STUDY DESIGN FOR EARLY PHASE CLINICAL TRIALS Christopher












- Slides: 46

STUDY DESIGN FOR EARLY PHASE CLINICAL TRIALS Christopher S. Coffey Professor, Department of Biostatistics Director, Clinical Trials Statistical and Data Management Center May 30, 2014

OUTLINE In this webinar, we will: I. Discuss the importance of adequate study planning for small clinical trials II. Describe some analytical approaches that have merit with small clinical trials III. Describe several proposed designs for small clinical trials 2

NOTICE OF RECORDING Ø This webinar is being recorded. Ø In accordance with our open access mission, we will be posting a video and the slides to our website and they will be publically available Ø You can talk to us using the “chat” function, and we will speak our responses Ø Also, please use audience response when prompted 3

OVERVIEW Before addressing some possible designs of interest, it is useful to review some key recommendations from the Executive Summary in the National Academy of Sciences document. 4

OVERVIEW Key Recommendations from the National Academy of Sciences Document – Small Clinical Trials, Issues and Challenges: 1) Define the research question 2) Tailor the design 3) Clarify methods when reporting trial results 4) Perform corroborative statistical analysis 5) Exercise caution in interpretation 6) More research on alternative designs is needed 5

OVERVIEW Key Recommendations from the National Academy of Sciences Document – Small Clinical Trials, Issues and Challenges: 1) Define the research question 2) Tailor the design 3) Clarify methods for reporting trial results 4) Perform corroborative statistical analysis 5) Exercise caution in interpretation 6) More research on alternative designs is needed So, why is this any different from other trials? 6

OVERVIEW Three basic requirements for any clinical trial: 1) Trial should examine an important research question 2) Trial should use a rigorous methodology that can answer the question of interest 3) Trial must be based on ethical considerations and assure that risks to subjects are minimized 7

OVERVIEW These three requirements should also apply to all early phase clinical trials. However, due to size limitations designing early phase clinical trials can be a formidable challenge. As a consequence, the importance of adequate study planning is magnified in small clinical trials. 8

OVERVIEW Ø Late phase RCT: • Large sample sizes • Many standard designs Ø Small populations • Efficiency is critical • Use models (but pre-specify) • Carefully evaluate alternative designs 9

EARLY PHASE DESIGNS Designs of interest in early phase clinical trials: Ø 3+3 Design Ø Continual Reassessment Method Ø Repeated measures design Ø Crossover design Ø N-of-1 design Ø Futility design Ø Ranking/Selection design Ø Adaptive designs 10

DOSE FINDING Very early learning phase designs may seek to determine the maximum tolerated dose (MTD). Accurate determination of the MTD is critical since it will likely be used as the maximum dose in future clinical development. Ø If dose too low, a potentially useful drug could be missed Ø If dose too high, participants in future studies could be put at risk 11

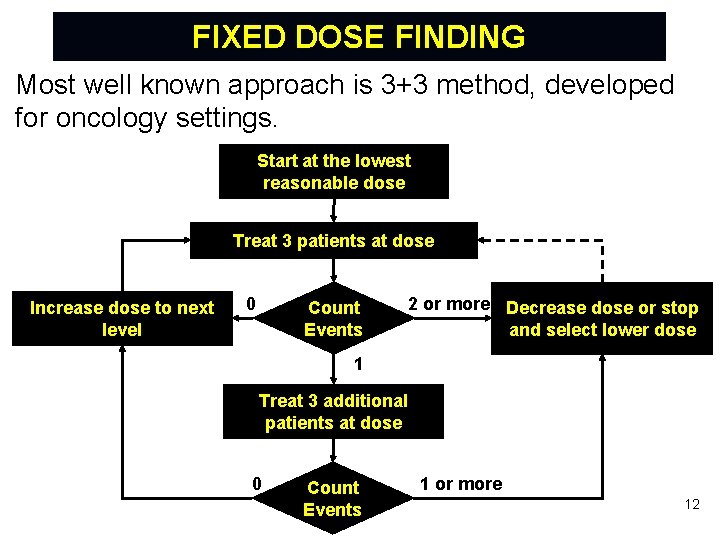
FIXED DOSE FINDING Most well known approach is 3+3 method, developed for oncology settings. Start at the lowest reasonable dose Treat 3 patients at dose Increase dose to next level 0 Count Events 2 or more Decrease dose or stop and select lower dose 1 Treat 3 additional patients at dose 0 Count Events 1 or more 12

