed event rates of 7. 7% for IPE and 10. 9% for Placebo.

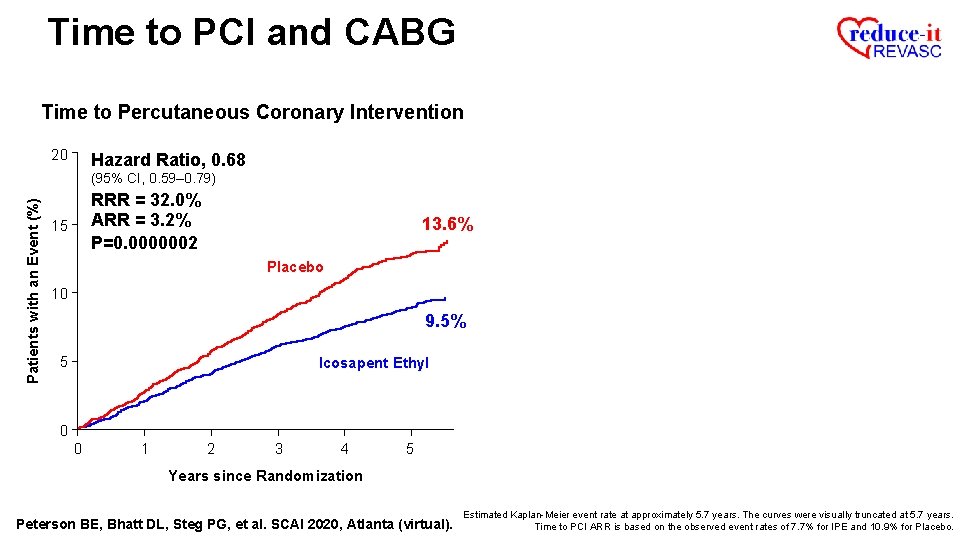
Time to PCI and CABG Time to Percutaneous Coronary Intervention 20 Hazard Ratio, 0. 68 Patients with an Event (%) (95% CI, 0. 59– 0. 79) RRR = 32. 0% ARR = 3. 2% P=0. 0000002 15 13. 6% Placebo 10 9. 5% 5 Icosapent Ethyl 0 0 1 2 3 4 5 Years since Randomization Peterson BE, Bhatt DL, Steg PG, et al. SCAI 2020, Atlanta (virtual). Estimated Kaplan-Meier event rate at approximately 5. 7 years. The curves were visually truncated at 5. 7 years. Time to PCI ARR is based on the observed event rates of 7. 7% for IPE and 10. 9% for Placebo.

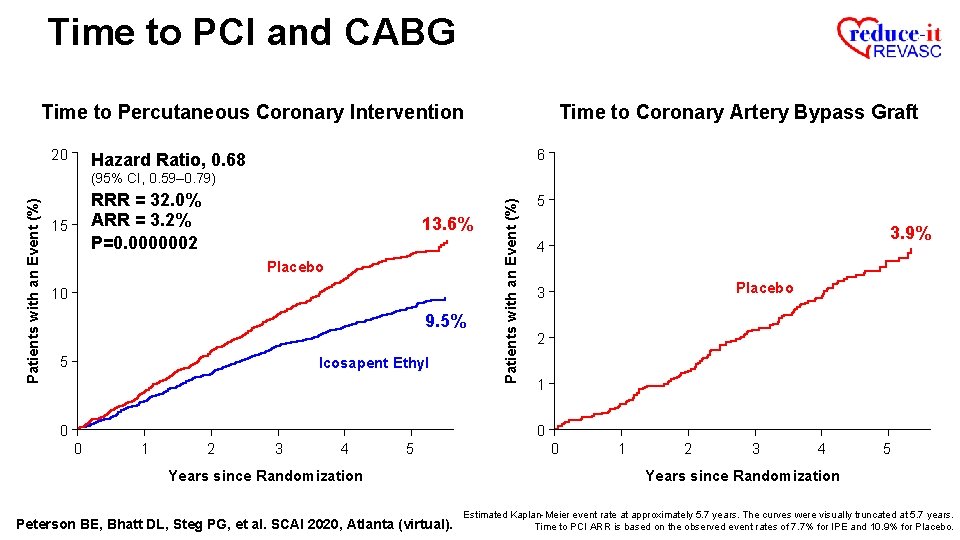
Time to PCI and CABG Time to Percutaneous Coronary Intervention 20 Time to Coronary Artery Bypass Graft 6 Hazard Ratio, 0. 68 RRR = 32. 0% ARR = 3. 2% P=0. 0000002 15 13. 6% Placebo 10 9. 5% 5 Icosapent Ethyl Patients with an Event (%) (95% CI, 0. 59– 0. 79) 5 3. 9% 4 Placebo 3 2 1 0 0 0 1 2 3 4 5 Years since Randomization Peterson BE, Bhatt DL, Steg PG, et al. SCAI 2020, Atlanta (virtual). 0 1 2 3 4 5 Years since Randomization Estimated Kaplan-Meier event rate at approximately 5. 7 years. The curves were visually truncated at 5. 7 years. Time to PCI ARR is based on the observed event rates of 7. 7% for IPE and 10. 9% for Placebo.

