th 5 edition NTP MANUAL OF PROCEDURES Case














- Slides: 62

th 5 edition NTP MANUAL OF PROCEDURES Case Holding

Case Holding Set of procedures that ensure patients complete their treatment • assignment of the appropriate treatment regimen • supervised drug intake • support to patients • monitoring response to treatment through follow-up DSSM

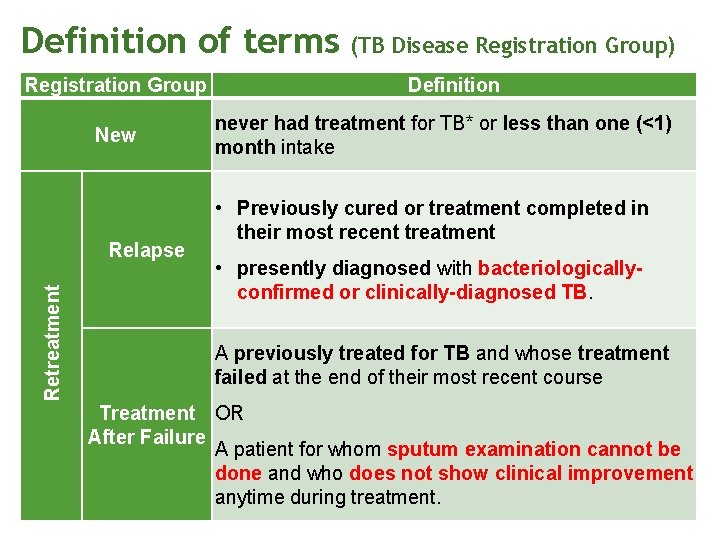
Definition of terms Registration Group New Retreatment Relapse (TB Disease Registration Group) Definition never had treatment for TB* or less than one (<1) month intake • Previously cured or treatment completed in their most recent treatment • presently diagnosed with bacteriologicallyconfirmed or clinically-diagnosed TB. A previously treated for TB and whose treatment failed at the end of their most recent course Treatment OR After Failure A patient for whom sputum examination cannot be done and who does not show clinical improvement anytime during treatment.

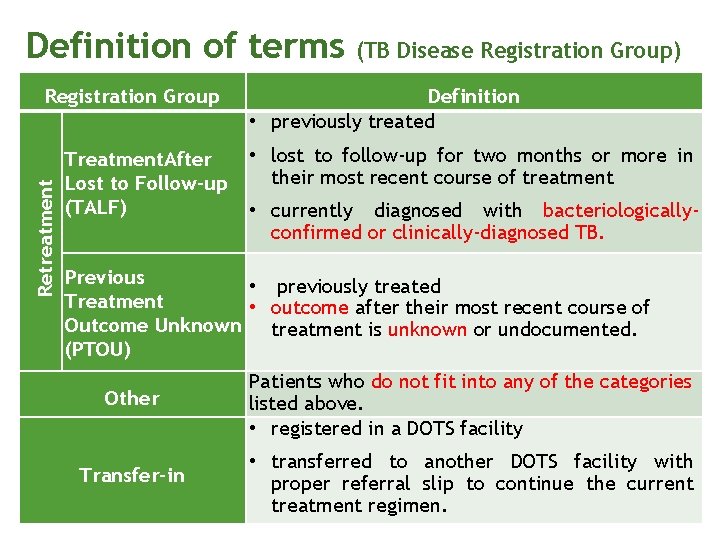
Definition of terms Retreatment Registration Group Treatment. After Lost to Follow-up (TALF) (TB Disease Registration Group) Definition • previously treated • lost to follow-up for two months or more in their most recent course of treatment • currently diagnosed with bacteriologicallyconfirmed or clinically-diagnosed TB. Previous • previously treated Treatment • outcome after their most recent course of Outcome Unknown treatment is unknown or undocumented. (PTOU) Other Patients who do not fit into any of the categories listed above. • registered in a DOTS facility Transfer-in • transferred to another DOTS facility with proper referral slip to continue the current treatment regimen.

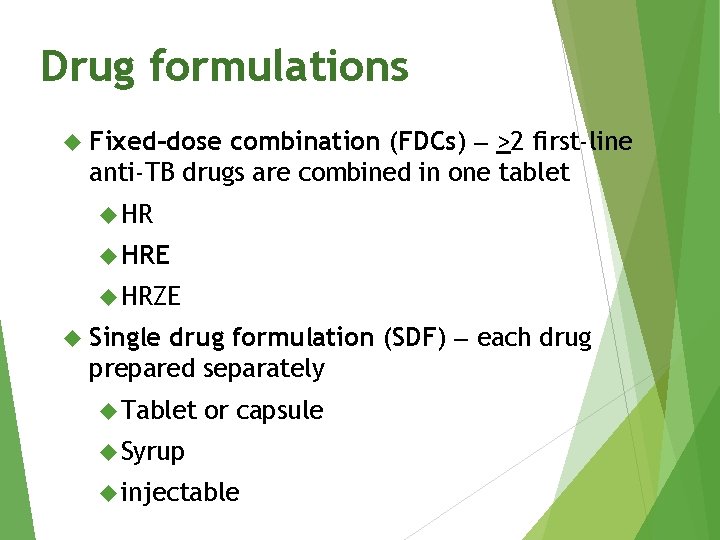
Drug formulations combination (FDCs) >2 first-line anti-TB drugs are combined in one tablet Fixed–dose HRE HRZE drug formulation (SDF) each drug prepared separately Single Tablet or capsule Syrup injectable

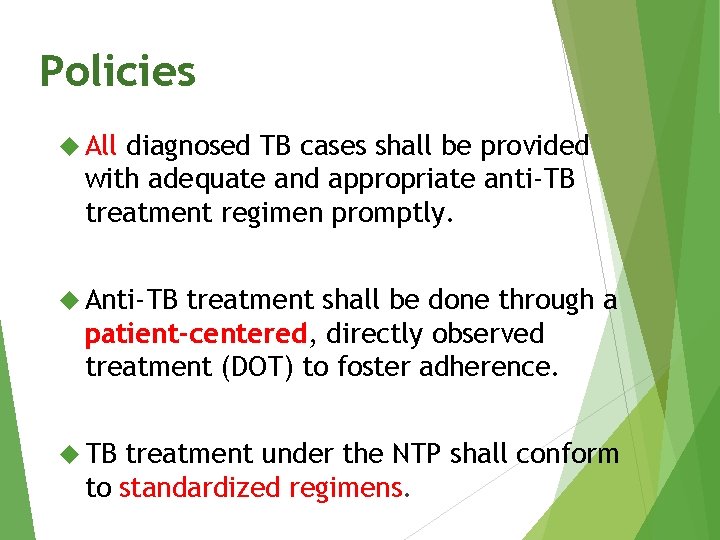
Policies All diagnosed TB cases shall be provided with adequate and appropriate anti-TB treatment regimen promptly. Anti-TB treatment shall be done through a patient-centered, directly observed treatment (DOT) to foster adherence. TB treatment under the NTP shall conform to standardized regimens.

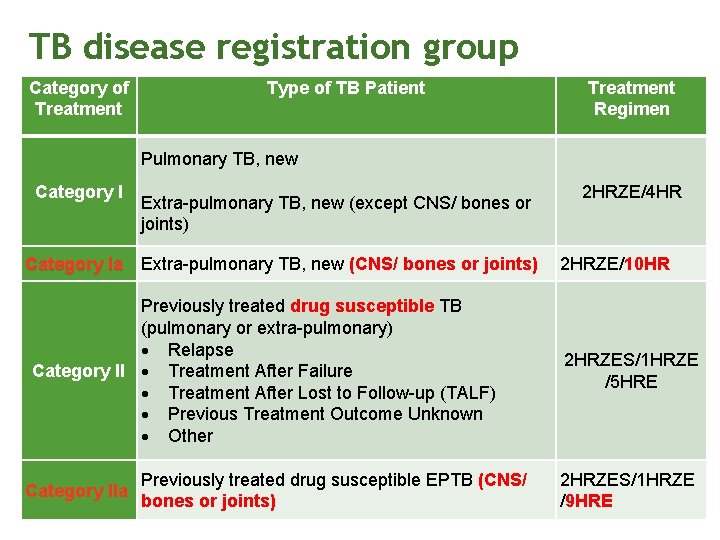
TB disease registration group Category of Treatment Type of TB Patient Treatment Regimen Pulmonary TB, new Category I Extra-pulmonary TB, new (except CNS/ bones or joints) Category Ia Extra-pulmonary TB, new (CNS/ bones or joints) Previously treated drug susceptible TB (pulmonary or extra-pulmonary) Relapse Category II Treatment After Failure Treatment After Lost to Follow-up (TALF) Previous Treatment Outcome Unknown Other Category IIa Previously treated drug susceptible EPTB (CNS/ bones or joints) 2 HRZE/4 HR 2 HRZE/10 HR 2 HRZES/1 HRZE /5 HRE 2 HRZES/1 HRZE /9 HRE

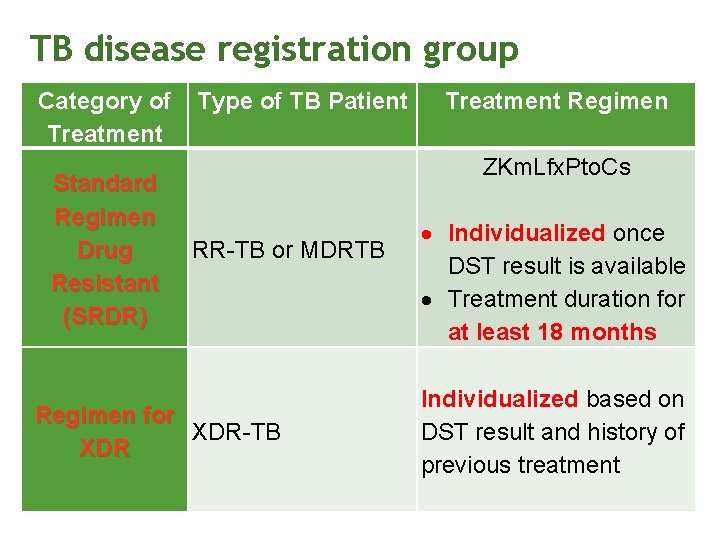
TB disease registration group Category of Treatment Standard Regimen Drug Resistant (SRDR) Type of TB Patient Treatment Regimen ZKm. Lfx. Pto. Cs RR-TB or MDRTB Regimen for XDR-TB XDR Individualized once DST result is available Treatment duration for at least 18 months Individualized based on DST result and history of previous treatment

