The CAPi TA ILI study Monitoring of influenzalike












- Slides: 26

The CAPi. TA ILI study Monitoring of influenza-like symptoms among eldery, an observational study” Marieke Bolkenbaas, MD, Ph. D candidate Julius Center for Health Sciences and Primary Care, UMC Utrecht, The Netherlands

Overview • Introduction and background • Design and practical aspects • Results

Introduction and background • Influenza, influenza-like illness (ILI) and lower respiratory tract infections (LRTI) are common and represent an important health care problem worldwide ü Influenza: global annual attack rate 5 -10% in adults, 20 -30% in children; 3 -5 million severe cases, 250 -300 k deaths 1 ü 3. 2 million people die of LRTI worldwide each year (ranking no. 4 in the WHO top 10 of death causes)2 ü At highest risk for a worse outcome are children and elderly 3

Introduction and background • Respiratory viruses and pneumococci are often found simultaneously during respiratory tract infections • It is widely assumed that respiratory viruses like influenza facilitate secondary bacterial infection of the (lower) respiratory tract 4

Introduction and background • Viral and bacterial respiratory tract infections often show a similar complex of symtoms and cannot easily be distinguished based on clinical signs • Unknown is to what extent pneumococci play a role in the symptomatology of milder respiratory tract infections • ≈80% of Dutch elderly receives annual flu vaccination 5 • Pneumococcal vaccination is not (yet) recommended for 65+ year olds in The Netherlands 6

Monitoring of influenza-like symptoms • Two ways of ILI monitoring in The Netherlands ü GP surveillance (sentinel network) • Diagnosis by GP • Max 2 nasopharynx/throatswabs each week for typing ü Internet-based monitoring • Since 2003/2004, Great Influenza Study 7 • Over 50, 000 participants, most participating >1 season • Self-reported symptoms of ILI and common cold • Weekly questionnaire • Anyone can registrate as a participant

Great Influenza Study

Internet surveillance vs GP surveillance • ILI results from both systems correlated well • GIS detected weekly ILI incidence trends 1 week before GP sentinel network • Only 1 in 6 patients with ILI symptoms visits GP, in elderly 1 in 4 • Elderly and very young relatively underrepresented in GIS • Influenza vaccination % in elderly in GIS comparable with general population

Introduction and background • CAPi. TA trial 2008 - 2013 • Double-blind randomized controlled trial • 84, 496 participants of 65+ year old • Pneumococcal vaccine (PCV 13) or placebo • 56 hospitals and 2, 200 GPs → Demonstrated effectivity of pneumococcal vaccination with PCV 13 in preventing hospitalisation or death due to VT pneumococcal CAP (Bonten et al, NEJM, accepted)

The ILI study - design • Observational study nested in the CAPi. TA trial Aims: • Explore the effect of pneumococcal vaccination on self-reported symptoms of ILI and LRTI • Determine the incidence of ILI and self-reported symptoms of LRTI (sr. LRTI) • Determine the proportions of episodes for which a GP is consulted

The ILI study – design • Computer-based random selection of candidates • Participation during autumn/winter seasons 2010/11 and 2011/12 • Letter with instructions and login code to secured website, digital informed consent • Single time questionnaire on: comorbidity, influenza vaccination status, smoking, contact with young children • Weekly questionnaire on symptoms: type, duration, perceived severity, GP visit, reasons to visit, treatment by GP, use of OTC drugs • Missing weekly questionnaires allowed • Not possible to fill in older questionnaires

The ILI study – practical aspects • Help and information: ü Telephone ü E-mail ü Website ü Paper user manual ü Weekly reminders with hyperlink

The ILI study – practical aspects

ILI study - definitions • ILI - Criteria European Centre for Disease Control (ECDC): ü Sudden onset of symptoms AND ü At least one of the following symptoms: fever or feverishness, malaise, headache, myalgia AND ü At least one respiratory symptom: cough, sore throat or shortness of breath • Self reported (possible) LRTI: ü Acute cough or acute worsening of cough ≥ 3 days AND ü At least one of the following symptoms: fever ≥ 38°C, shortness of breath, wheezing, chest pain or sputum production

