The concept of essential drugs and the WHO















- Slides: 26

The concept of essential drugs and the WHO Model List of Essential Medicines Hans V. Hogerzeil, MD, Ph. D, FRCP Edin Department of Essential Drugs and Medicines Policy World Health Organization, March 2004

Essential medicines n The concept of essential medicines A limited range of carefully selected essential medicines leads to better health care, better drug management, and lower costs n Definition of essential medicines Essential medicines are those that satisfy the priority health care needs of the population (Report to WHO Executive Board, January 2002) EDC/Model List 2 Department of Essential Drugs and Medicines Policy

History of the WHO Model List of Essential Drugs n 1977 First Model list published, ± 200 active substances n List is revised every two years by WHO Expert Committee n 2002 Revised procedures approved by WHO n Last revision (April 2003) contains 315 active substances The first list was a major breakthrough in the history of medicine, pharmacy and public health Médecins sans Frontières, 2000 EDC/Model List 3 Department of Essential Drugs and Medicines Policy

Full description of essential drugs (Expert Committee Report, April 2002) Definition: Essential medicines are those that satisfy the priority health care needs of the population Selection criteria: Essential medicines are selected with due regard to disease prevalence, evidence on efficacy and safety, and comparative cost-effectiveness Purpose: Essential medicines are intended to be available within the context of functioning health systems at all times, in adequate amounts, in the appropriate dosage forms, with assured quality, and at a price the individual and the community can afford. Implementation: The implementation of the concept of essential medicines is intended to be flexible and adaptable to many different situations; exactly which medicines are regarded as essential remains a national responsibility. EDC/Model List 4 Department of Essential Drugs and Medicines Policy

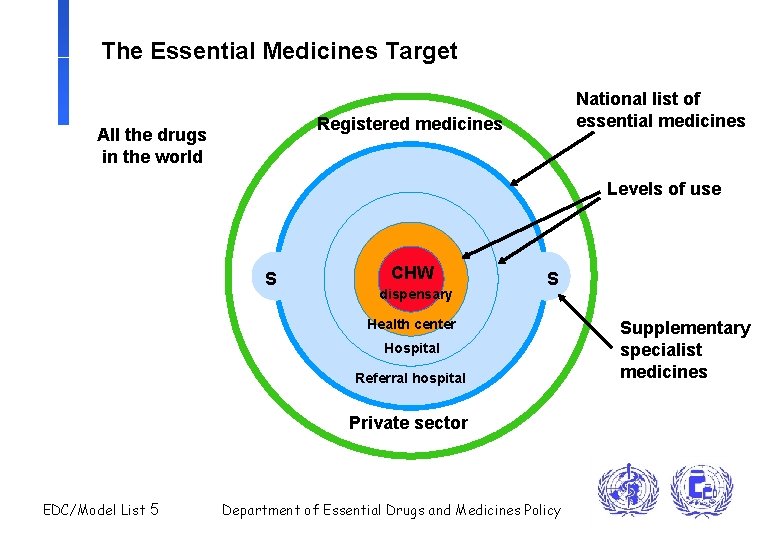
The Essential Medicines Target National list of essential medicines Registered medicines All the drugs in the world Levels of use S CHW dispensary S Health center Hospital Referral hospital Private sector EDC/Model List 5 Department of Essential Drugs and Medicines Policy Supplementary specialist medicines