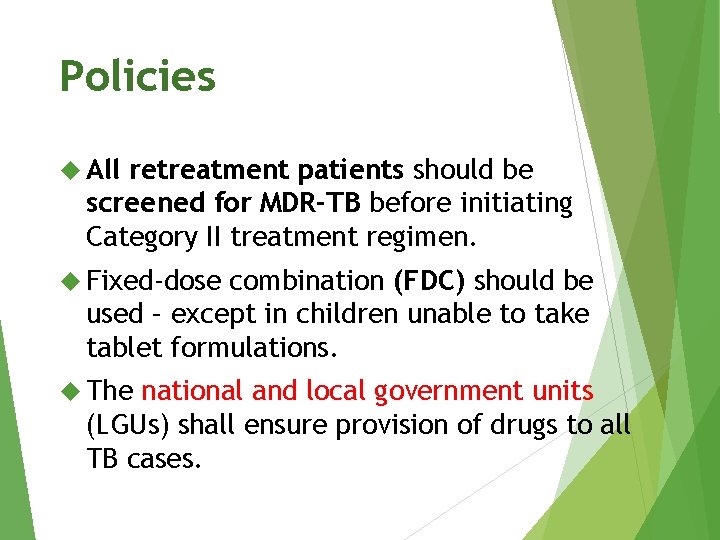
Policies All retreatment patients should be screened for MDR-TB before initiating Category II treatment regimen. Fixed-dose combination (FDC) should be used – except in children unable to take tablet formulations. The national and local government units (LGUs) shall ensure provision of drugs to all TB cases.

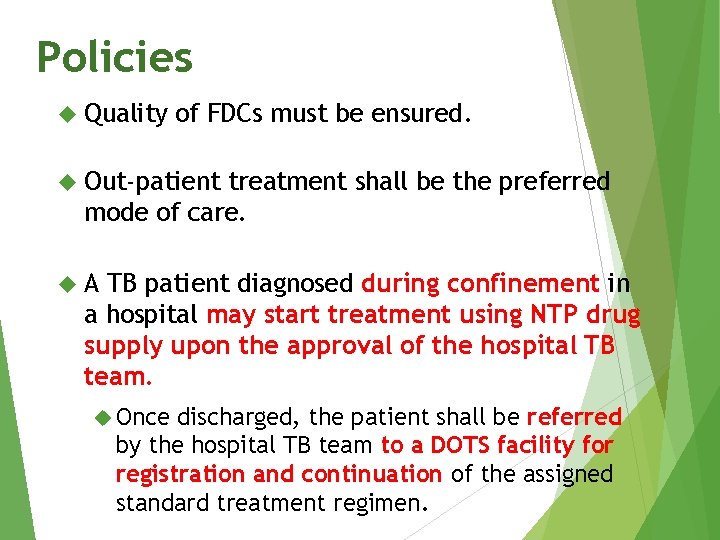
Policies Quality of FDCs must be ensured. Out-patient treatment shall be the preferred mode of care. A TB patient diagnosed during confinement in a hospital may start treatment using NTP drug supply upon the approval of the hospital TB team. Once discharged, the patient shall be referred by the hospital TB team to a DOTS facility for registration and continuation of the assigned standard treatment regimen.

Policies Treatment response of PTB patients shall be monitored through follow-up DSSM and clinical signs and symptoms. Tracking mechanism for patients lost to follow-up shall be put in place. Appropriate infection control measures shall be observed at all times based on TB Infection Control guidelines.

Policies All registered TB patients in Category A and B sites, shall be offered Provider-initiated HIV Counselling and Testing (PICT). All confirmed drug resistant TB cases shall be offered PICT.

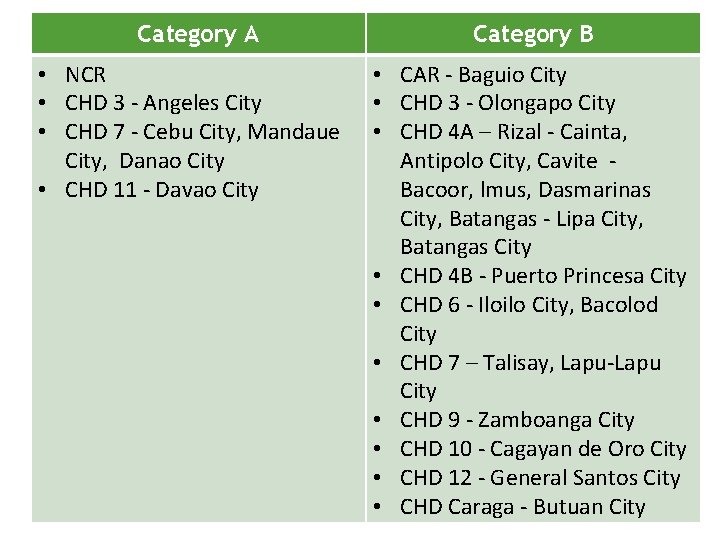
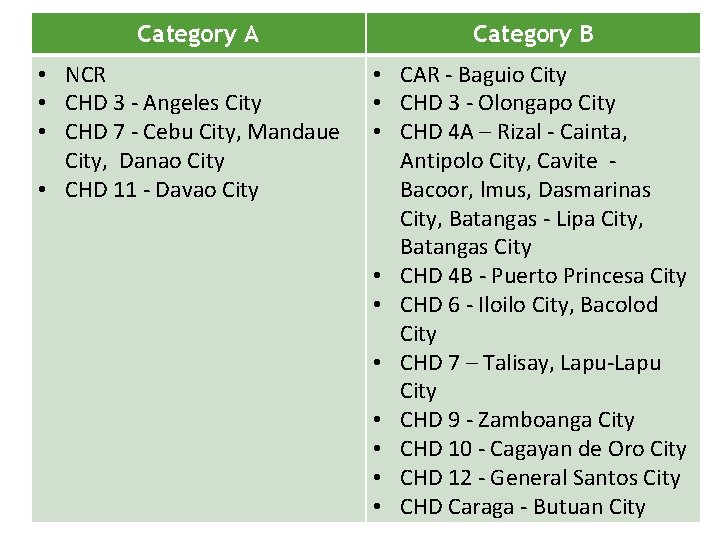
Category A • NCR • CHD 3 - Angeles City • CHD 7 - Cebu City, Mandaue City, Danao City • CHD 11 - Davao City Category B • CAR - Baguio City • CHD 3 - Olongapo City • CHD 4 A – Rizal - Cainta, Antipolo City, Cavite Bacoor, lmus, Dasmarinas City, Batangas - Lipa City, Batangas City • CHD 4 B - Puerto Princesa City • CHD 6 - Iloilo City, Bacolod City • CHD 7 – Talisay, Lapu-Lapu City • CHD 9 - Zamboanga City • CHD 10 - Cagayan de Oro City • CHD 12 - General Santos City • CHD Caraga - Butuan City

Treatment and registration Do pre-treatment evaluation. Address all pertinent health issues appropriately Assign the corresponding treatment regimen based on the patient’s disease site and registration group. Open and accomplish Form 4. TB Treatment/ IPT Card and two (2) Form 5. NTP ID Cards one for the patient and the other for the treatment partner.