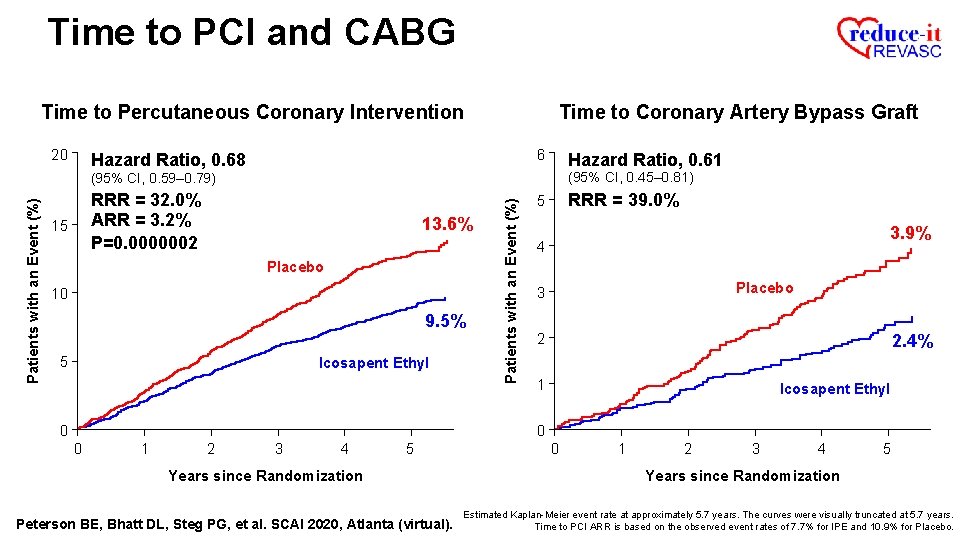
Time to PCI and CABG Time to Percutaneous Coronary Intervention 20 Time to Coronary Artery Bypass Graft 6 Hazard Ratio, 0. 68 Hazard Ratio, 0. 61 (95% CI, 0. 45– 0. 81) RRR = 32. 0% ARR = 3. 2% P=0. 0000002 15 13. 6% Placebo 10 9. 5% 5 Icosapent Ethyl Patients with an Event (%) (95% CI, 0. 59– 0. 79) 5 3. 9% 4 Placebo 3 2 2. 4% 1 Icosapent Ethyl 0 0 0 1 2 3 4 5 Years since Randomization Peterson BE, Bhatt DL, Steg PG, et al. SCAI 2020, Atlanta (virtual). 0 1 2 3 4 5 Years since Randomization Estimated Kaplan-Meier event rate at approximately 5. 7 years. The curves were visually truncated at 5. 7 years. Time to PCI ARR is based on the observed event rates of 7. 7% for IPE and 10. 9% for Placebo.