FIXED DOSE FINDING Strengths of Conventional 3+3 Designs: Ø Simple and intuitive algorithm Ø Easy to implement and monitor – requires no computer program Ø Familiar to many clinicians However, the method has been criticized for treating many patients at low, ineffective doses and not producing a good estimate of the MTD. 13

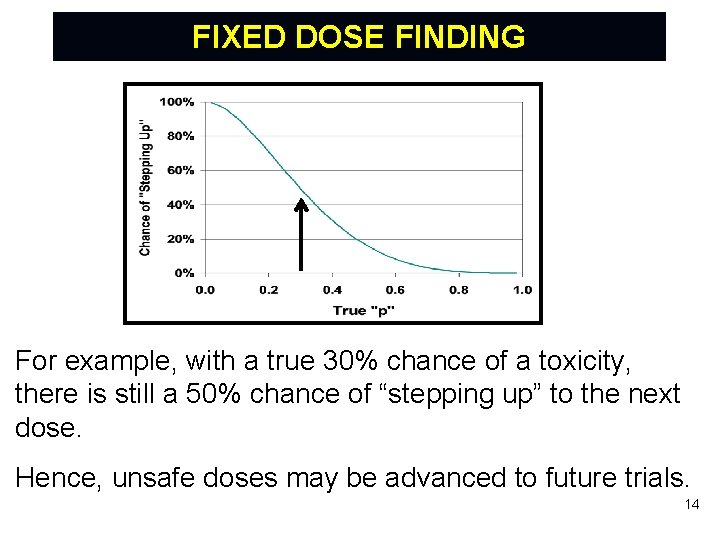
FIXED DOSE FINDING For example, with a true 30% chance of a toxicity, there is still a 50% chance of “stepping up” to the next dose. Hence, unsafe doses may be advanced to future trials. 14

ADAPTIVE DOSE FINDING Adaptive dose finding methods offer more efficient ways to learn about dose response. Most common approach is Continual Reassessment Method [CRM - See Garrett-Mayer (Statistics in Medicine, 2006) for an excellent tutorial]. Ø Originated as a Bayesian method for phase I cancer trials of cytotoxic agents. Ø Assumes a particular model (such as logistic function), and probabilities of both efficacy and toxicity increase with increasing dose Ø Assignment of doses converges to the MTD. 15

ADAPTIVE DOSE FINDING Steps for implementing CRM: 1) Begin with assumed a priori dose-toxicity curve and a chosen target toxicity rate 2) Assign first subject(s) dose most likely to be associated with target toxicity level 3) Updated dose-toxicity curve is refit (shifted slightly up or down) depending on whether or not first subject(s) experienced a DLT 4) Next subject assigned dose closest to target toxicity level based on updated curve 5) Continue until some pre-defined stopping criteria are met 16

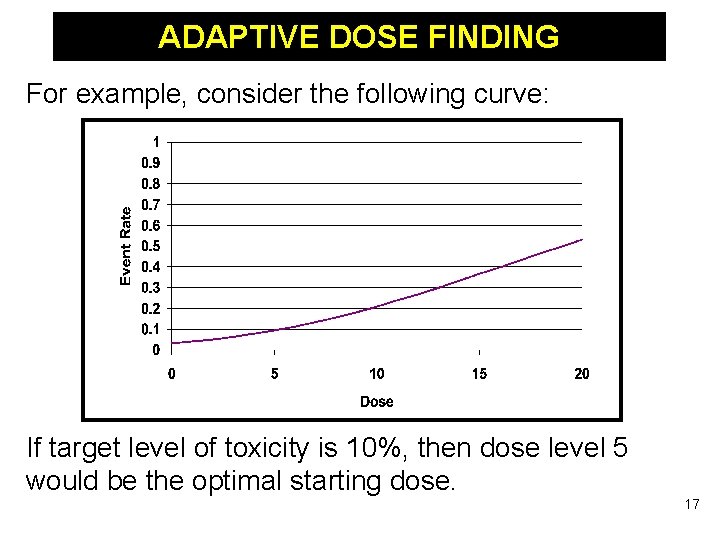
ADAPTIVE DOSE FINDING For example, consider the following curve: If target level of toxicity is 10%, then dose level 5 would be the optimal starting dose. 17