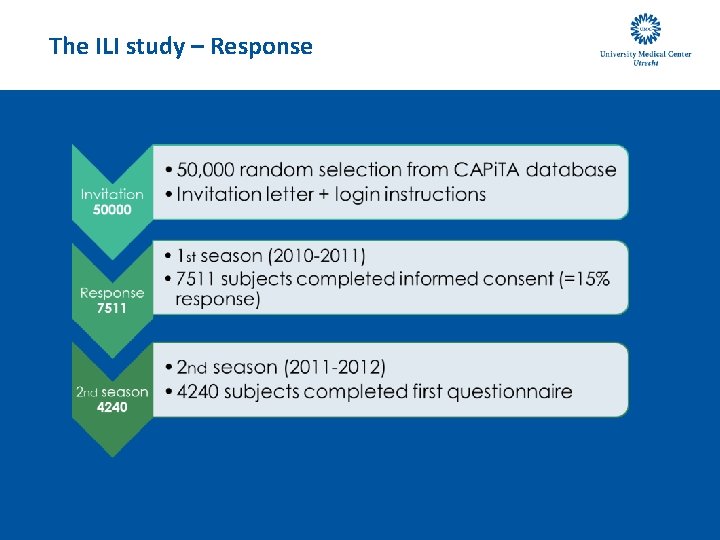
The ILI study – Response

The ILI study – Response • 2010 -2011 • 2011 -2012 • N=7511 • N=4240 • No. of questionnaires: 95. 954 • No of questionnaires: 72. 589 • Median no. of questionnaires: 15 • Median no. of questionnaires: 20 • No. single questionnaires: 548 • No. single questionnaires : 161 • Median interval questionnaires: 8 days • Median study duration: 126 days (7 -188) • Median study duration: 170 days (7 – 212)

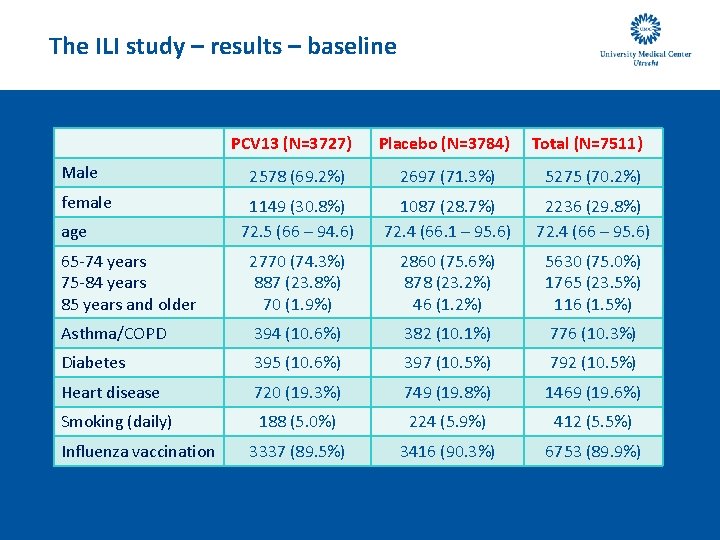
The ILI study – results – baseline PCV 13 (N=3727) Male Placebo (N=3784) Total (N=7511) 2578 (69. 2%) 2697 (71. 3%) 5275 (70. 2%) 1149 (30. 8%) 72. 5 (66 – 94. 6) 1087 (28. 7%) 72. 4 (66. 1 – 95. 6) 2236 (29. 8%) 72. 4 (66 – 95. 6) 65 -74 years 75 -84 years 85 years and older 2770 (74. 3%) 887 (23. 8%) 70 (1. 9%) 2860 (75. 6%) 878 (23. 2%) 46 (1. 2%) 5630 (75. 0%) 1765 (23. 5%) 116 (1. 5%) Asthma/COPD 394 (10. 6%) 382 (10. 1%) 776 (10. 3%) Diabetes 395 (10. 6%) 397 (10. 5%) 792 (10. 5%) Heart disease 720 (19. 3%) 749 (19. 8%) 1469 (19. 6%) Smoking (daily) 188 (5. 0%) 224 (5. 9%) 412 (5. 5%) 3337 (89. 5%) 3416 (90. 3%) 6753 (89. 9%) female age Influenza vaccination