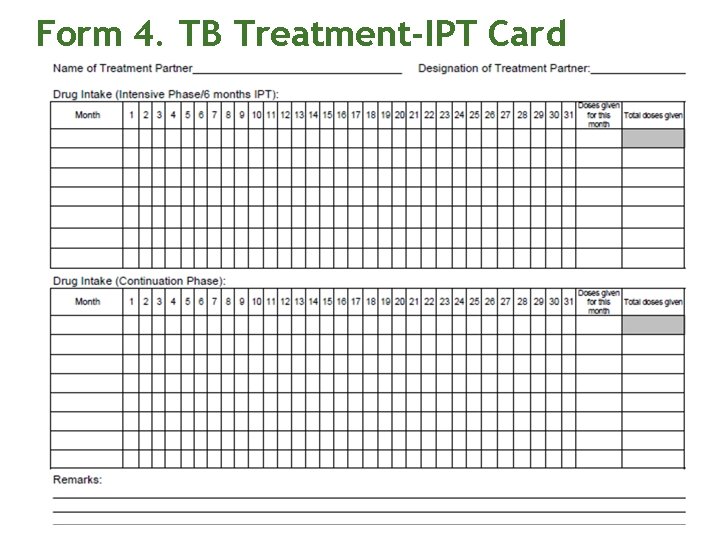
Form 4. TB Treatment-IPT Card

Form 4. TB Treatment-IPT Card

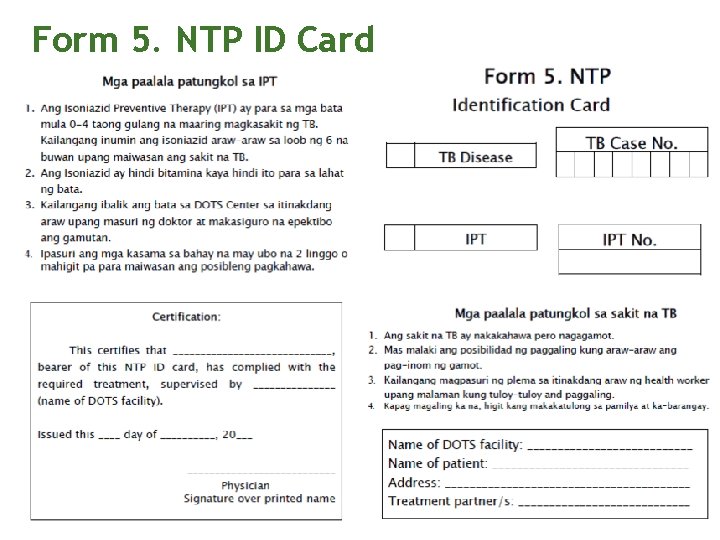
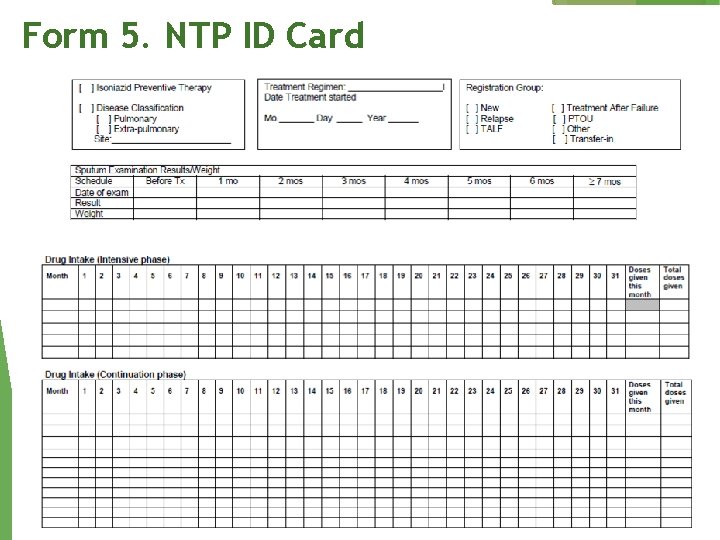
Form 5. NTP ID Card

Form 5. NTP ID Card

Revised contents of the treatment card SOURCE OF PATIENT Public health centers Other government facilities/hospitals Private hospitals/clinics/physician/NGO Community clinics Phil. Health number Household contacts Treatment card for adults and children integrated

Treatment and registration Discuss with the patient and decide who will be the most appropriate treatment partner and where the treatment will be administered Treatment DOTS a partner: facility staff, or trained community member, such as the barangay health worker (BHW), local government official, or a former TB patient.

Treatment and registration Trained family members as the sole treatment partner in special/exceptional cases: Poor access to a DOTS facility due to geographical barriers (including temporarily displaced populations) Debilitated and/or bed-ridden patients DOT schedule is in conflict with the patient’s work/school schedule and unable to access other DOTS facilities Cultural beliefs that limit the choice of a treatment partner (e. g. , indigenous people) Treatment of children

Treatment and registration Trained family members as the sole treatment partner drug supply on a weekly basis or as agreed between health worker and family member. Streptomycin IM injections by trained and authorized health personnel. If no access during weekends/holidays, may forego streptomycin doses during weekends/holidays provided they still complete the recommended number of doses (i. e. , 56 doses).

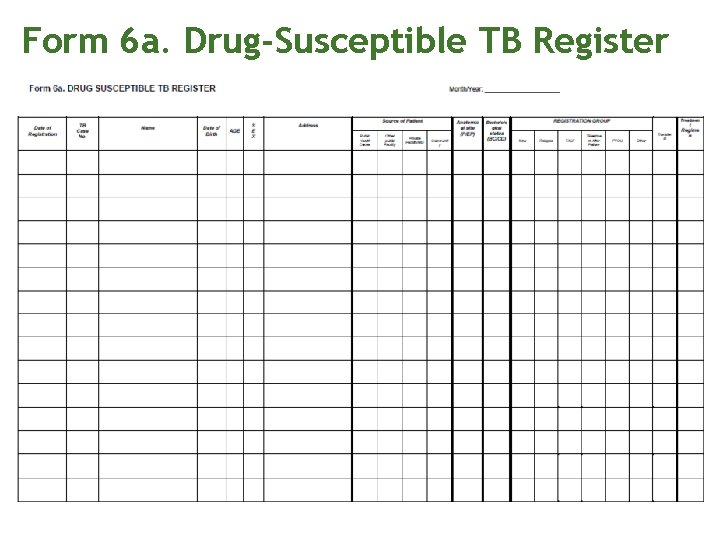
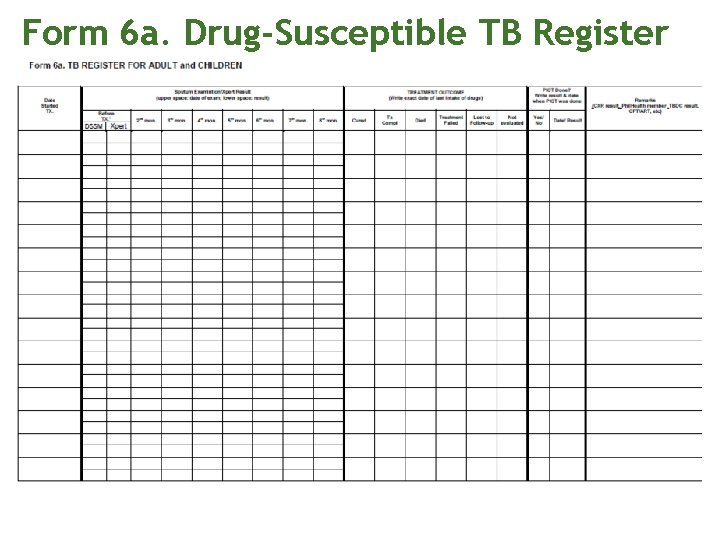
Treatment and registration Ask if the patient is a Phil. Health member or a qualified dependent Open the appropriate Standard Regimen and watch the patient take the initial dose of medications. Record intake in treatment and ID card Register the patient in the Form 6 a. Drug Susceptible TB register. Assign a TB case number.

Form 6 a. Drug-Susceptible TB Register

Form 6 a. Drug-Susceptible TB Register

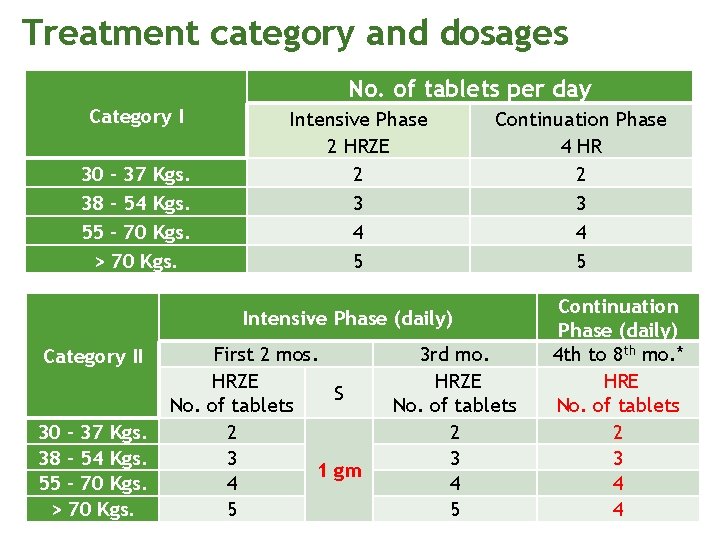
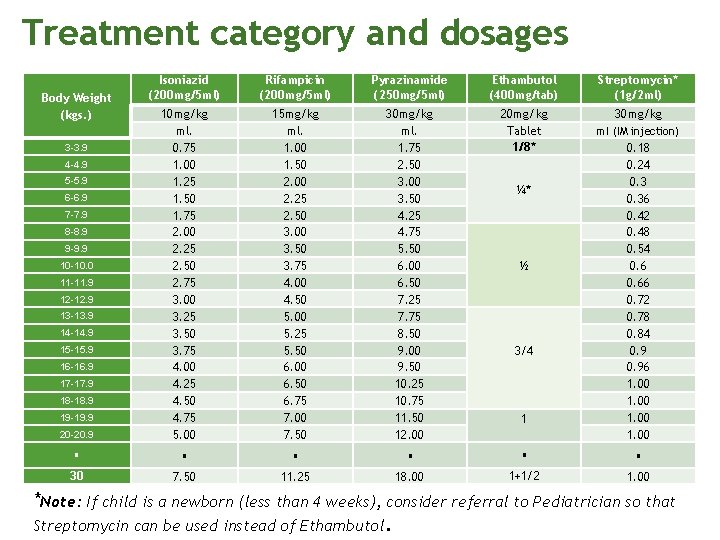
Treatment category and dosages No. of tablets per day Category I 30 – 37 Kgs. 38 – 54 Kgs. 55 – 70 Kgs. > 70 Kgs. Intensive Phase 2 HRZE 2 3 4 5 Continuation Phase 4 HR 2 3 4 5 Intensive Phase (daily) Category II 30 – 37 Kgs. 38 – 54 Kgs. 55 – 70 Kgs. > 70 Kgs. First 2 mos. HRZE S No. of tablets 2 3 1 gm 4 5 3 rd mo. HRZE No. of tablets 2 3 4 5 Continuation Phase (daily) 4 th to 8 th mo. * HRE No. of tablets 2 3 4 4

