States of matter What does a scientist look

















- Slides: 21

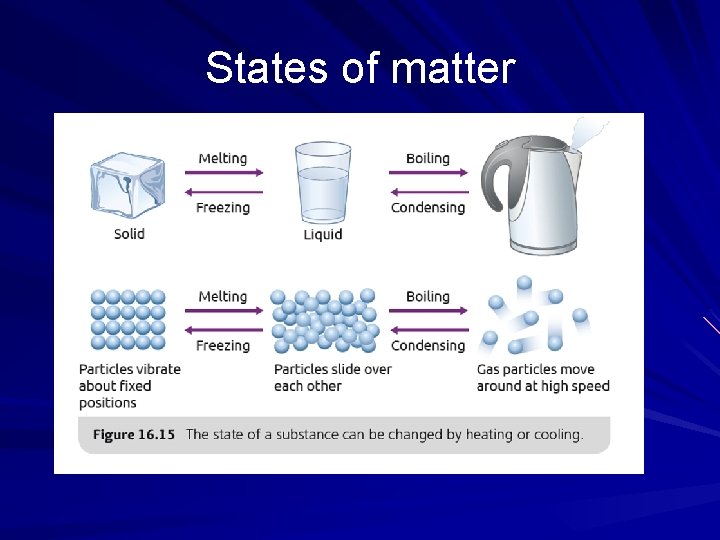
States of matter

What does a scientist look like?

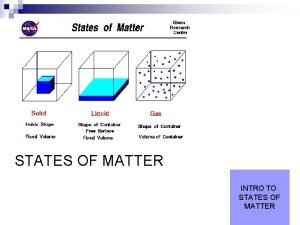
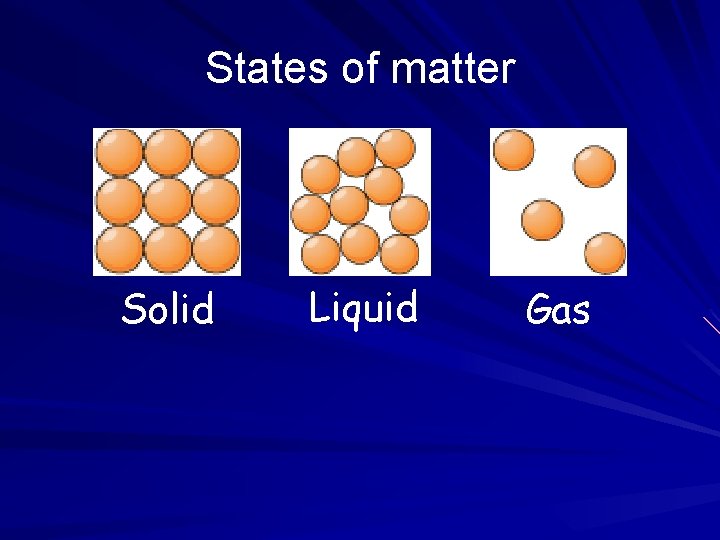
States of matter Solid Liquid Gas


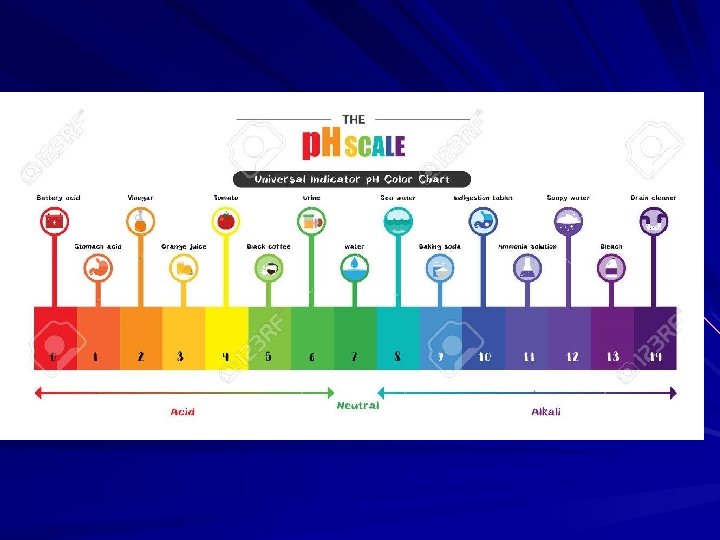
Acidic nature of carbon dioxide solution Add dry ice to water in a beaker or graduated cylinder at room temperature containing some universal indicator solution.

Explanation The indicator turns red implying an acidic solution. The green colour can be restored using an alkali. The carbon dioxide reacts with the water to form carbonic acid.

