Soil Chemistry Soil Chemistry Mineral salts From weathered



















- Slides: 30

Soil Chemistry

Soil Chemistry • Mineral salts – From weathered rocks – Break down of Organic Matter (OM) – Addition of fertilizer

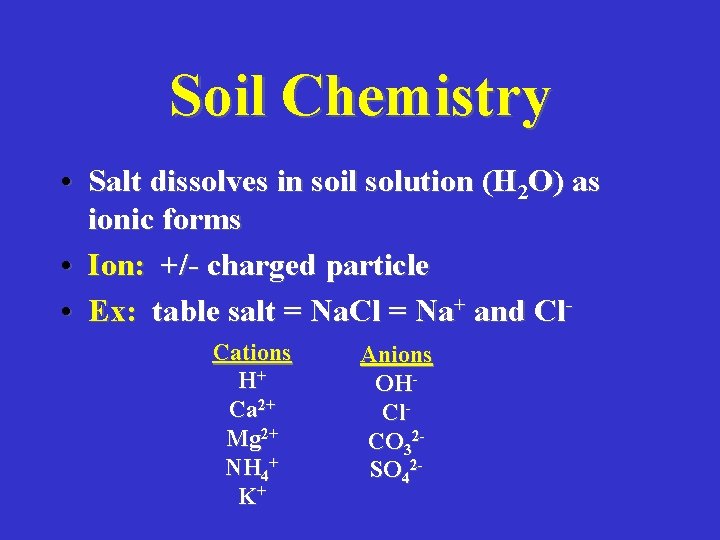
Soil Chemistry • Salt dissolves in soil solution (H 2 O) as ionic forms • Ion: +/- charged particle • Ex: table salt = Na. Cl = Na+ and Cl. Cations H+ Ca 2+ Mg 2+ NH 4+ K+ Anions OHCl. CO 32 SO 42 -

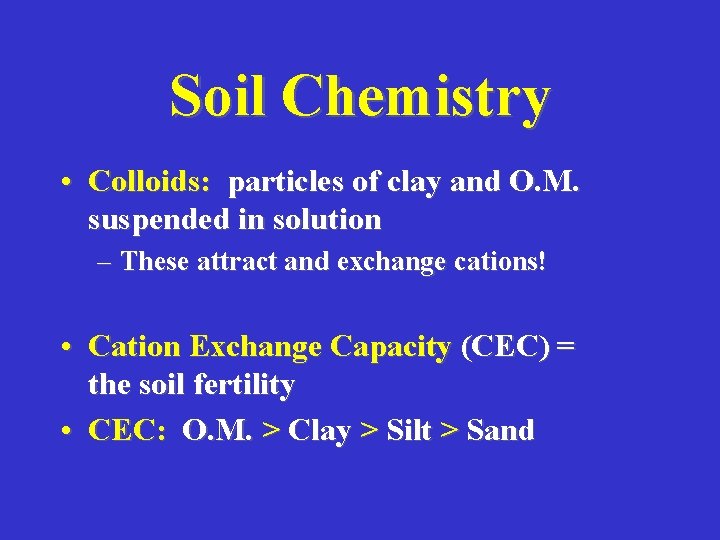
Soil Chemistry • Colloids: particles of clay and O. M. suspended in solution – These attract and exchange cations! • Cation Exchange Capacity (CEC) = the soil fertility • CEC: O. M. > Clay > Silt > Sand

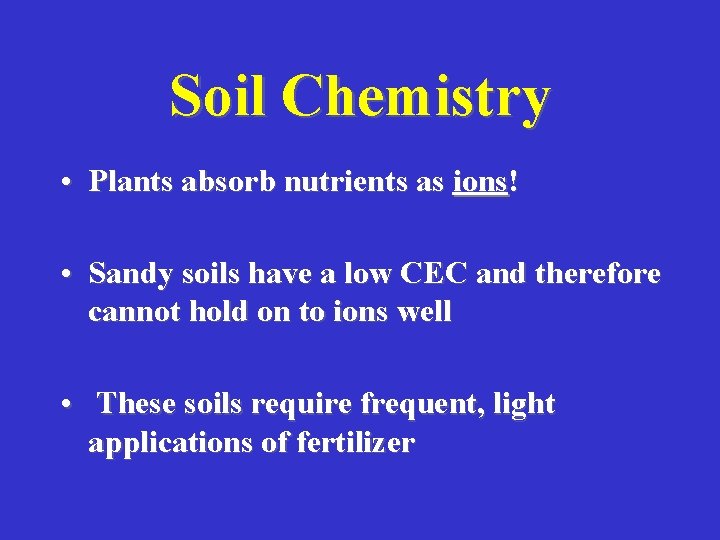
Soil Chemistry • Plants absorb nutrients as ions! • Sandy soils have a low CEC and therefore cannot hold on to ions well • These soils require frequent, light applications of fertilizer