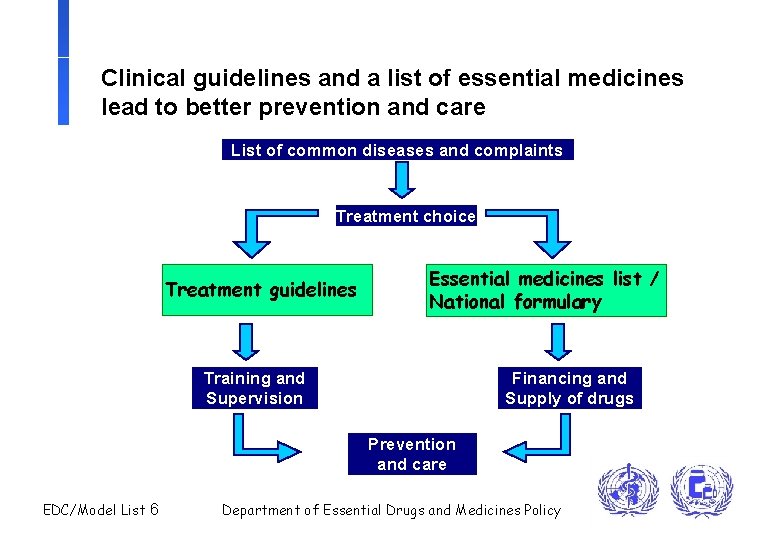
Clinical guidelines and a list of essential medicines lead to better prevention and care List of common diseases and complaints Treatment choice Treatment guidelines Essential medicines list / National formulary Training and Supervision Financing and Supply of drugs Prevention and care EDC/Model List 6 Department of Essential Drugs and Medicines Policy

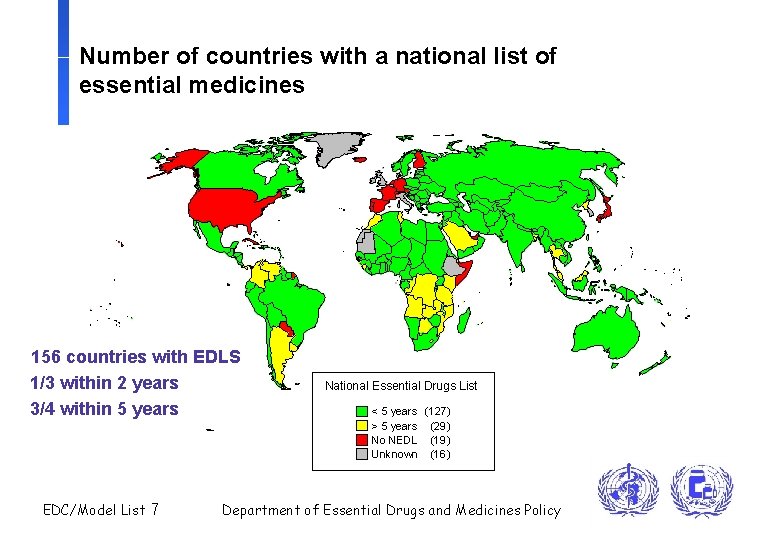
Number of countries with a national list of essential medicines 156 countries with EDLS 1/3 within 2 years 3/4 within 5 years EDC/Model List 7 National Essential Drugs List < 5 years (127) > 5 years (29) No NEDL (19) Unknown (16) Department of Essential Drugs and Medicines Policy

Use of the WHO Model List of Essential Drugs n 156 countries have a national list of essential drugs, of which 81% have been updated in the last 5 years n Major international agencies (UNICEF, UNHCR, IDA) base their catalogue on the WHO Model List n Sub-sets: UN list of recommended essential drugs for emergency relief (85 drugs); interagency New Emergency Health Kit (55 drugs for 10, 000 consultations) n Normative tools: WHO Model Formulary, International Pharmacopoea, Basic Quality Tests, and development of reference standards follow the WHO Model List EDC/Model List 8 Department of Essential Drugs and Medicines Policy