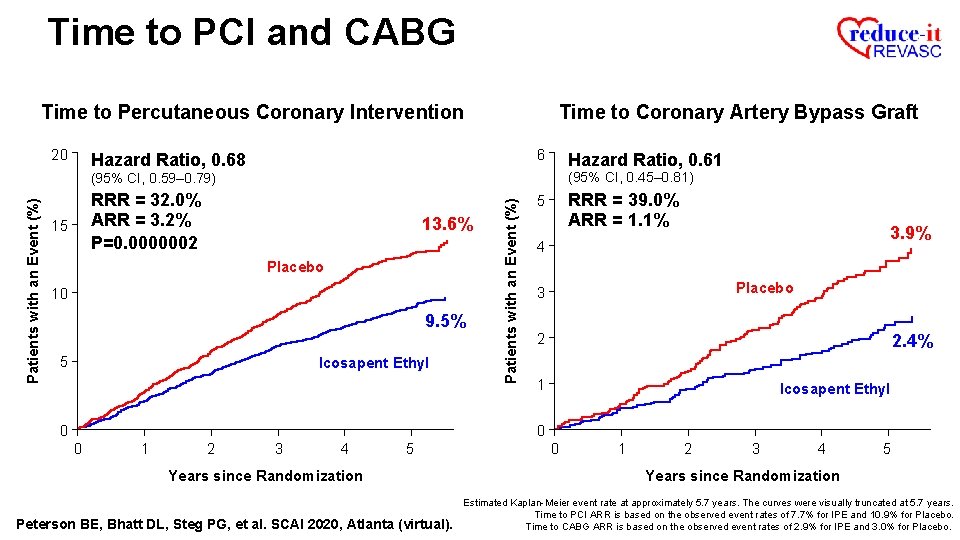
Time to PCI and CABG Time to Percutaneous Coronary Intervention 20 Time to Coronary Artery Bypass Graft 6 Hazard Ratio, 0. 68 Hazard Ratio, 0. 61 (95% CI, 0. 45– 0. 81) RRR = 32. 0% ARR = 3. 2% P=0. 0000002 15 13. 6% Placebo 10 9. 5% 5 Icosapent Ethyl Patients with an Event (%) (95% CI, 0. 59– 0. 79) RRR = 39. 0% 5 3. 9% 4 Placebo 3 2 2. 4% 1 Icosapent Ethyl 0 0 0 1 2 3 4 5 Years since Randomization Peterson BE, Bhatt DL, Steg PG, et al. SCAI 2020, Atlanta (virtual). 0 1 2 3 4 5 Years since Randomization Estimated Kaplan-Meier event rate at approximately 5. 7 years. The curves were visually truncated at 5. 7 years. Time to PCI ARR is based on the observed event rates of 7. 7% for IPE and 10. 9% for Placebo.

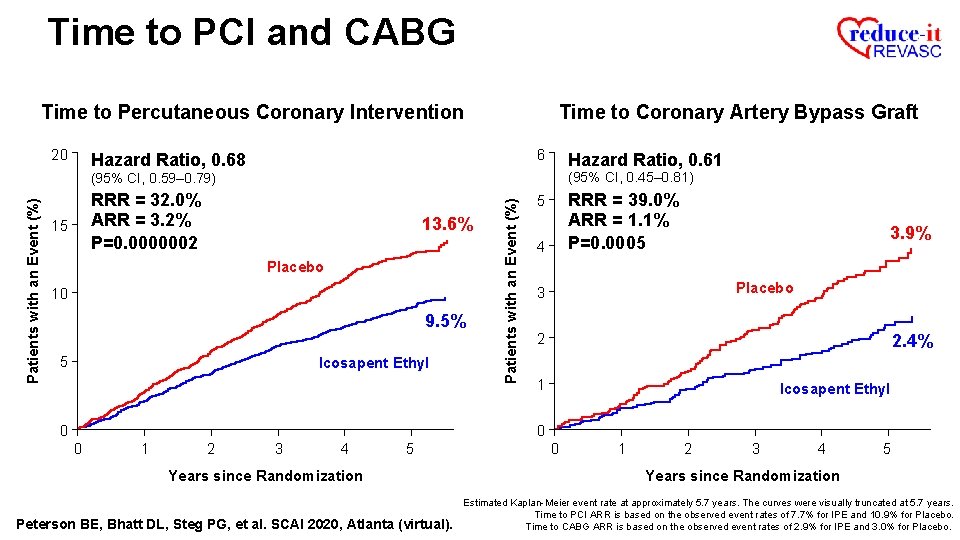
Time to PCI and CABG Time to Percutaneous Coronary Intervention 20 Time to Coronary Artery Bypass Graft 6 Hazard Ratio, 0. 68 Hazard Ratio, 0. 61 (95% CI, 0. 45– 0. 81) RRR = 32. 0% ARR = 3. 2% P=0. 0000002 15 13. 6% Placebo 10 9. 5% 5 Icosapent Ethyl Patients with an Event (%) (95% CI, 0. 59– 0. 79) RRR = 39. 0% ARR = 1. 1% 5 3. 9% 4 Placebo 3 2 2. 4% 1 Icosapent Ethyl 0 0 0 1 2 3 4 5 Years since Randomization Peterson BE, Bhatt DL, Steg PG, et al. SCAI 2020, Atlanta (virtual). 0 1 2 3 4 5 Years since Randomization Estimated Kaplan-Meier event rate at approximately 5. 7 years. The curves were visually truncated at 5. 7 years. Time to PCI ARR is based on the observed event rates of 7. 7% for IPE and 10. 9% for Placebo. Time to CABG ARR is based on the observed event rates of 2. 9% for IPE and 3. 0% for Placebo.