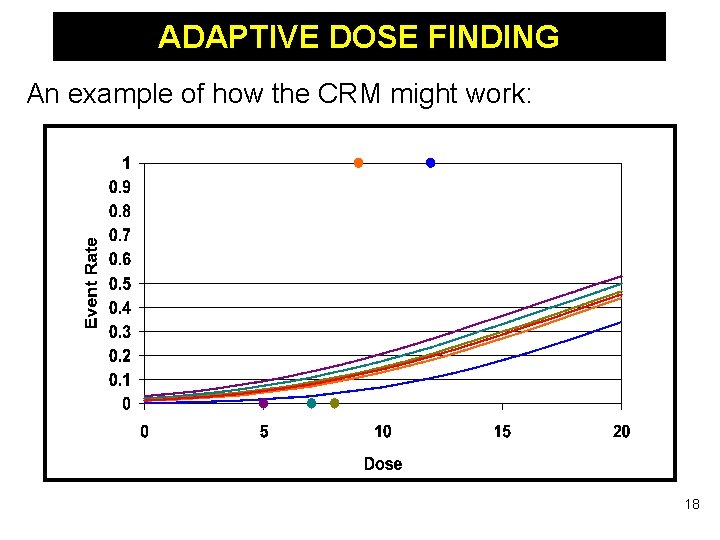
ADAPTIVE DOSE FINDING An example of how the CRM might work: 18

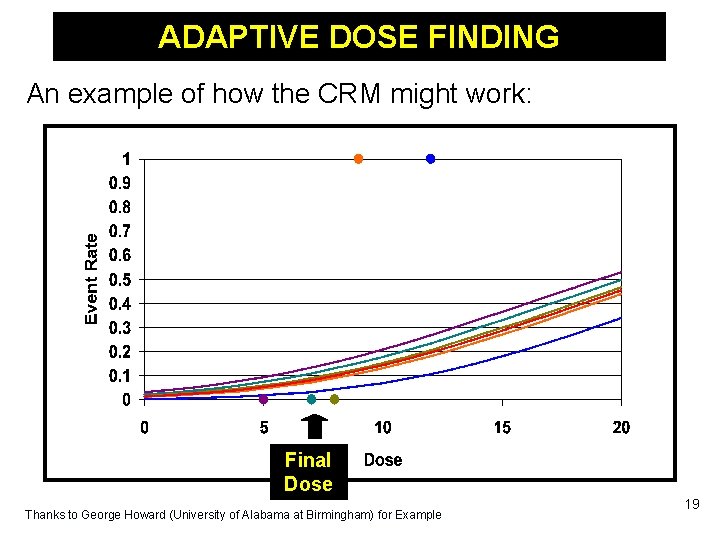
ADAPTIVE DOSE FINDING An example of how the CRM might work: Final Dose Thanks to George Howard (University of Alabama at Birmingham) for Example 19

ADAPTIVE DOSE FINDING Strengths of CRM: Ø “Learns” from information gained at early time points in the study – all participants studied contribute to the estimated dose. Ø Generally more efficient/safer than 3+3 design • Can more accurately estimate the MTD as compared to standard 3+3 designs • More likely to treat participants at doses around the MTD • Less likely to treat participants at ineffective doses • Less likely to treat participants at toxic doses – tends to incur fewer dose-limiting toxicities. 20

ADAPTIVE DOSE FINDING Drawbacks of CRM: Ø Implementation requires a substantial collaboration between the investigator and statistician Ø Mathematical and statistical complexities make it difficult for many clinical investigators to understand. Ø Properties must be assessed via simulation. Ø Safety concern with original CRM: Large dose escalations can occur early based on limited information. 21

ADAPTIVE DOSE FINDING Several modified CRM approaches have been developed to address these concerns: Ø Always start at lowest dose level under consideration Ø Enroll 2 -3 patients in each cohort Ø Any given dose escalation cannot increase by more than one level. 22