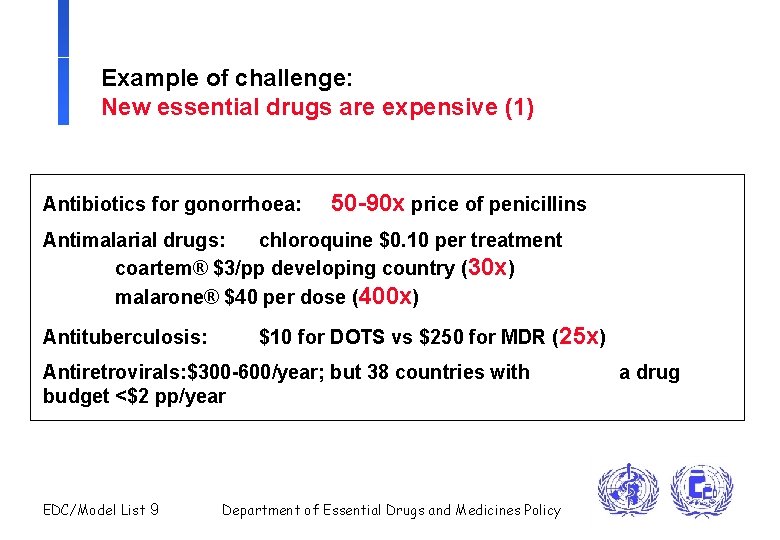
Example of challenge: New essential drugs are expensive (1) Antibiotics for gonorrhoea: 50 -90 x price of penicillins Antimalarial drugs: chloroquine $0. 10 per treatment coartem® $3/pp developing country (30 x) malarone® $40 per dose (400 x) Antituberculosis: $10 for DOTS vs $250 for MDR (25 x) Antiretrovirals: $300 -600/year; but 38 countries with budget <$2 pp/year EDC/Model List 9 Department of Essential Drugs and Medicines Policy a drug

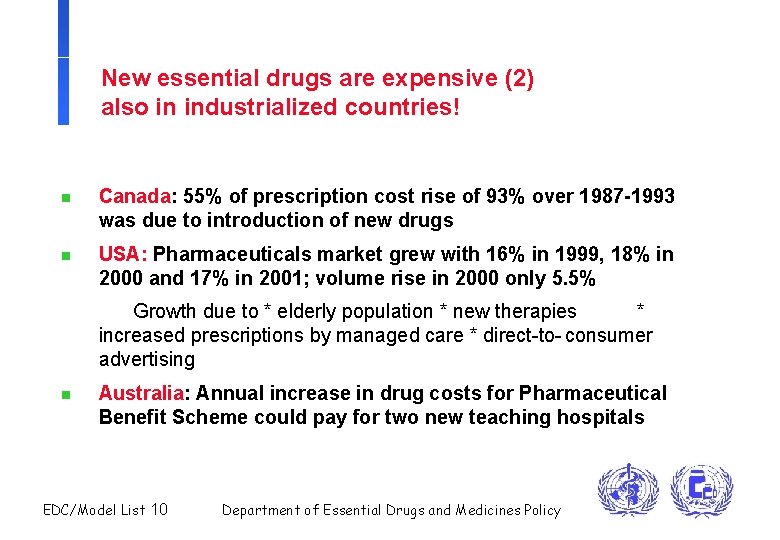
New essential drugs are expensive (2) also in industrialized countries! n Canada: 55% of prescription cost rise of 93% over 1987 -1993 was due to introduction of new drugs n USA: Pharmaceuticals market grew with 16% in 1999, 18% in 2000 and 17% in 2001; volume rise in 2000 only 5. 5% Growth due to * elderly population * new therapies * increased prescriptions by managed care * direct-to- consumer advertising n Australia: Annual increase in drug costs for Pharmaceutical Benefit Scheme could pay for two new teaching hospitals EDC/Model List 10 Department of Essential Drugs and Medicines Policy

The WHO Model List of Essential Medicines is a model process, model product and public health tool n n n Independent Membership of the Committee, careful consideration of conflict of interest Transparent process, standard application, review Link to evidence-based treatment recommendations, in accordance with WHO Recommended Process for Developing Clinical Practice Guidelines Systematic review of comparative efficacy, safety and costeffectiveness, and review of public health relevance Rapid dissemination, electronic access Regular review EDC/Model List 11 Department of Essential Drugs and Medicines Policy