Change of state - condensation

Using the concept of condensation to explain the formation of the cloud of water vapour The very cold dry ice causes the warm water vapour to condense into droplets of water to form the cloud.

Making soapy bubbles using dry ice

Explanation The soap solution traps the water vapour and carbon dioxide gas in the form of a bubble. The pressure of the gas forces the bubbles out of the graduated cylinder containing warm soapy water. Note that the bubbles fall down due to fact that density of carbon dioxide is higher than that of air.

Adding Dry Ice to balloons and lab gloves

Why is carbon dioxide used in fire extinguishers? Place some pellets of dry ice at the bottom of a transparent tank for a few minutes – do this at the beginning of the class. Place some lighting candles of various heights in the tank. Note what happens to each candle.

Explanation The carbon dioxide sublimes and the invisible carbon dioxide displaces the air and fills the tank. Since carbon dioxide does not support combustion, the candles lower down are extinguished.

Floating air bubbles on CO 2 14

Explanation The bubble contains air. The trough is filled with invisible carbon dioxide which is more dense than air The less dense bubble floats on the more dense carbon dioxide.

Making Boo Bubbles

Explanation The carbon dioxide gets trapped in the detergent forming bubbles. The density of carbon dioxide causes the bubbles to fall downwards.

Making a crystal ball bubble using dry ice

Explanation Moving the piece of cotton cloth across the mouth of the bowl, creates a thin layer of soap film. The diffusion of carbon dioxide gas away from the water causes it to be trapped within the bubble.

Rocket launcher Place some warm water in a plastic bottle. The apparatus shown in this experiment was purchased from Chillisticks. Add some dry ice to the warm water. Quickly insert the rubber stopper firmly into the bottle and place the stoppered bottle in the jug with the stopper at the end of the jug as shown. The jug should be pointing away from the audience. The pressure of carbon dioxide launches the bottle into the air!

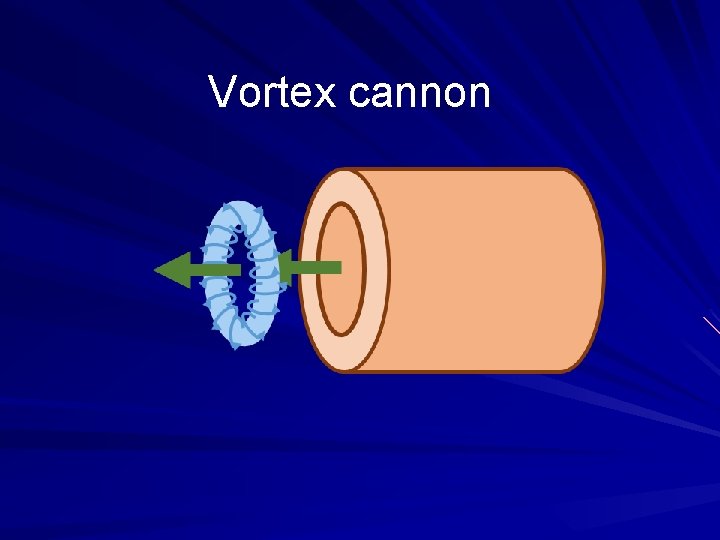
Vortex cannon
 Look down look up
Look down look up States of matter foldable
States of matter foldable Four states of matter
Four states of matter Four states of matter
Four states of matter States of matter
States of matter Thermal energy in states of matter
Thermal energy in states of matter Changing state
Changing state Phet states of matter basics
Phet states of matter basics 5 states of matter
5 states of matter Solid liquid venn diagram
Solid liquid venn diagram The kinetic theory of matter states that
The kinetic theory of matter states that There were 11
There were 11 Southern states vs northern states
Southern states vs northern states Classification of matter section 1 composition of matter
Classification of matter section 1 composition of matter Chapter 12 states of matter study guide
Chapter 12 states of matter study guide Chapter 10 review states of matter section 4
Chapter 10 review states of matter section 4 States of matter: basics
States of matter: basics States of matter foldable
States of matter foldable Thermal energy vs heat
Thermal energy vs heat Uses of heat
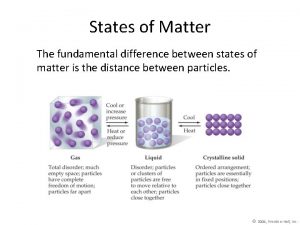
Uses of heat The fundamental difference between states of matter is the
The fundamental difference between states of matter is the Stayes of matter
Stayes of matter