REPEATED MEASURES DESIGNS Multiple observations or response variables are obtained for each subject. - Repeated measurements over time (longitudinal) - Multiple measurements on same subject Allows both between-subject and within-subject comparisons. Can reduce the required sample size needed to obtain a specific target power. 23

REPEATED MEASURES DESIGNS Suppose you are measuring over time: Ø STANDARD: Final value – Baseline value Ø BETTER: Final value, with baseline value as a covariate Ø STILL BETTER: Longitudinal • Differentiate “through” vs. “at” • Think about variance/covariance structure • Think how you want to model time 24

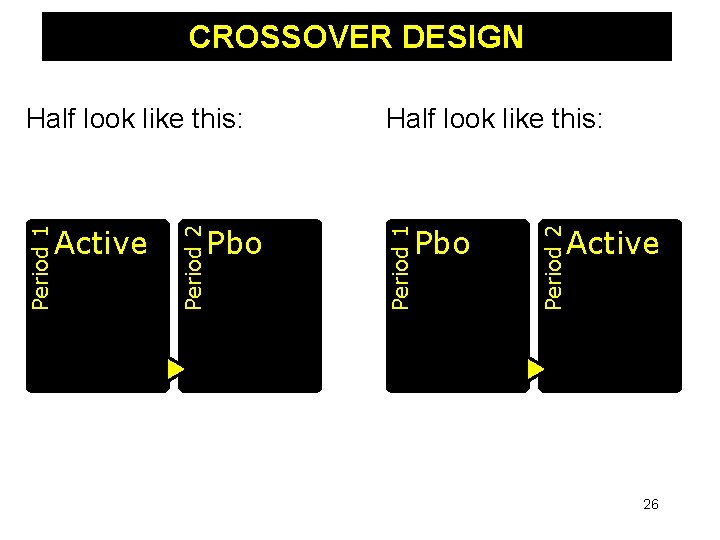
CROSSOVER DESIGN Each subject exposed to all treatments - Order of treatments randomized - First may show better (or worse) effect Prognostic factors balanced – self vs. self Required sample size reduced considerably due to self vs. self comparisons Each participant receives the active treatment at some point during the study 25

CROSSOVER DESIGN Pbo Period 2 Pbo Half look like this: Period 1 Active Period 2 Period 1 Half look like this: Active 26

CROSSOVER DESIGN Disadvantages: Ø Disease needs to be long-term Ø Treatment must be taken regularly over time Ø Relevant outcomes must occur and be measured over time Ø Not relevant for acute treatments Ø Concerns due to a ‘carryover effect’ 27

N-OF-1 DESIGN Special case of a crossover/repeated measures design, where a single subject undergoes treatment for several pairs of periods. For each pair: Ø Subject receives experimental treatment for one part of each pair Ø Subject receives alternative treatment for other pair Ø Order of two treatments within each pair is randomized 28

N-OF-1 DESIGN The final outcome of the trial is a determination about the best treatment for the particular subject under study. Most feasible for treatments with rapid onset that stop acting soon after discontinuation. Results of a series of N-of-1 trials may be combined using meta-analysis. 29

SELECTION DESIGNS Selection (ranking) designs compare parameters of multiple (k) study populations. Generally require smaller sample sizes than trials designed to estimate and test treatment effects. Selection designs can be used to: Ø Select the treatment with the best response out of k potential treatments Ø Rank treatments in order of preference Ø Rule out poor treatments for further study (Helpful with ‘pipeline’ problem) 30

FUTILITY DESIGN A futility design is a screening tool to identify agents that should not be candidates for phase III trials while minimizing costs/sample size. The main intent is to identify ineffective treatments, not to establish efficacy. To use a futility design, a research must define an effect of interest. If study shows unlikely for future trials to obtain desired effect, it is deemed ‘futile’ to conduct future trials. 31

FUTILITY DESIGN For example, suppose a 10% increase in favorable response rates is clinically meaningful. A futility design would assess the following hypothesis: H 0: Treatment improves outcome by at least 10% compared to placebo (p. T – p. P ≥ 0. 10) versus H 1: Treatment does not improve outcome by at least 10% compared to placebo (p. T – p. P < 0. 10) – futile to consider in a phase III trial 32