Model process (1): Seven steps to get a new medicine on the WHO Model List of Essential Drugs 1. Identification of public-health need for a medicine 2. Development of the medicine; phase I - III trials 3. Regulatory approval in a number of countries > Effective and safe medicine on the market 4. More experience under different field circumstances; post-marketing surveillance 5. Price indication for public sector use 6. Review by WHO disease programme; define comparative effectiveness and safety in real-life situations, comparative costeffectiveness and public health relevance > Medicine included in WHO treatment guideline 7. Submission to WHO Expert Committee on Essential Drugs > Medicine included in WHO Model List EDC/Model List 12 Department of Essential Drugs and Medicines Policy

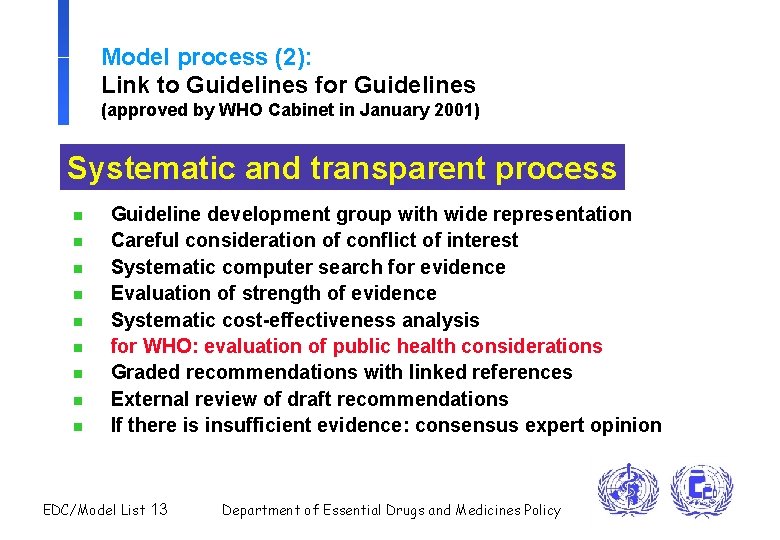
Model process (2): Link to Guidelines for Guidelines (approved by WHO Cabinet in January 2001) Systematic and transparent process n n n n n Guideline development group with wide representation Careful consideration of conflict of interest Systematic computer search for evidence Evaluation of strength of evidence Systematic cost-effectiveness analysis for WHO: evaluation of public health considerations Graded recommendations with linked references External review of draft recommendations If there is insufficient evidence: consensus expert opinion EDC/Model List 13 Department of Essential Drugs and Medicines Policy

Model process (3): Steps in review of applications to the Model List 1 Summary of application posted on WHO Medicines web site 2 Specialist assessment of comparative efficacy, safety and cost-effectiveness 3 Review of assessments by Expert Committee member (“presenter”); formulation of draft recommendation 4 Review of draft recommendation by relevant Expert Advisory Panel members; and posted on WHO Medicines web site 5 Review by presenter, prepares final draft recommendation 6 Discussion, recommendation by Expert Committee EDC/Model List 14 Department of Essential Drugs and Medicines Policy