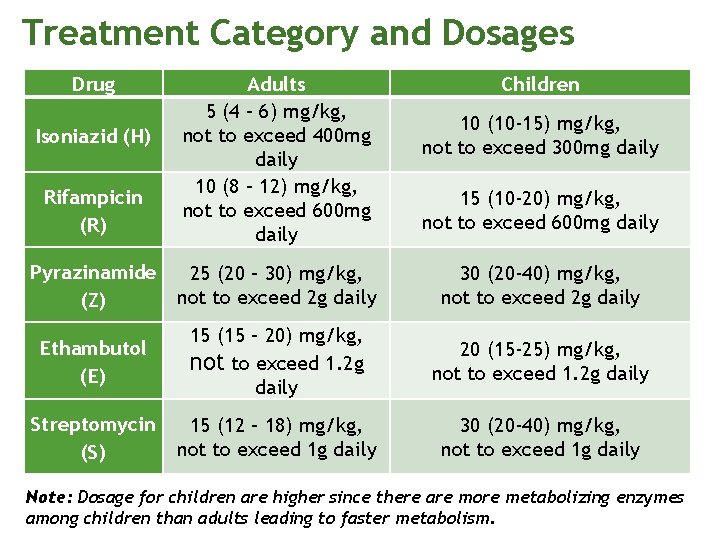
Treatment Category and Dosages Drug Rifampicin (R) Adults 5 (4 – 6) mg/kg, not to exceed 400 mg daily 10 (8 – 12) mg/kg, not to exceed 600 mg daily Pyrazinamide (Z) 25 (20 – 30) mg/kg, not to exceed 2 g daily 30 (20 -40) mg/kg, not to exceed 2 g daily Ethambutol (E) 15 (15 – 20) mg/kg, not to exceed 1. 2 g daily 20 (15 -25) mg/kg, not to exceed 1. 2 g daily Streptomycin (S) 15 (12 – 18) mg/kg, not to exceed 1 g daily 30 (20 -40) mg/kg, not to exceed 1 g daily Isoniazid (H) Children 10 (10 -15) mg/kg, not to exceed 300 mg daily 15 (10 -20) mg/kg, not to exceed 600 mg daily Note: Dosage for children are higher since there are more metabolizing enzymes among children than adults leading to faster metabolism.

Treatment category and dosages Isoniazid (200 mg/5 ml) Rifampicin (200 mg/5 ml) Pyrazinamide (250 mg/5 ml) Ethambutol (400 mg/tab) Streptomycin* (1 g/2 ml) 15 mg/kg ml. 1. 00 1. 50 2. 00 2. 25 2. 50 3. 00 3. 50 3. 75 4. 00 4. 50 5. 00 5. 25 5. 50 6. 00 6. 50 6. 75 7. 00 7. 50 30 mg/kg ml. 1. 75 2. 50 3. 00 3. 50 4. 25 4. 75 5. 50 6. 00 6. 50 7. 25 7. 75 8. 50 9. 00 9. 50 10. 25 10. 75 11. 50 12. 00 20 mg/kg Tablet 1/8* 30 mg/kg 20 -20. 9 10 mg/kg ml. 0. 75 1. 00 1. 25 1. 50 1. 75 2. 00 2. 25 2. 50 2. 75 3. 00 3. 25 3. 50 3. 75 4. 00 4. 25 4. 50 4. 75 5. 00 1 0. 18 0. 24 0. 36 0. 42 0. 48 0. 54 0. 66 0. 72 0. 78 0. 84 0. 96 1. 00 30 7. 50 11. 25 18. 00 1+1/2 1. 00 Body Weight (kgs. ) 3 -3. 9 4 -4. 9 5 -5. 9 6 -6. 9 7 -7. 9 8 -8. 9 9 -9. 9 10 -10. 0 11 -11. 9 12 -12. 9 13 -13. 9 14 -14. 9 15 -15. 9 16 -16. 9 17 -17. 9 18 -18. 9 19 -19. 9 ¼* ½ 3/4 ml (IM injection) *Note: If child is a newborn (less than 4 weeks), consider referral to Pediatrician so that Streptomycin can be used instead of Ethambutol.

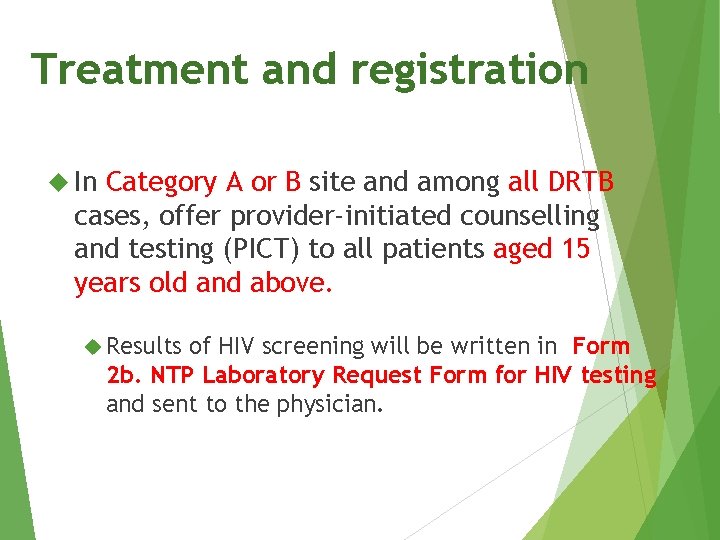
Treatment and registration In Category A or B site and among all DRTB cases, offer provider-initiated counselling and testing (PICT) to all patients aged 15 years old and above. Results of HIV screening will be written in Form 2 b. NTP Laboratory Request Form for HIV testing and sent to the physician.

Category A • NCR • CHD 3 - Angeles City • CHD 7 - Cebu City, Mandaue City, Danao City • CHD 11 - Davao City Category B • CAR - Baguio City • CHD 3 - Olongapo City • CHD 4 A – Rizal - Cainta, Antipolo City, Cavite Bacoor, lmus, Dasmarinas City, Batangas - Lipa City, Batangas City • CHD 4 B - Puerto Princesa City • CHD 6 - Iloilo City, Bacolod City • CHD 7 – Talisay, Lapu-Lapu City • CHD 9 - Zamboanga City • CHD 10 - Cagayan de Oro City • CHD 12 - General Santos City • CHD Caraga - Butuan City

Form 2 b. NTP Laboratory Result Form for HIV Screening of TB Patients

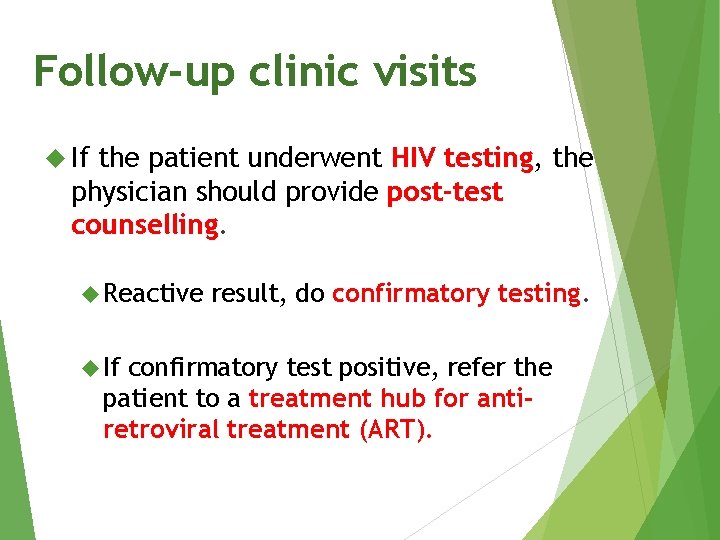
Follow-up clinic visits If the patient underwent HIV testing, the physician should provide post-test counselling. Reactive If result, do confirmatory testing. confirmatory test positive, refer the patient to a treatment hub for antiretroviral treatment (ART).

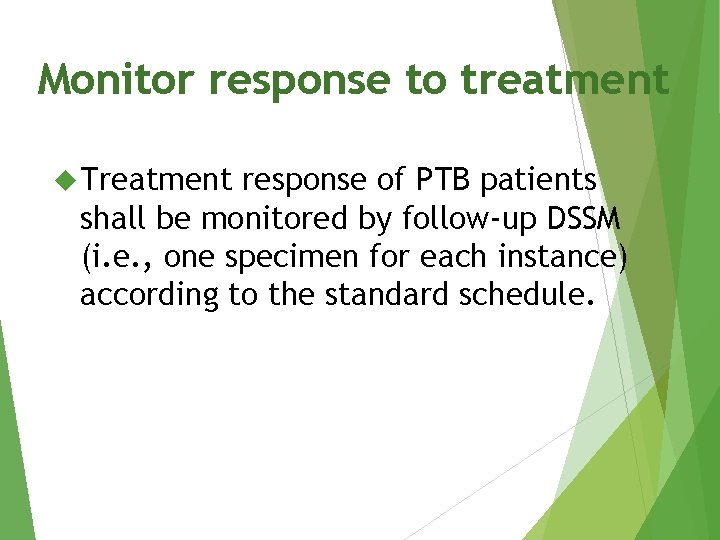
Monitor response to treatment Treatment response of PTB patients shall be monitored by follow-up DSSM (i. e. , one specimen for each instance) according to the standard schedule.