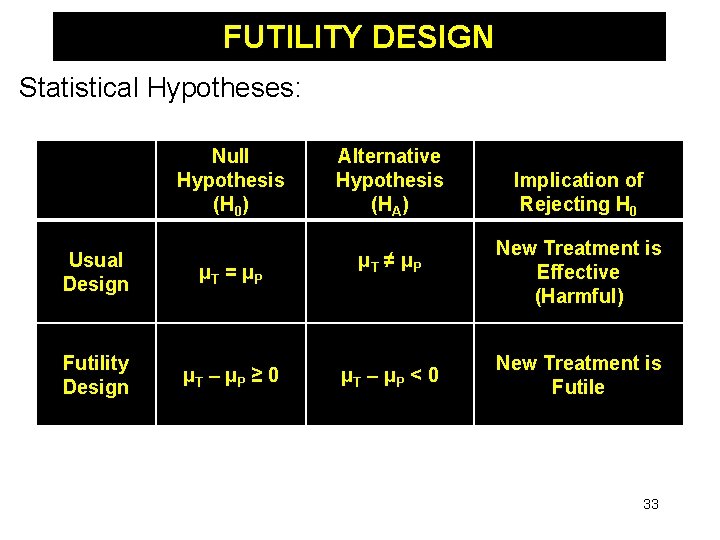
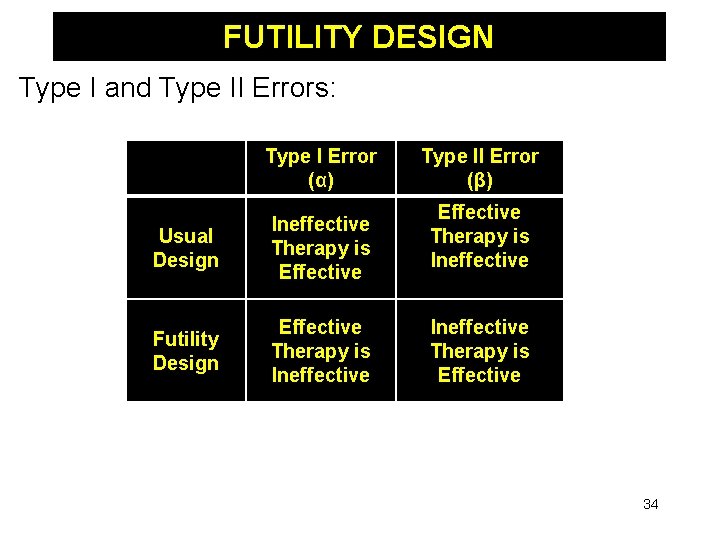
FUTILITY DESIGN Statistical Hypotheses: Null Hypothesis (H 0) Usual Design μT = μP Futility Design μT – μP ≥ 0 Alternative Hypothesis (HA) μT ≠ μP μT – μP < 0 Implication of Rejecting H 0 New Treatment is Effective (Harmful) New Treatment is Futile 33

FUTILITY DESIGN Type I and Type II Errors: Type I Error (α) Usual Design Ineffective Therapy is Effective Futility Design Effective Therapy is Ineffective Type II Error (β) Effective Therapy is Ineffective Therapy is Effective 34

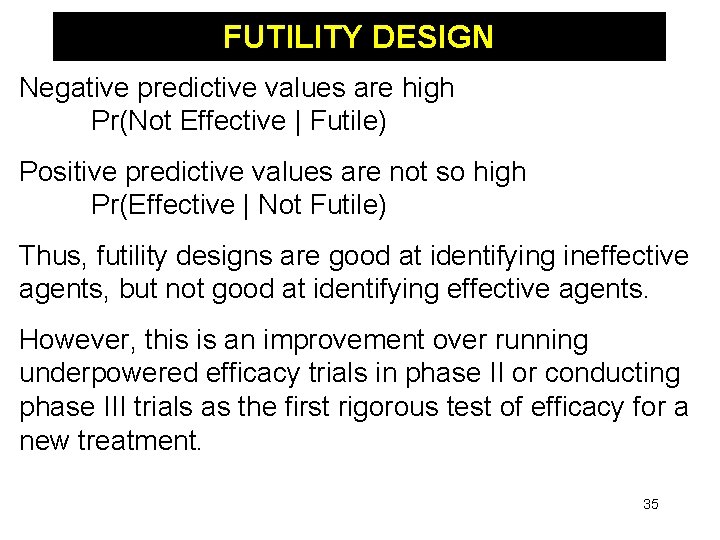
FUTILITY DESIGN Negative predictive values are high Pr(Not Effective | Futile) Positive predictive values are not so high Pr(Effective | Not Futile) Thus, futility designs are good at identifying ineffective agents, but not good at identifying effective agents. However, this is an improvement over running underpowered efficacy trials in phase II or conducting phase III trials as the first rigorous test of efficacy for a new treatment. 35