Time to PCI and CABG Time to Percutaneous Coronary Intervention 20 Time to Coronary Artery Bypass Graft 6 Hazard Ratio, 0. 68 Hazard Ratio, 0. 61 (95% CI, 0. 45– 0. 81) RRR = 32. 0% ARR = 3. 2% P=0. 0000002 15 13. 6% Placebo 10 9. 5% 5 Icosapent Ethyl Patients with an Event (%) (95% CI, 0. 59– 0. 79) RRR = 39. 0% ARR = 1. 1% P=0. 0005 5 4 3. 9% Placebo 3 2 2. 4% 1 Icosapent Ethyl 0 0 0 1 2 3 4 5 Years since Randomization Peterson BE, Bhatt DL, Steg PG, et al. SCAI 2020, Atlanta (virtual). 0 1 2 3 4 5 Years since Randomization Estimated Kaplan-Meier event rate at approximately 5. 7 years. The curves were visually truncated at 5. 7 years. Time to PCI ARR is based on the observed event rates of 7. 7% for IPE and 10. 9% for Placebo. Time to CABG ARR is based on the observed event rates of 2. 9% for IPE and 3. 0% for Placebo.

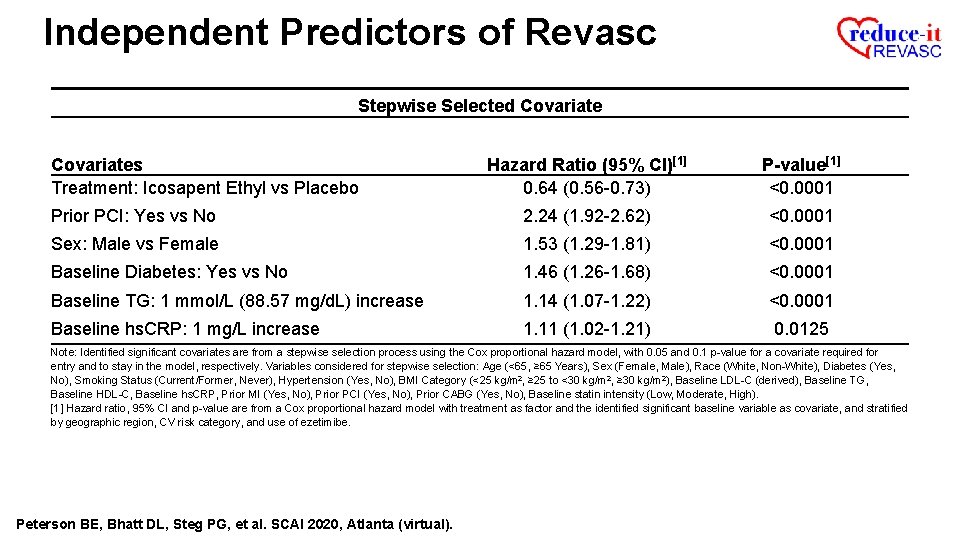
Independent Predictors of Revasc Stepwise Selected Covariates Treatment: Icosapent Ethyl vs Placebo Hazard Ratio (95% CI)[1] 0. 64 (0. 56 -0. 73) P-value[1] <0. 0001 Prior PCI: Yes vs No 2. 24 (1. 92 -2. 62) <0. 0001 Sex: Male vs Female 1. 53 (1. 29 -1. 81) <0. 0001 Baseline Diabetes: Yes vs No 1. 46 (1. 26 -1. 68) <0. 0001 Baseline TG: 1 mmol/L (88. 57 mg/d. L) increase 1. 14 (1. 07 -1. 22) <0. 0001 Baseline hs. CRP: 1 mg/L increase 1. 11 (1. 02 -1. 21) 0. 0125 Note: Identified significant covariates are from a stepwise selection process using the Cox proportional hazard model, with 0. 05 and 0. 1 p-value for a covariate required for entry and to stay in the model, respectively. Variables considered for stepwise selection: Age (<65, ≥ 65 Years), Sex (Female, Male), Race (White, Non-White), Diabetes (Yes, No), Smoking Status (Current/Former, Never), Hypertension (Yes, No), BMI Category (<25 kg/m 2, ≥ 25 to <30 kg/m 2, ≥ 30 kg/m 2), Baseline LDL-C (derived), Baseline TG, Baseline HDL-C, Baseline hs. CRP, Prior MI (Yes, No), Prior PCI (Yes, No), Prior CABG (Yes, No), Baseline statin intensity (Low, Moderate, High). [1] Hazard ratio, 95% CI and p-value are from a Cox proportional hazard model with treatment as factor and the identified significant baseline variable as covariate, and stratified by geographic region, CV risk category, and use of ezetimibe. Peterson BE, Bhatt DL, Steg PG, et al. SCAI 2020, Atlanta (virtual).