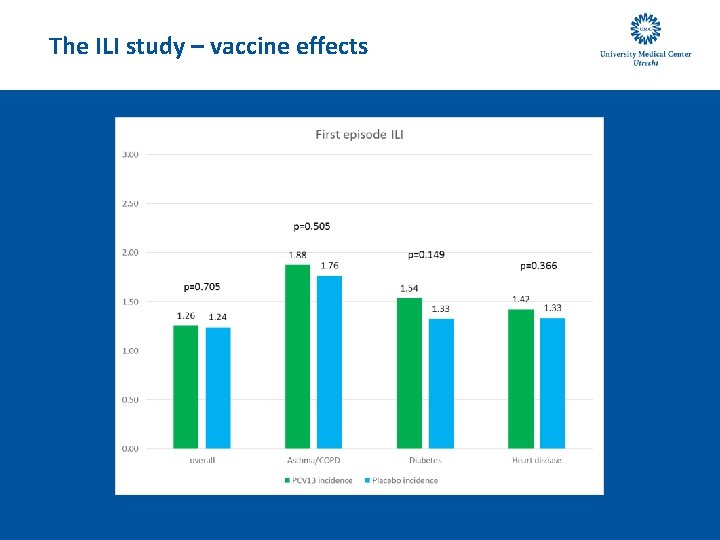
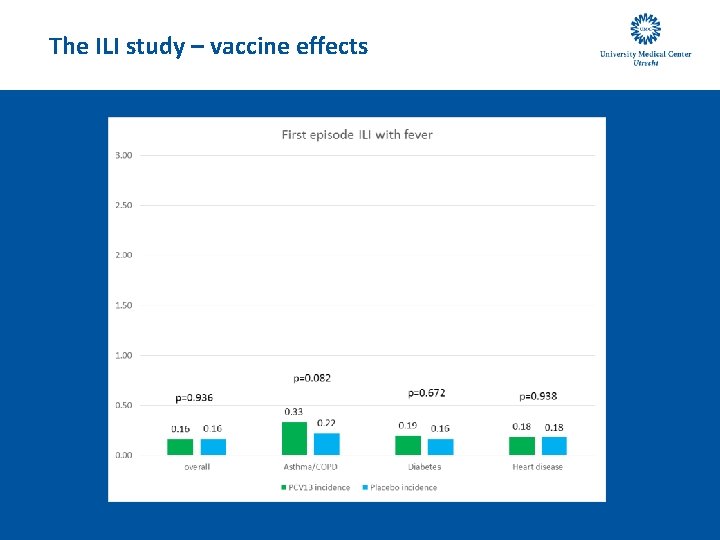
The ILI study – vaccine effects

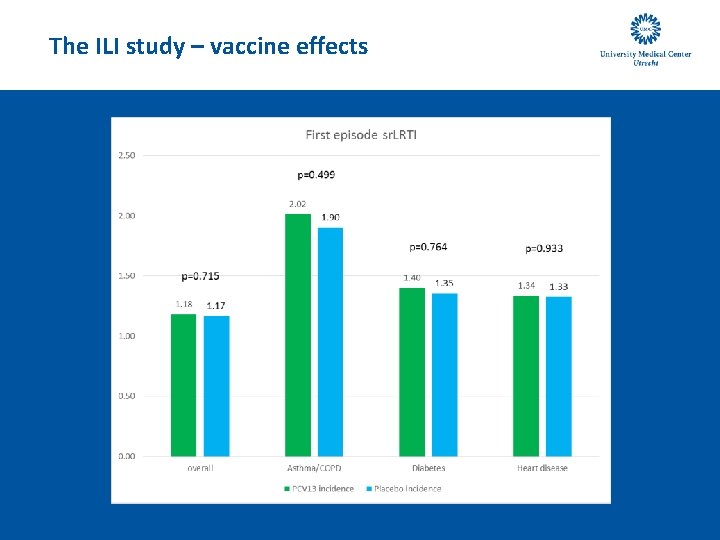
The ILI study – vaccine effects

The ILI study – vaccine effects

The ILI study – vaccine effects - conclusion • There is no statistically significant effect of PCV 13 vaccination on the incidence of ILI and self-reported LRTI symptoms