ADAPTIVE DESIGNS There may be limited information to guide initial choices for the design of a study. Since more knowledge will accrue as the study progresses, adaptive designs allow these elements to be reviewed during the trial. An adaptive design allows for changing or modifying the characteristics of a trial based on cumulative information. 36

ADAPTIVE DESIGNS Ph. RMA Working Group on Adaptive Designs (2006): “By adaptive design we refer to a clinical study design that uses accumulating data to modify aspects of the study as it continues, without undermining the validity and integrity of the trial. …changes are made by design, and not on an ad hoc basis…not a remedy for inadequate planning. ” FDA Draft Guidance Document (2010): “… a study that includes a prospectively planned opportunity for modification of one or more specified aspects of the study design and hypotheses based on analysis of data (usually interim data) from subjects in the study. ” “Adaptive By Design” 37

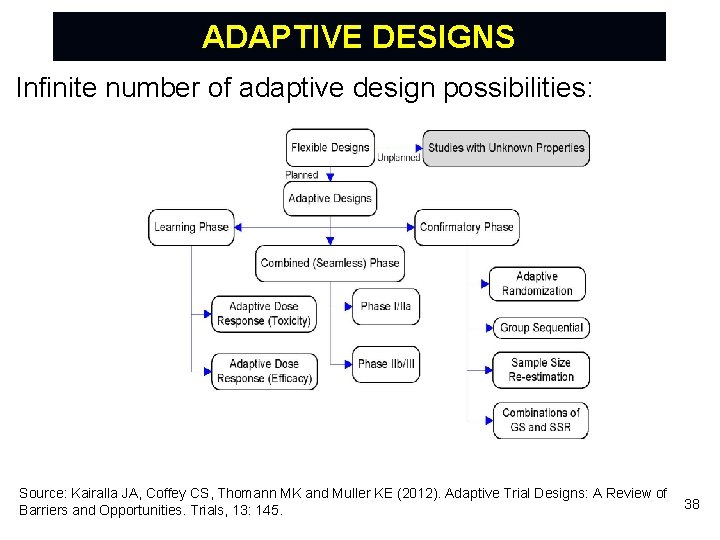
ADAPTIVE DESIGNS Infinite number of adaptive design possibilities: Source: Kairalla JA, Coffey CS, Thomann MK and Muller KE (2012). Adaptive Trial Designs: A Review of Barriers and Opportunities. Trials, 13: 145. 38

GROUP SEQUENTIAL DESIGNS Given this definition, group sequential designs (that allow early termination for efficacy or futility) are one of the most commonly used ADs in clinical trials. Several approaches have been proposed to adjust for repeated testing and control overall type I error rate. It is important to recognize that many of these techniques are based on large sample theory. For small clinical trials, the distributions used for these calculations become increasingly inaccurate and may lead to poor performance of these ‘standard’ techniques. 39