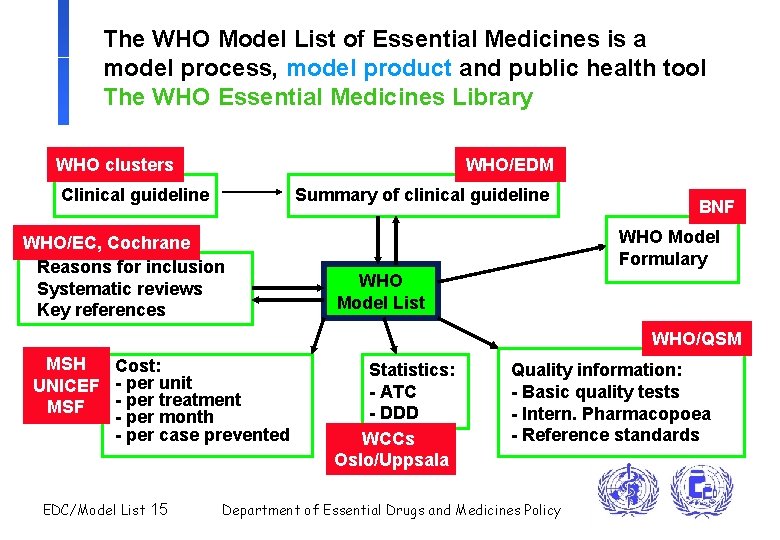
The WHO Model List of Essential Medicines is a model process, model product and public health tool The WHO Essential Medicines Library WHO clusters WHO/EDM Clinical guideline Summary of clinical guideline WHO/EC, Cochrane Reasons for inclusion Systematic reviews Key references BNF WHO Model Formulary WHO Model List WHO/QSM MSH Cost: UNICEF - per unit - per treatment MSF - per month - per case prevented EDC/Model List 15 Statistics: - ATC - DDD WCCs Oslo/Uppsala Quality information: - Basic quality tests - Intern. Pharmacopoea - Reference standards Department of Essential Drugs and Medicines Policy

Example of a link: chloramphenicol Chloramphenicol is recommended for the following indications: Injectable: Severe pneumonia in children*, severe asthmapneumonia*, brain abscess*, meningitis in children with ARI*, epiglottitis*, granuloma inguinale*, mastoiditis*, meningitis (various kinds), obstetrics, septicaemia* Oral: severe pneumonia in children, asthma pneumonia*, meningitis in children*, cholera*, louse borne typhus*, measles pneumonia, meningitis (empirical and meningococcal), abortion care, plague*, relapsing fever*, Rickettsia, typhoid fever* *recommended as alternative drug EDC/Model List 16 Department of Essential Drugs and Medicines Policy

WHO Model Formulary n n n n First edition issued in 2002; (SFr 40, SFr 20) Web version as PDF file and searchable database CD-ROM (searchable and downloadable) Translations ongoing in Arabic, Chinese, French, Russian, Spanish; electronic versions only (on CD) Edition 2004 follows 2003 Model List 5 -year commitment to RPS and BMJ Manual “How to develop a national formulary” EDC/Model List 17 Department of Essential Drugs and Medicines Policy

The New Emergency Health Kit 1984, 1990, 1998 Essential medicines and supplies for 10, 000 people for three months Consensus between WHO, UNICEF, UNHCR, UNFPA, Red Cross, MSF, OXFAM, missions, IDA EDC/Model List 18 Department of Essential Drugs and Medicines Policy

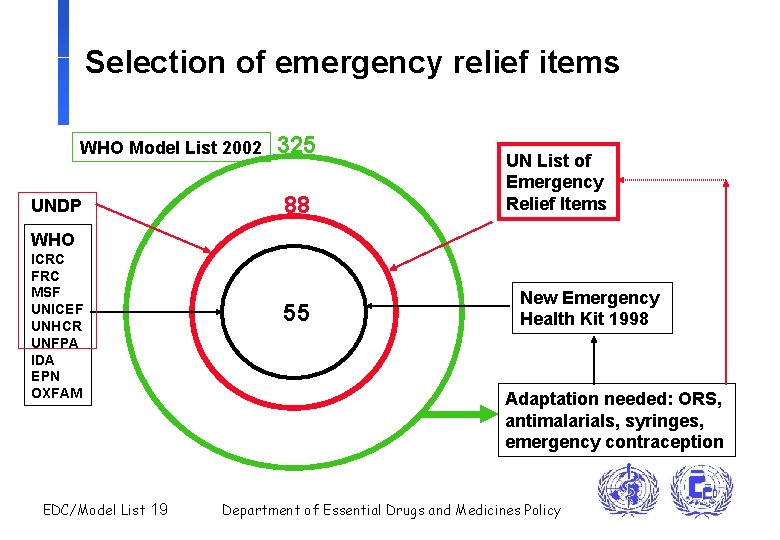
Selection of emergency relief items WHO Model List 2002 UNDP 325 88 UN List of Emergency Relief Items WHO ICRC FRC MSF UNICEF UNHCR UNFPA IDA EPN OXFAM EDC/Model List 19 55 New Emergency Health Kit 1998 Adaptation needed: ORS, antimalarials, syringes, emergency contraception Department of Essential Drugs and Medicines Policy