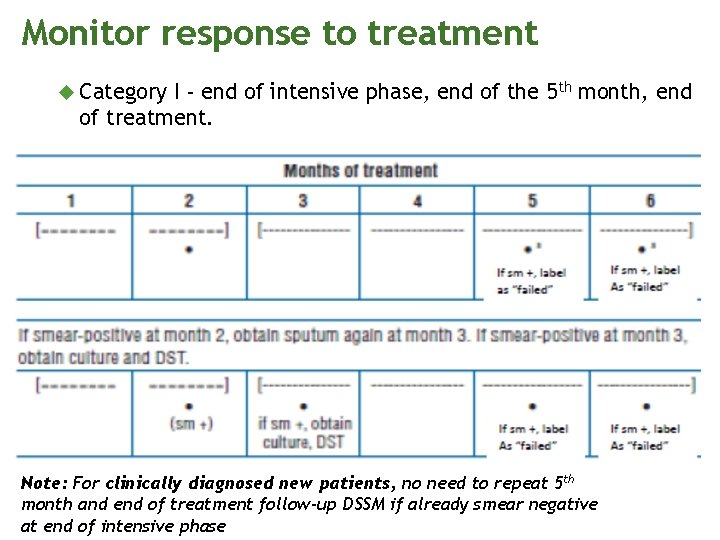
Monitor response to treatment Category I - end of intensive phase, end of the 5 th month, end of treatment. Note: For clinically diagnosed new patients, no need to repeat 5 th month and end of treatment follow-up DSSM if already smear negative at end of intensive phase

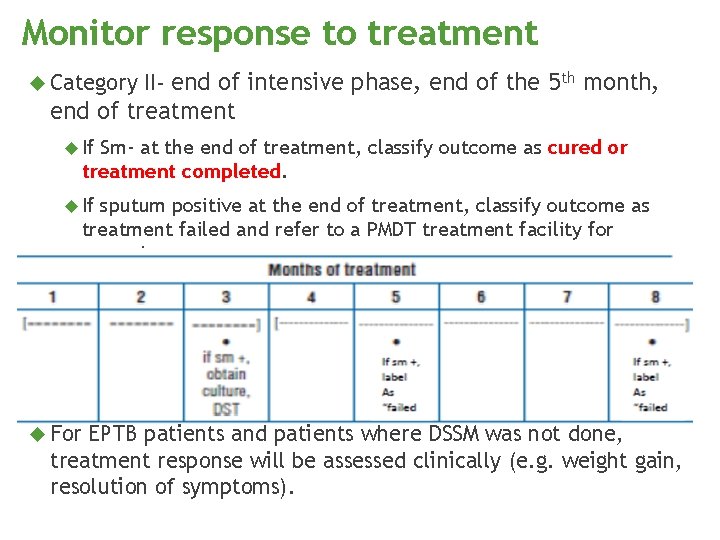
Monitor response to treatment Category II- end of intensive phase, end of the 5 th month, end of treatment If Sm- at the end of treatment, classify outcome as cured or treatment completed. If sputum positive at the end of treatment, classify outcome as treatment failed and refer to a PMDT treatment facility for screening. For EPTB patients and patients where DSSM was not done, treatment response will be assessed clinically (e. g. weight gain, resolution of symptoms).

th 5 edition NTP MANUAL OF PROCEDURES Case Holding II

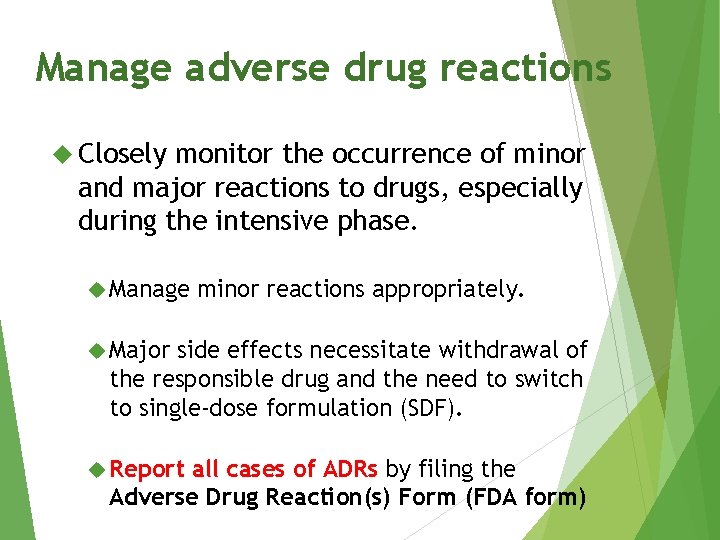
Manage adverse drug reactions Closely monitor the occurrence of minor and major reactions to drugs, especially during the intensive phase. Manage minor reactions appropriately. Major side effects necessitate withdrawal of the responsible drug and the need to switch to single-dose formulation (SDF). Report all cases of ADRs by filing the Adverse Drug Reaction(s) Form (FDA form)

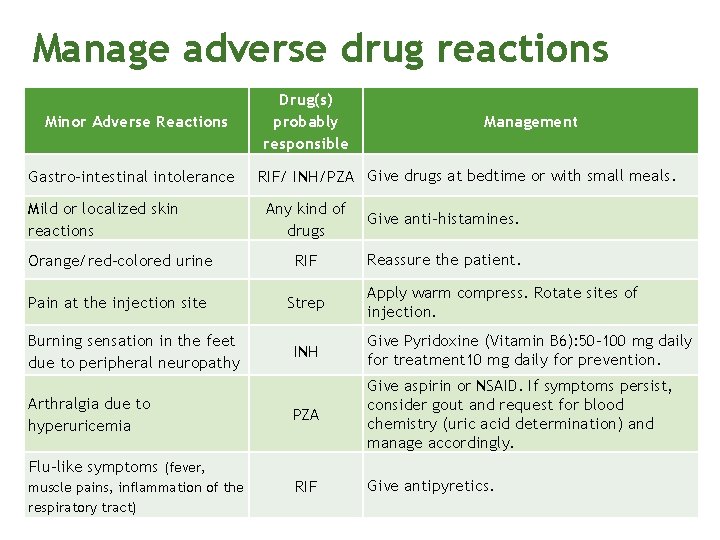
Manage adverse drug reactions Minor Adverse Reactions Gastro-intestinal intolerance Mild or localized skin reactions Drug(s) probably responsible Management RIF/ INH/PZA Give drugs at bedtime or with small meals. Any kind of drugs Give anti-histamines. Orange/red-colored urine RIF Reassure the patient. Pain at the injection site Strep Burning sensation in the feet due to peripheral neuropathy Arthralgia due to hyperuricemia INH Give Pyridoxine (Vitamin B 6): 50 -100 mg daily for treatment 10 mg daily for prevention. PZA Give aspirin or NSAID. If symptoms persist, consider gout and request for blood chemistry (uric acid determination) and manage accordingly. RIF Give antipyretics. Flu-like symptoms (fever, muscle pains, inflammation of the respiratory tract) Apply warm compress. Rotate sites of injection.

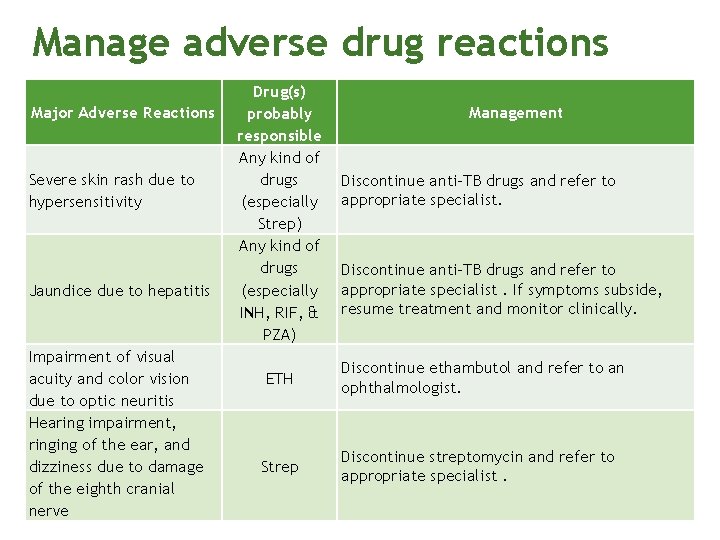
Manage adverse drug reactions Major Adverse Reactions Severe skin rash due to hypersensitivity Jaundice due to hepatitis Impairment of visual acuity and color vision due to optic neuritis Hearing impairment, ringing of the ear, and dizziness due to damage of the eighth cranial nerve Drug(s) probably responsible Any kind of drugs (especially Strep) Any kind of drugs (especially INH, RIF, & PZA) Management Discontinue anti-TB drugs and refer to appropriate specialist. If symptoms subside, resume treatment and monitor clinically. ETH Discontinue ethambutol and refer to an ophthalmologist. Strep Discontinue streptomycin and refer to appropriate specialist.

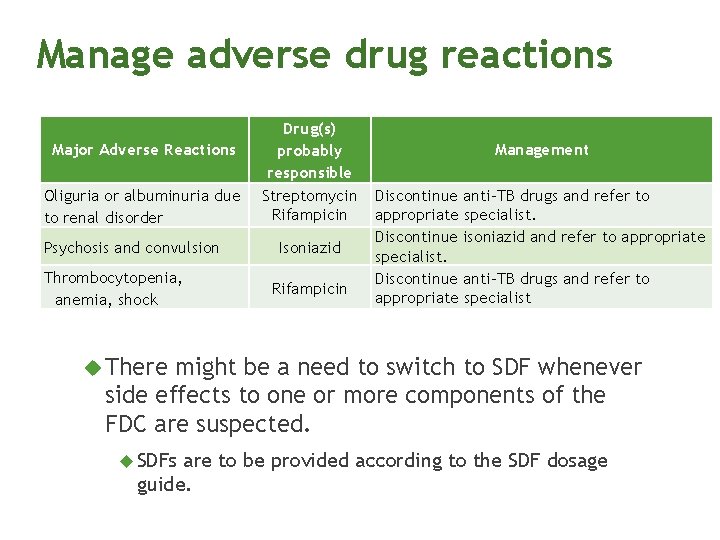
Manage adverse drug reactions Major Adverse Reactions Oliguria or albuminuria due to renal disorder Psychosis and convulsion Thrombocytopenia, anemia, shock Drug(s) probably responsible Streptomycin Rifampicin Isoniazid Rifampicin Management Discontinue anti-TB drugs and refer to appropriate specialist. Discontinue isoniazid and refer to appropriate specialist. Discontinue anti-TB drugs and refer to appropriate specialist There might be a need to switch to SDF whenever side effects to one or more components of the FDC are suspected. SDFs are to be provided according to the SDF dosage guide.