First and Subsequent Revasc Events Number of Primary Composite Endpoint Events Full Dataset RR 0. 64 36% Reduction in Total Revasc (95% CI, 0. 56 -0. 74) 800 731 57 600 130 P=0. 000005 ≥ 3 Events RR 0. 51 (95% CI, 0. 27 -0. 99) P=0. 05 2 nd Events HR 0. 49 (95% CI, 0. 37 -0. 66) P=0. 000003 400 544 200 1 st Events HR 0. 67 (95% CI, 0. 58 -0. 76) P=0. 00001 No. of Fewer Cases 473 -258 31 66 -26 -64 376 705 -168 0 Icosapent Ethyl [N=4089] Placebo [N=4090] Full Dataset Event No. 1 st Peterson BE, Bhatt DL, Steg PG, et al. SCAI 2020, Atlanta (virtual). 2 nd ≥ 3 Note: WLW method for the 1 st events and 2 nd events categories; Negative binomial model for ≥ 3 rd events and overall treatment comparison.

Limitations Coronary revascularization as an endpoint can be considered subjective Peterson BE, Bhatt DL, Steg PG, et al. SCAI 2020, Atlanta (virtual).

Limitations Coronary revascularization as an endpoint can be considered subjective • 537 (58. 4%) of the first revascularization events were urgent or emergent, suggestive largely of acute coronary syndromes Peterson BE, Bhatt DL, Steg PG, et al. SCAI 2020, Atlanta (virtual).

Limitations Coronary revascularization as an endpoint can be considered subjective • 537 (58. 4%) of the first revascularization events were urgent or emergent, suggestive largely of acute coronary syndromes • Each subtype of revascularization was similarly and statistically reduced Peterson BE, Bhatt DL, Steg PG, et al. SCAI 2020, Atlanta (virtual).

Limitations Coronary revascularization as an endpoint can be considered subjective • 537 (58. 4%) of the first revascularization events were urgent or emergent, suggestive largely of acute coronary syndromes • Each subtype of revascularization was similarly and statistically reduced • Revascularization endpoints were adjudicated by an independent, blinded clinical endpoint committee evaluating data from a randomized, double-blind, placebo-controlled trial – therefore, no risk of bias Peterson BE, Bhatt DL, Steg PG, et al. SCAI 2020, Atlanta (virtual).