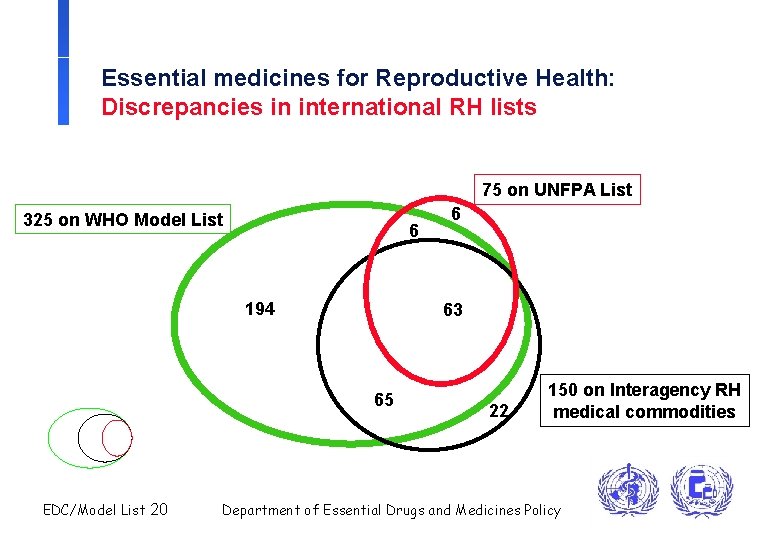
Essential medicines for Reproductive Health: Discrepancies in international RH lists 75 on UNFPA List 325 on WHO Model List 6 194 63 65 EDC/Model List 20 6 22 150 on Interagency RH medical commodities Department of Essential Drugs and Medicines Policy

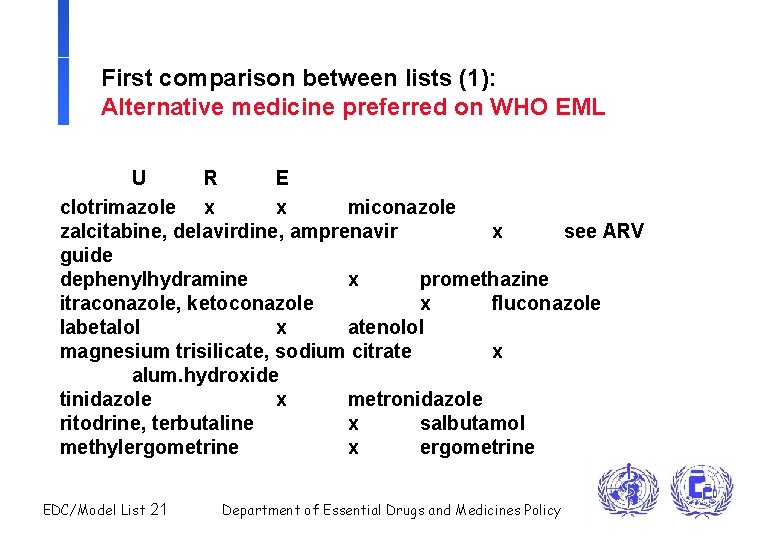
First comparison between lists (1): Alternative medicine preferred on WHO EML U R E clotrimazole x x miconazole zalcitabine, delavirdine, amprenavir x see ARV guide dephenylhydramine x promethazine itraconazole, ketoconazole x fluconazole labetalol x atenolol magnesium trisilicate, sodium citrate x alum. hydroxide tinidazole x metronidazole ritodrine, terbutaline x salbutamol methylergometrine x ergometrine EDC/Model List 21 Department of Essential Drugs and Medicines Policy