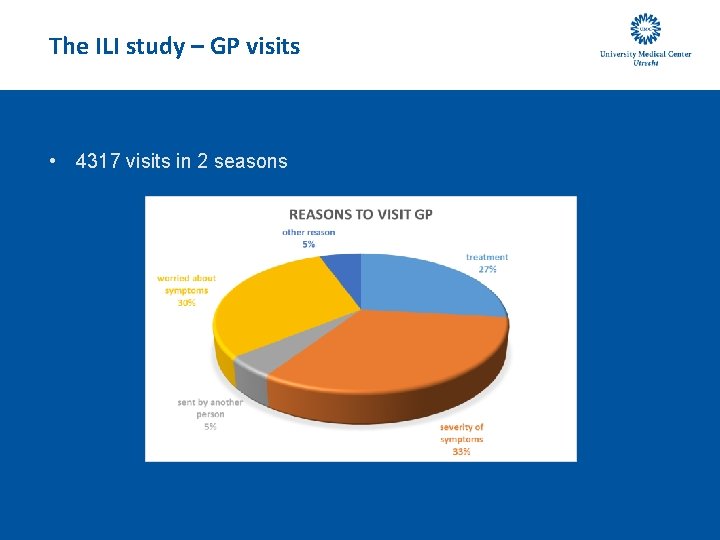
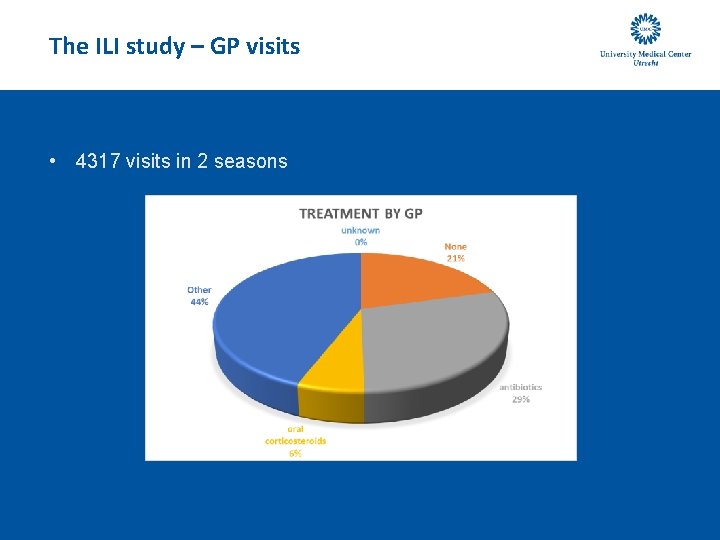
The ILI study – GP visits • 4317 visits in 2 seasons

The ILI study – GP visits • 4317 visits in 2 seasons

The ILI study – what next? • Duration of symptoms • Effects of ILI and sr. LRTI on daily activity level • Coupling of data with pneumonia/LRTI data of GP practice and hospital pneumonia data -> comprehensive view on clinical course of lower respiratory tract infections

Questions?

Reference list 1. WHO, Factsheet no. 211 Seasonal Influenza, March 2014 www. who. int 2. WHO, Factsheet no. 310 The Leading 10 Causes of Death, May 2014 www. who. int 3. Voordouw AC, Sturkenboom MC, Dieleman JP, Stijnen T, Smith DJ, van der LJ, et al. Annual revaccination against influenza and mortality risk in community-dwelling elderly persons. JAMA 2004 Nov 3; 292(17): 2089 -95. 4. Morens DM, Taubenberger JK, Fauci AS. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J Infect Dis 2008 Oct 1; 198(7): 962 -70. 5. Jansen AG, Sanders EA, Nichol AL, Van Loon AM, Hoes AW, Hak E. Decline in influenzaassociated mortality among Dutch elderly following the introduction of a nationwide vaccination program. Vaccine 2008 Oct 16; 26(44): 5567 -74 6. Health Council of the Netherlands. Pneumococcal vaccine in elderly adults and risk groups. Health Council of the Netherlands. 18 -8 -2003. 7. Friesema IH, Koppeschaar CE, Donker GA, Dijkstra F, van Noort SP, Smallenburg R, et al. Internet-based monitoring of influenza-like illness in the general population: experience of five influenza seasons in The Netherlands. Vaccine 2009 Oct 23; 27(45): 6353 -7 8. ECDC. Influenza case definitions. http: //www. ecdc. europa. eu/en/activities/surveillance/EISN/surveillance/Pages/influen za_case_definitions. aspx
 Slavko kolar breza analiza likova
Slavko kolar breza analiza likova Türkiye cumhuriyeti mührü
Türkiye cumhuriyeti mührü 1 liranın çapı
1 liranın çapı Capi system
Capi system Capi papi
Capi papi Atom çapı nasıl artar
Atom çapı nasıl artar Elektron ilgisi nasıl artar
Elektron ilgisi nasıl artar Venöz hum üfürümü
Venöz hum üfürümü Onbirgen
Onbirgen Codice deontologico infermieri capi
Codice deontologico infermieri capi Capi mode
Capi mode Capi export
Capi export Omurgalılarda sinir şeridi
Omurgalılarda sinir şeridi Open capi
Open capi Capi survey
Capi survey Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Slidetodoc
Slidetodoc Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Voi kéo gỗ như thế nào
Voi kéo gỗ như thế nào Glasgow thang điểm
Glasgow thang điểm Chúa sống lại
Chúa sống lại Các môn thể thao bắt đầu bằng từ đua
Các môn thể thao bắt đầu bằng từ đua Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tính thế năng
Công thức tính thế năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ

















