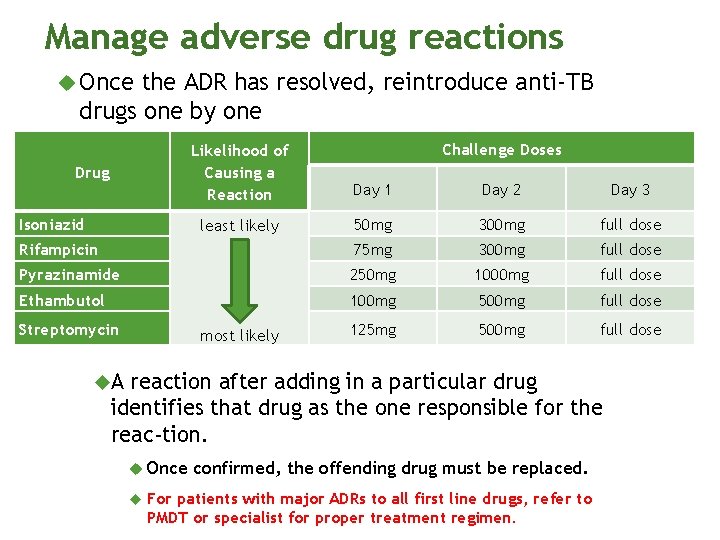
Manage adverse drug reactions Once the ADR has resolved, reintroduce anti-TB drugs one by one Drug Isoniazid Day 1 Day 2 Day 3 least likely 50 mg 300 mg full dose 75 mg 300 mg full dose 250 mg 1000 mg full dose 100 mg 500 mg full dose 125 mg 500 mg full dose Rifampicin most likely Pyrazinamide Ethambutol Streptomycin Challenge Doses Likelihood of Causing a Reaction A reaction after adding in a particular drug identifies that drug as the one responsible for the reac tion. Once confirmed, the offending drug must be replaced. For patients with major ADRs to all first line drugs, refer to PMDT or specialist for proper treatment regimen.

Deciding when a PTB patient is no longer infectious during treatment For bacteriologically confirmed patients, DSSM can be done month after start of treatment for purposes of certifying that the patient can return to work. No bacteriologically confirmed case should be allowed to return to work without a negative followup smear examination.

Deciding when a PTB patient is no longer infectious during treatment For clinically diagnosed patients (smear negative or smear not done), it is possible to clear him after 2 weeks as long as treatment compliance is assured there is clinical improvement or no clinical deterioration Once appropriate, issue a certificate that the patient is no longer infectious and can safely return to work.

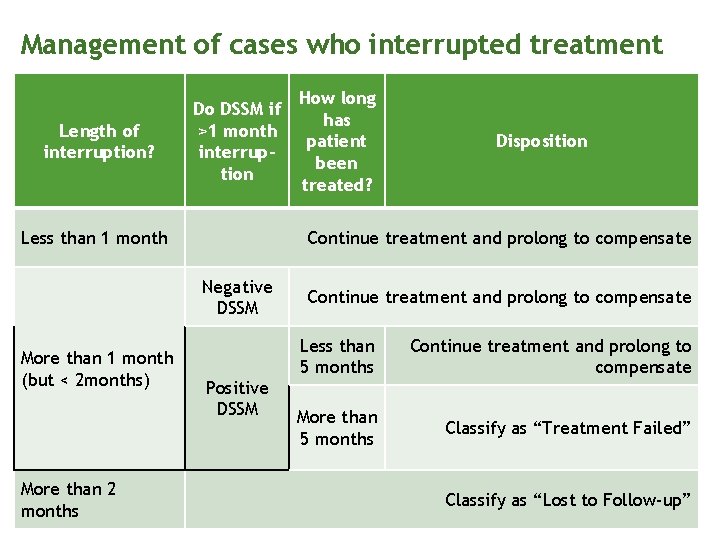
Management of cases who interrupted treatment Patients who fail to follow up as scheduled should be immediately traced through: telephone call, text message or home/workplace visit. Assess the cause of interruption and agree on solutions.

Management of cases who interrupted treatment Length of interruption? Do DSSM if >1 month interruption Less than 1 month More than 2 months Disposition Continue treatment and prolong to compensate Negative DSSM More than 1 month (but < 2 months) How long has patient been treated? Positive DSSM Continue treatment and prolong to compensate Less than 5 months Continue treatment and prolong to compensate More than 5 months Classify as “Treatment Failed” Classify as “Lost to Follow-up”

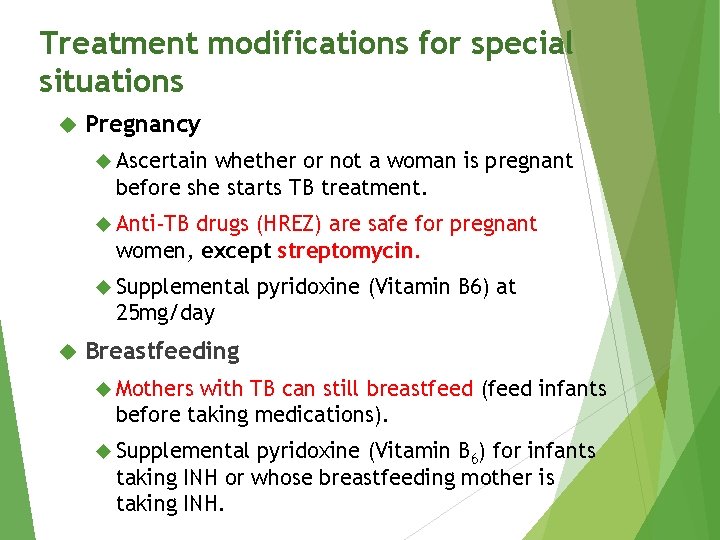
Treatment modifications for special situations Pregnancy Ascertain whether or not a woman is pregnant before she starts TB treatment. Anti-TB drugs (HREZ) are safe for pregnant women, except streptomycin. Supplemental pyridoxine (Vitamin B 6) at 25 mg/day Breastfeeding Mothers with TB can still breastfeed (feed infants before taking medications). Supplemental pyridoxine (Vitamin B 6) for infants taking INH or whose breastfeeding mother is taking INH.

Treatment modifications for special situations Oral Contraceptives Rifampicin interacts with oral contraceptive medications with a risk of decreased protective efficacy against pregnancy. take an oral contraceptive pill containing a higher dose of estrogen (50 ), following consultation with a clinician; or use another form of contraception.

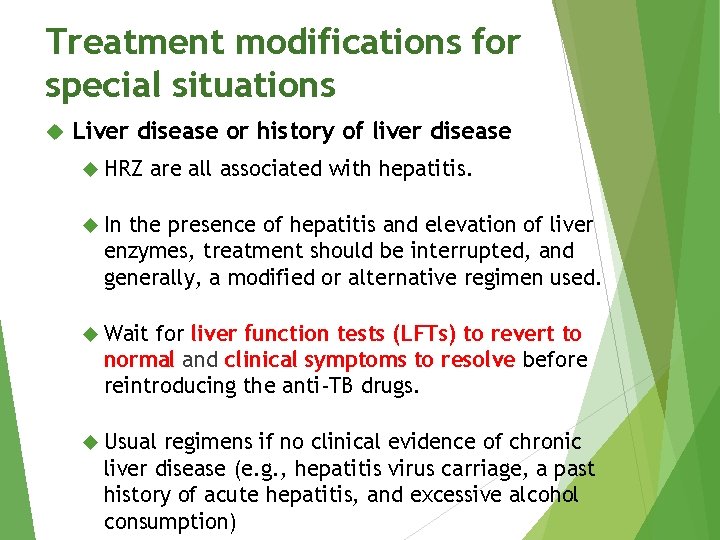
Treatment modifications for special situations Liver disease or history of liver disease HRZ are all associated with hepatitis. In the presence of hepatitis and elevation of liver enzymes, treatment should be interrupted, and generally, a modified or alternative regimen used. Wait for liver function tests (LFTs) to revert to normal and clinical symptoms to resolve before reintroducing the anti-TB drugs. Usual regimens if no clinical evidence of chronic liver disease (e. g. , hepatitis virus carriage, a past history of acute hepatitis, and excessive alcohol consumption)

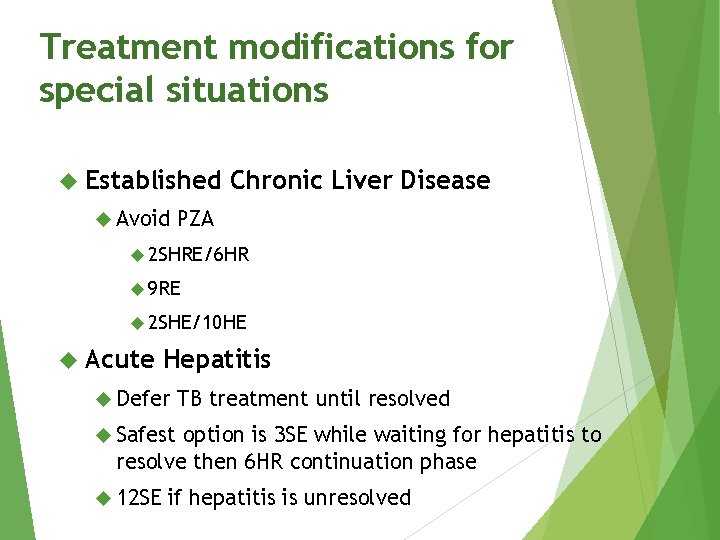
Treatment modifications for special situations Established Avoid Chronic Liver Disease PZA 2 SHRE/6 HR 9 RE 2 SHE/10 HE Acute Hepatitis Defer TB treatment until resolved Safest option is 3 SE while waiting for hepatitis to resolve then 6 HR continuation phase 12 SE if hepatitis is unresolved