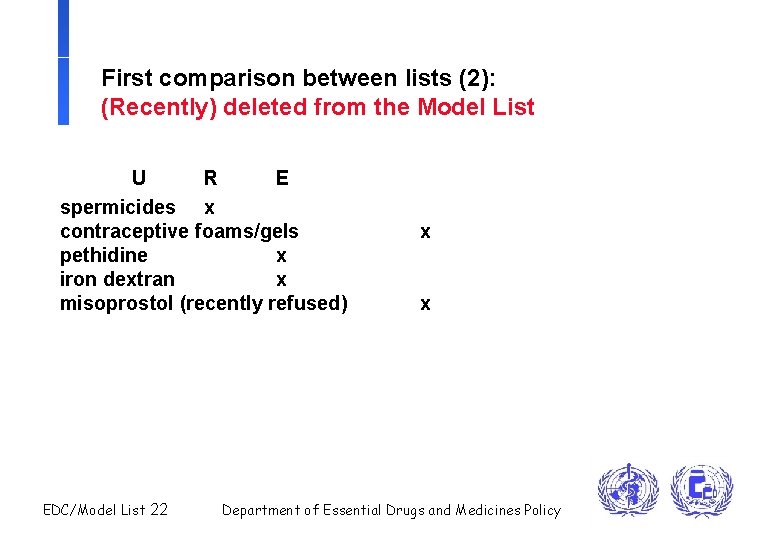
First comparison between lists (2): (Recently) deleted from the Model List U R E spermicides x contraceptive foams/gels pethidine x iron dextran x misoprostol (recently refused) EDC/Model List 22 x x Department of Essential Drugs and Medicines Policy

To include or not to include? Suggested for systematic review and submission to WHO Expert Committee U R E levonorgestrel-IUDs x norethisterone enantate + valerate x oestradiol cyprionate +med. prog. acetate indometacin (tocolytic) x cefazolin (geneal surgical prophylaxis) cefixime (gonorhoea) x prostaglandins x subdermal contraceptive inplants hydralazine* x x x * Recently discussed but needs more review EDC/Model List 23 Department of Essential Drugs and Medicines Policy

The WHO Model List of Essential Medicines is a model process, model product and public health tool Main public health advocacy messages: n Essential drugs are the most cost-effective drugs for a given condition n A limited range of carefully selected medicines can cater for most health care needs n There is much waste through irrational selection and use n Access to health care is a Human Right to be progressively realized. This includes access to essential medicines n The essential medicines concept is globally applicable EDC/Model List 24 Department of Essential Drugs and Medicines Policy

Conclusion n The concept of essential medicines is a global concept n WHO clinical guidelines are the foundation for the Model List of Essential Drugs; the Model List remains a strong public health tool n The WHO Essential Medicines Library is a valuable information base for all Member States, international organisations, drugs and therapeutic committees and health insurance organisations EDC/Model List 25 Department of Essential Drugs and Medicines Policy

WHO Department of Essential Drugs and Medicines Policy www. who. int / medicines Thank you
 Concept of essential drugs
Concept of essential drugs Provision of essential drugs
Provision of essential drugs Characteristics of lipids
Characteristics of lipids Differentiate between real self and ideal self
Differentiate between real self and ideal self Pengertian marketing concept
Pengertian marketing concept Remains annotated poem
Remains annotated poem Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Ng-html
Ng-html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Glasgow thang điểm
Glasgow thang điểm Chúa sống lại
Chúa sống lại Môn thể thao bắt đầu bằng từ đua
Môn thể thao bắt đầu bằng từ đua Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tính độ biến thiên đông lượng
Công thức tính độ biến thiên đông lượng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Cách giải mật thư tọa độ
Cách giải mật thư tọa độ Làm thế nào để 102-1=99
Làm thế nào để 102-1=99 độ dài liên kết
độ dài liên kết Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thể thơ truyền thống
Thể thơ truyền thống Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng nó xinh thế
Cái miệng nó xinh thế Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau


















