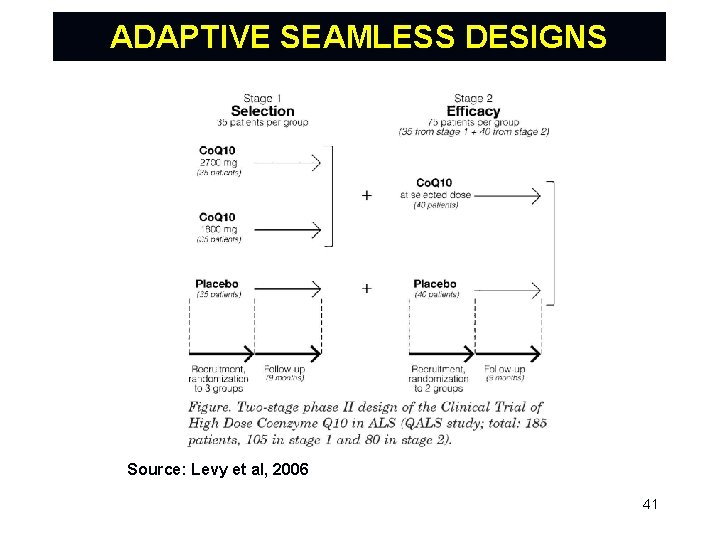
ADAPTIVE SEAMLESS DESIGNS A seamless design combines objectives traditionally addressed in separate trials into a single trial. An adaptive seamless design combines trials and uses data from patients enrolled before and after the adaptation for the final analysis. Most interest to date has been with a seamless transition between phase IIb (learning) and III (confirming). However, there also opportunities for seamless designs in early development (phase I/IIa). 40

ADAPTIVE SEAMLESS DESIGNS Source: Levy et al, 2006 41

ADAPTIVE SEAMLESS DESIGNS Adaptive seamless designs have the potential to improve the drug development process by reducing the timelines for approval. However, analysis requires specialized methods to correct for bias introduced because data from the first stage are used for both decision making and final analysis. Hence, extra planning is necessary when implementing an adaptive seamless design protocol. The potential benefits should be carefully weighed against the challenges of such designs. 42

ADAPTIVE DESIGNS It is important to be clear about what an adaptive design can and cannot do in the rare disease setting. An AD cannot “change the answer” regarding the effectiveness of a particular treatment, but can increase the efficiency in finding an answer. An AD design cannot make a drug more effective. Rather, one of the biggest benefits of an AD is ability to identify ineffective treatments in a more timely manner. 43

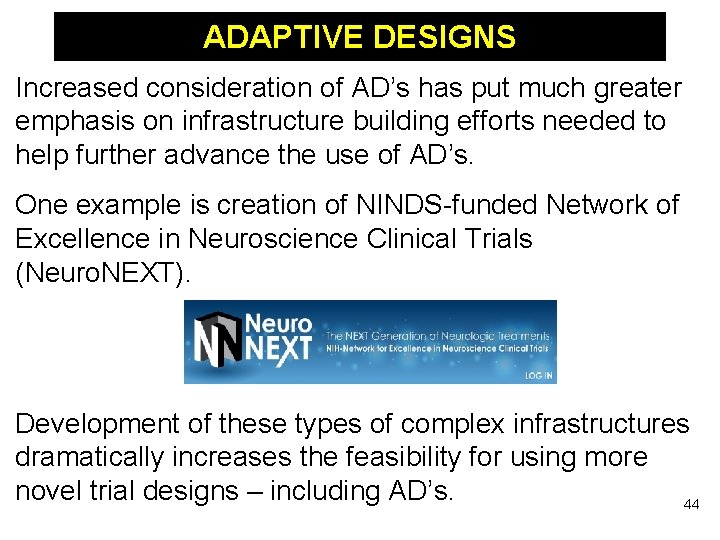
ADAPTIVE DESIGNS Increased consideration of AD’s has put much greater emphasis on infrastructure building efforts needed to help further advance the use of AD’s. One example is creation of NINDS-funded Network of Excellence in Neuroscience Clinical Trials (Neuro. NEXT). Development of these types of complex infrastructures dramatically increases the feasibility for using more novel trial designs – including AD’s. 44

SUMMARY An appropriate study design has sufficient sample size, adequate power, and proper control of bias to allow a meaningful interpretation of the results. Although early phase trials pose important limitations, the above issues cannot be ignored. One of the objectives of this course is to give researchers the tools they need to successfully design these types of trials. 45

EVALUATIONS Ø Please consider going to the following website to evaluate this webinar: https: //umichumhs. qualtrics. com/SE/? SID=SV_9 Hyyo. Tw. DYEHsf 2 J 46