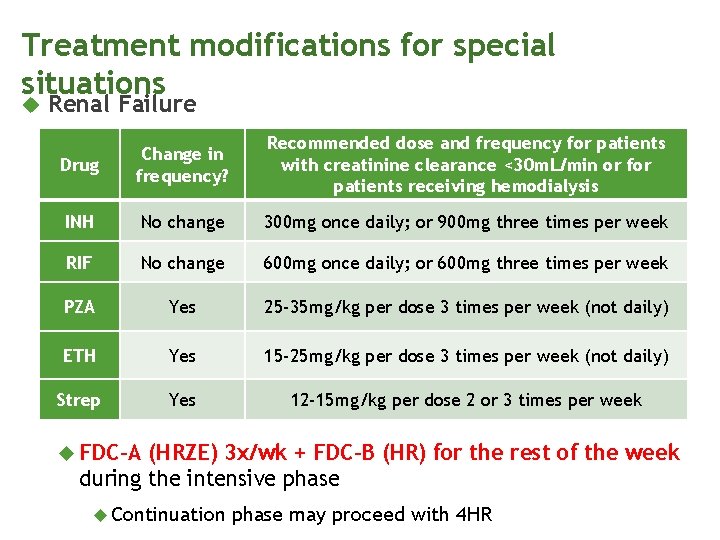
Treatment modifications for special situations Renal Failure Drug Change in frequency? Recommended dose and frequency for patients with creatinine clearance <30 m. L/min or for patients receiving hemodialysis INH No change 300 mg once daily; or 900 mg three times per week RIF No change 600 mg once daily; or 600 mg three times per week PZA Yes 25 -35 mg/kg per dose 3 times per week (not daily) ETH Yes 15 -25 mg/kg per dose 3 times per week (not daily) Strep Yes 12 -15 mg/kg per dose 2 or 3 times per week FDC-A (HRZE) 3 x/wk + FDC-B (HR) for the rest of the week during the intensive phase Continuation phase may proceed with 4 HR

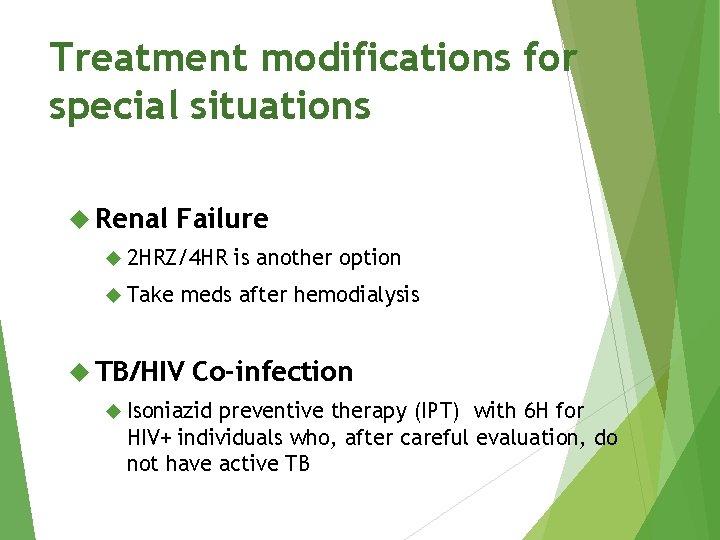
Treatment modifications for special situations Renal Failure 2 HRZ/4 HR Take is another option meds after hemodialysis TB/HIV Co-infection Isoniazid preventive therapy (IPT) with 6 H for HIV+ individuals who, after careful evaluation, do not have active TB

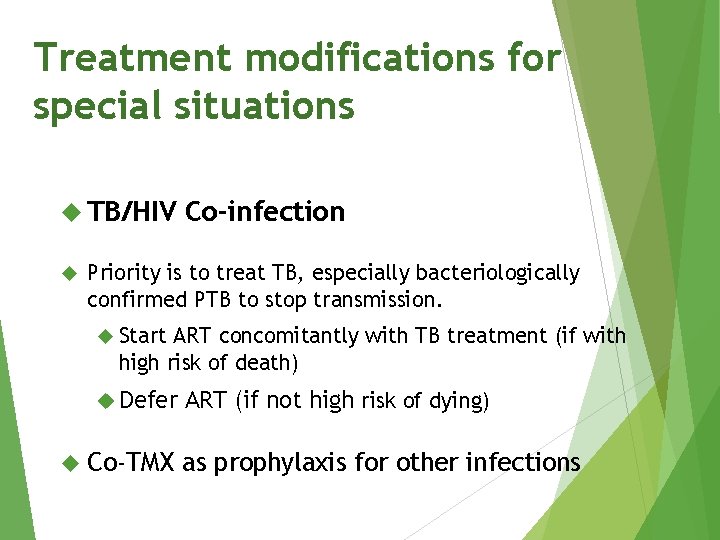
Treatment modifications for special situations TB/HIV Co-infection Priority is to treat TB, especially bacteriologically confirmed PTB to stop transmission. Start ART concomitantly with TB treatment (if with high risk of death) Defer Co-TMX ART (if not high risk of dying) as prophylaxis for other infections

Drug interactions during TB treatment Elderly individuals with significant co-morbidities, as well as the immune-compromised patients (e. g. , HIV/AIDS patients) at higher risk To minimize drug interactions, it is advisable that drugs be administered 12 hours apart. Listing of drug-drug interactions is available in the MOP

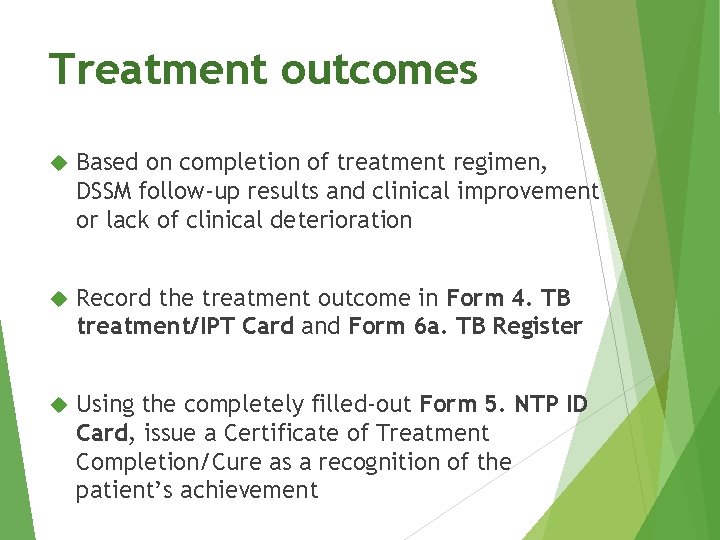
Treatment outcomes Based on completion of treatment regimen, DSSM follow-up results and clinical improvement or lack of clinical deterioration Record the treatment outcome in Form 4. TB treatment/IPT Card and Form 6 a. TB Register Using the completely filled-out Form 5. NTP ID Card, issue a Certificate of Treatment Completion/Cure as a recognition of the patient’s achievement

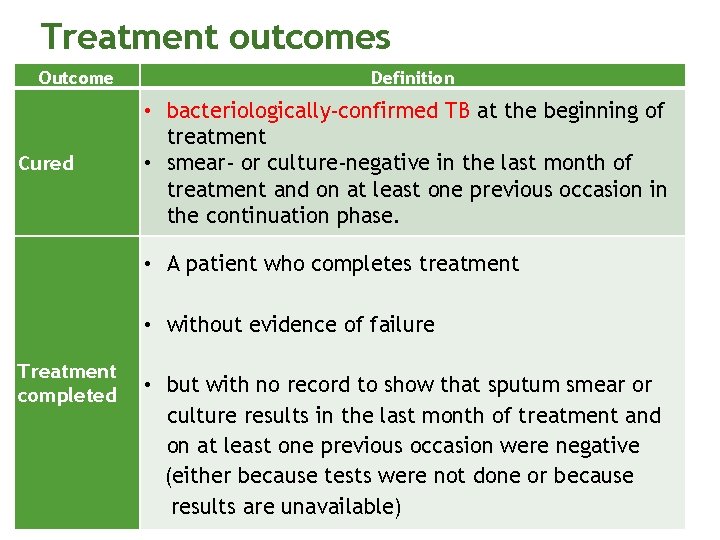
Treatment outcomes Outcome Cured Definition • bacteriologically-confirmed TB at the beginning of treatment • smear- or culture-negative in the last month of treatment and on at least one previous occasion in the continuation phase. • A patient who completes treatment • without evidence of failure Treatment completed • but with no record to show that sputum smear or culture results in the last month of treatment and on at least one previous occasion were negative (either because tests were not done or because results are unavailable)

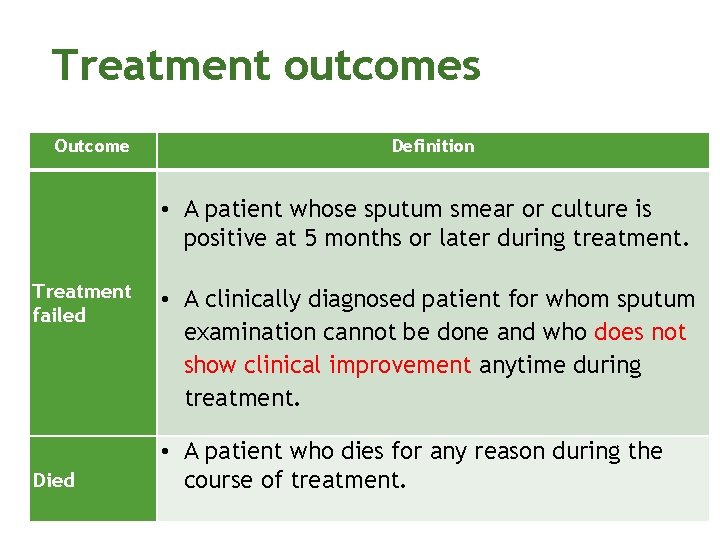
Treatment outcomes Outcome Definition • A patient whose sputum smear or culture is positive at 5 months or later during treatment. Treatment failed Died • A clinically diagnosed patient for whom sputum examination cannot be done and who does not show clinical improvement anytime during treatment. • A patient who dies for any reason during the course of treatment.