Conclusions Compared with placebo, icosapent ethyl 4 g/day significantly reduced first and total revascularization events by 34% and 36%, respectively Peterson BE, Bhatt DL, Steg PG, et al. SCAI 2020, Atlanta (virtual).

Conclusions Compared with placebo, icosapent ethyl 4 g/day significantly reduced first and total revascularization events by 34% and 36%, respectively This reduction was consistent across urgent, emergent, and elective revascularization categories, as well as for PCI and CABG individually Peterson BE, Bhatt DL, Steg PG, et al. SCAI 2020, Atlanta (virtual).

Conclusions Compared with placebo, icosapent ethyl 4 g/day significantly reduced first and total revascularization events by 34% and 36%, respectively This reduction was consistent across urgent, emergent, and elective revascularization categories, as well as for PCI and CABG individually To the best of our knowledge, this is the first non-LDL cholesterol intervention in a major randomized trial in which statin-treated patients underwent fewer CABG surgeries Peterson BE, Bhatt DL, Steg PG, et al. SCAI 2020, Atlanta (virtual).

Conclusions Compared with placebo, icosapent ethyl 4 g/day significantly reduced first and total revascularization events by 34% and 36%, respectively This reduction was consistent across urgent, emergent, and elective revascularization categories, as well as for PCI and CABG individually To the best of our knowledge, this is the first non-LDL cholesterol intervention in a major randomized trial in which statin-treated patients underwent fewer CABG surgeries These data highlight the substantial impact of icosapent ethyl on the underlying atherothrombotic burden in the at-risk REDUCE-IT population Peterson BE, Bhatt DL, Steg PG, et al. SCAI 2020, Atlanta (virtual).

We thank the investigators, the study coordinators, and especially the 8, 179 patients in REDUCE-IT! L-MARC

Slides available for free download: www. scai. org
 Best treatment for hypertriglyceridemia
Best treatment for hypertriglyceridemia Familial hypertriglyceridemia
Familial hypertriglyceridemia Dealing with challenging patients
Dealing with challenging patients Patients rights charter
Patients rights charter Non-infective
Non-infective Some patients shout in pain while ______ an injection.
Some patients shout in pain while ______ an injection. Handling patients
Handling patients Daily intentional nurse leader rounding on patients
Daily intentional nurse leader rounding on patients What are the 7 patient rights?
What are the 7 patient rights? Classification of soft palate
Classification of soft palate Semi prone position in nursing
Semi prone position in nursing Chapter 36 patients with special challenges
Chapter 36 patients with special challenges Jack in the box
Jack in the box Urgent move emt
Urgent move emt Define inadequate
Define inadequate Management of patients with neurologic trauma
Management of patients with neurologic trauma Lifting and transporting patients
Lifting and transporting patients Malaysian safety goals
Malaysian safety goals Moving and handling dementia patients
Moving and handling dementia patients Rostering patients
Rostering patients Dr carlson advises his depressed patients
Dr carlson advises his depressed patients Leischker
Leischker Prevention of vte in nonorthopedic surgical patients
Prevention of vte in nonorthopedic surgical patients Periodontal therapy in female patients
Periodontal therapy in female patients How does nurse ratched manipulate the patients
How does nurse ratched manipulate the patients Chapter 58 care of patients with liver problems
Chapter 58 care of patients with liver problems Ems lifting techniques
Ems lifting techniques Hygiene fundamentals of nursing
Hygiene fundamentals of nursing Cataracts ncp
Cataracts ncp Rocking chair therapy for dementia patients
Rocking chair therapy for dementia patients Nursing care of male patients with genitourinary disorders
Nursing care of male patients with genitourinary disorders Leader rounding on patients
Leader rounding on patients Tena shampoo cap
Tena shampoo cap Lifting and moving patient in bed
Lifting and moving patient in bed Daily nutritional requirements chart pdf
Daily nutritional requirements chart pdf Chapter 8 lifting and moving patients
Chapter 8 lifting and moving patients The factors of care that patients can expect to receive
The factors of care that patients can expect to receive Admission of a patient
Admission of a patient Ventriculostomy care
Ventriculostomy care