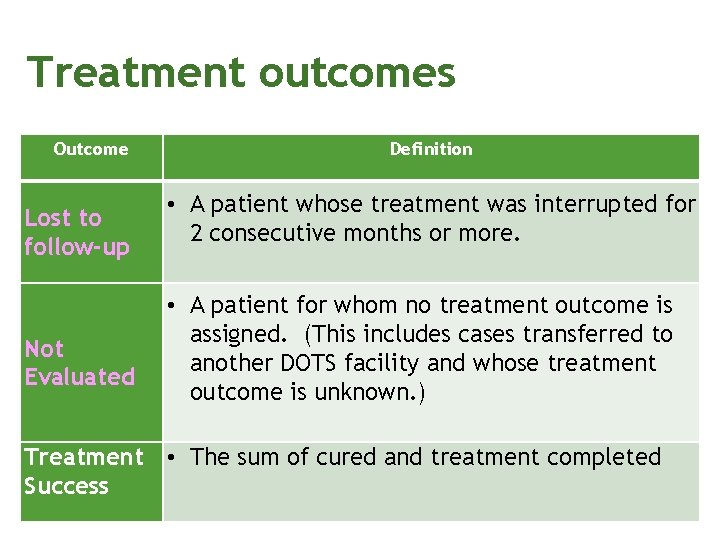
Treatment outcomes Outcome Lost to follow-up Not Evaluated Definition • A patient whose treatment was interrupted for 2 consecutive months or more. • A patient for whom no treatment outcome is assigned. (This includes cases transferred to another DOTS facility and whose treatment outcome is unknown. ) Treatment • The sum of cured and treatment completed Success

Treatment outcomes A patient who is diagnosed to have DR-TB anytime during the course of treatment shall be excluded from the cohort and is not assigned an outcome if they are started on second line drug regimen. However, if treatment with a second-line drug regimen is not possible, the patient is kept in the main TB cohort and assigned an outcome from among those listed above.

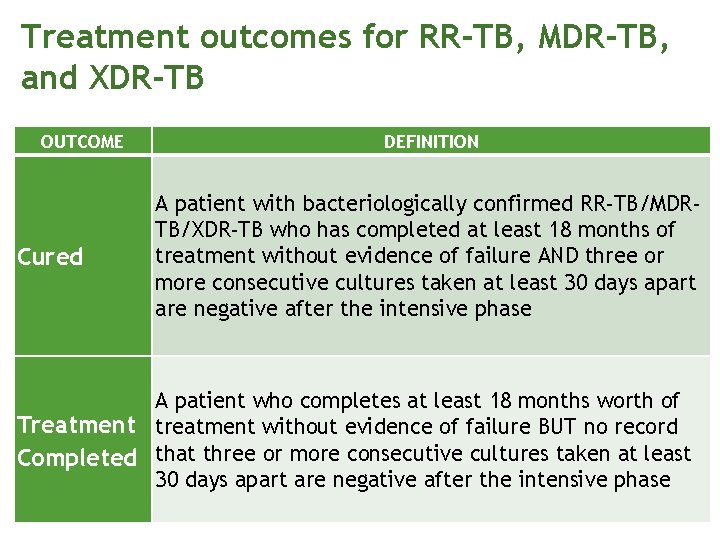
Treatment outcomes for RR-TB, MDR-TB, and XDR-TB OUTCOME Cured DEFINITION A patient with bacteriologically confirmed RR-TB/MDRTB/XDR-TB who has completed at least 18 months of treatment without evidence of failure AND three or more consecutive cultures taken at least 30 days apart are negative after the intensive phase A patient who completes at least 18 months worth of Treatment treatment without evidence of failure BUT no record Completed that three or more consecutive cultures taken at least 30 days apart are negative after the intensive phase

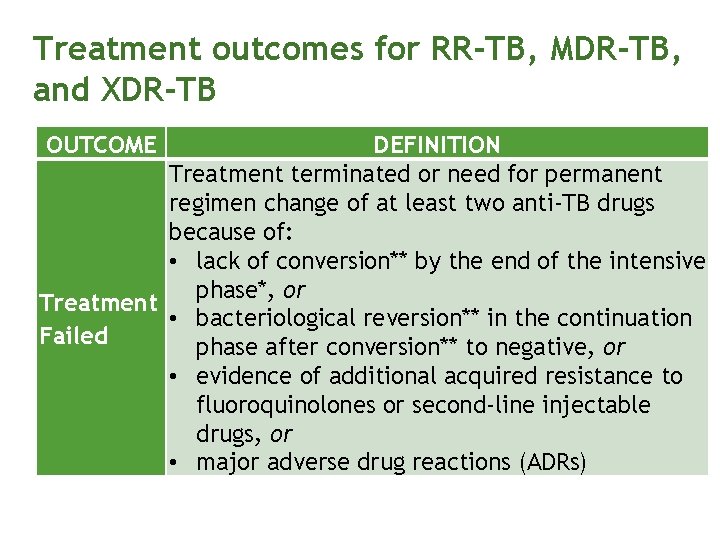
Treatment outcomes for RR-TB, MDR-TB, and XDR-TB OUTCOME DEFINITION Treatment terminated or need for permanent regimen change of at least two anti-TB drugs because of: • lack of conversion** by the end of the intensive phase*, or Treatment • bacteriological reversion** in the continuation Failed phase after conversion** to negative, or • evidence of additional acquired resistance to fluoroquinolones or second-line injectable drugs, or • major adverse drug reactions (ADRs)

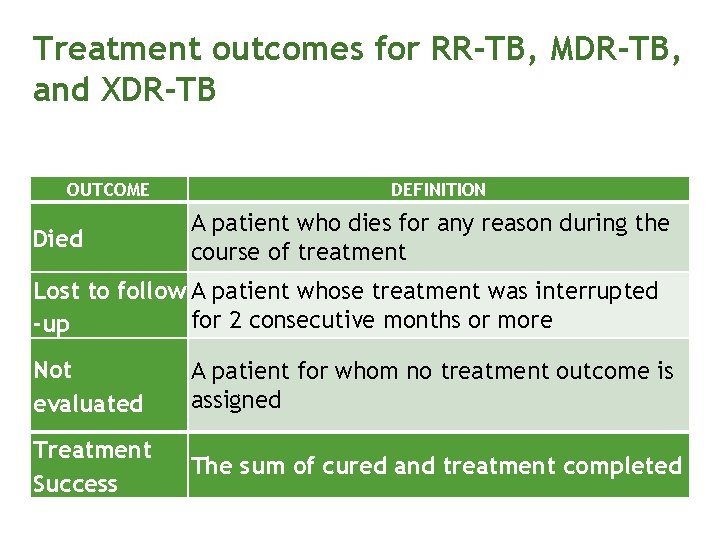
Treatment outcomes for RR-TB, MDR-TB, and XDR-TB OUTCOME Died DEFINITION A patient who dies for any reason during the course of treatment Lost to follow A patient whose treatment was interrupted for 2 consecutive months or more -up Not evaluated A patient for whom no treatment outcome is assigned Treatment Success The sum of cured and treatment completed

Thank you.
 Best worst and average case
Best worst and average case Gwisnet
Gwisnet Using mis (10th edition) 10th edition
Using mis (10th edition) 10th edition Report
Report Manual of structural kinesiology 18th edition
Manual of structural kinesiology 18th edition Engineering economy 16th edition chapter 5 solutions
Engineering economy 16th edition chapter 5 solutions Engineering economy 16th edition solution manual chapter 5
Engineering economy 16th edition solution manual chapter 5 Engineering economy 16th edition solution manual chapter 3
Engineering economy 16th edition solution manual chapter 3 Ntp yellow
Ntp yellow Nts 144 minsa 2018
Nts 144 minsa 2018 Ntp 601
Ntp 601 Ntp 544
Ntp 544 Ntp 534
Ntp 534 Ntp carga mental
Ntp carga mental 10. sınıf ntp
10. sınıf ntp Ntp online learning
Ntp online learning Ntp montacargas
Ntp montacargas Ntp conditions
Ntp conditions Ntp treatment card
Ntp treatment card Simple ntp
Simple ntp David mills ntp
David mills ntp David mills ntp
David mills ntp David mills ntp
David mills ntp Derechos asertivos básicos
Derechos asertivos básicos Solaris 10 ntp
Solaris 10 ntp Ntp 321
Ntp 321 Respuesta
Respuesta Sirim product testing
Sirim product testing David l. mills
David l. mills David l. mills
David l. mills Ntp 476
Ntp 476 Ntp 17025
Ntp 17025 Tipos de resguardo
Tipos de resguardo Ntp 17025
Ntp 17025 Ntp stratum levels cisco
Ntp stratum levels cisco Ntp iso 17025
Ntp iso 17025 Que es ntp
Que es ntp Ct 83/2010
Ct 83/2010 Ocr investigation
Ocr investigation Difference between short case and long case
Difference between short case and long case Binary search average case
Binary search average case Case western reserve university case school of engineering
Case western reserve university case school of engineering Bubble sort best case and worst case
Bubble sort best case and worst case Hershey's erp failure
Hershey's erp failure Bubble sort best case and worst case
Bubble sort best case and worst case Bubble sort best case and worst case
Bubble sort best case and worst case How are the asa case and the saa case differ
How are the asa case and the saa case differ Work study techniques
Work study techniques Uncontrolled aerodrome procedures
Uncontrolled aerodrome procedures Army tlps
Army tlps Troop leading procedures
Troop leading procedures Sample timekeeping policy and procedures
Sample timekeeping policy and procedures Data gathering procedure in research example
Data gathering procedure in research example Telephone answering procedures
Telephone answering procedures Classification of audit
Classification of audit Standards vs procedures
Standards vs procedures How to disassemble an engine step by step
How to disassemble an engine step by step Rnav procedures
Rnav procedures Provider performed microscopy
Provider performed microscopy Mutually exclusive codes
Mutually exclusive codes Classroom procedures
Classroom procedures Module 12 emergency procedures
Module 12 emergency procedures Family medicine procedures
Family medicine procedures