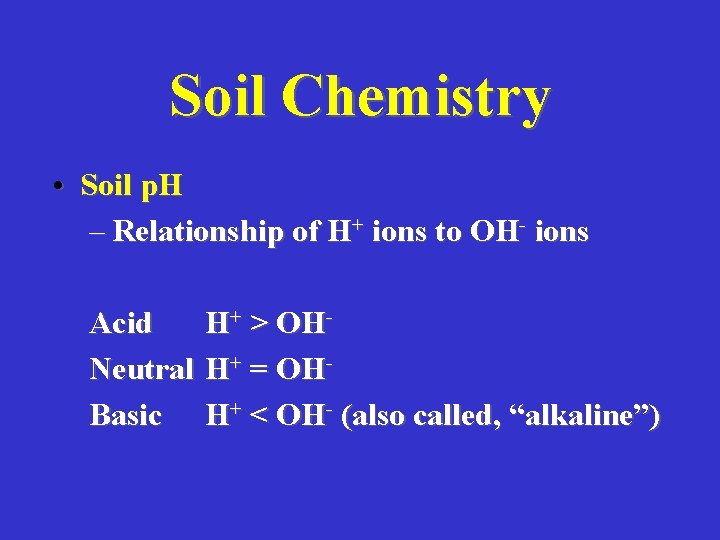
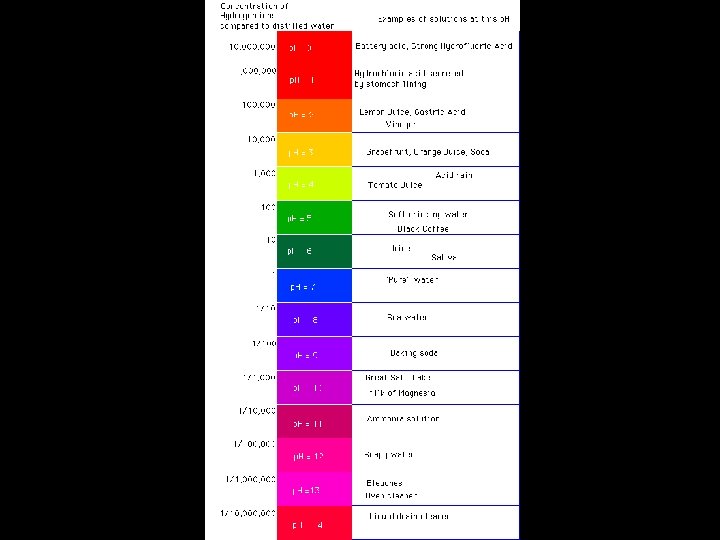
Soil Chemistry • Soil p. H – Relationship of H+ ions to OH- ions Acid Neutral Basic H+ > OHH+ = OHH+ < OH- (also called, “alkaline”)

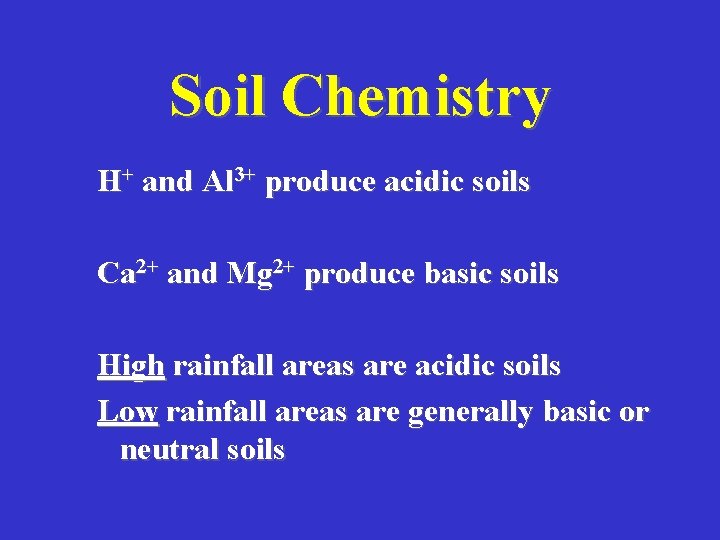
Soil Chemistry H+ and Al 3+ produce acidic soils Ca 2+ and Mg 2+ produce basic soils High rainfall areas are acidic soils Low rainfall areas are generally basic or neutral soils

Soil Chemistry Salt build-up in low-rainfall location damages crops

• Fungi most active if p. H<5. 5 • Bacteria most active if p. H>5. 5 Properly nodulated legumes add 55 to 300 pounds of nitrogen per acre to soil.



p. H of various liquids household ammonia 11. 9 sea water 8. 5 human blood 7. 4 milk 6. 4 orange juice 3. 5 lemon juice 2. 3 vinegar 2. 8


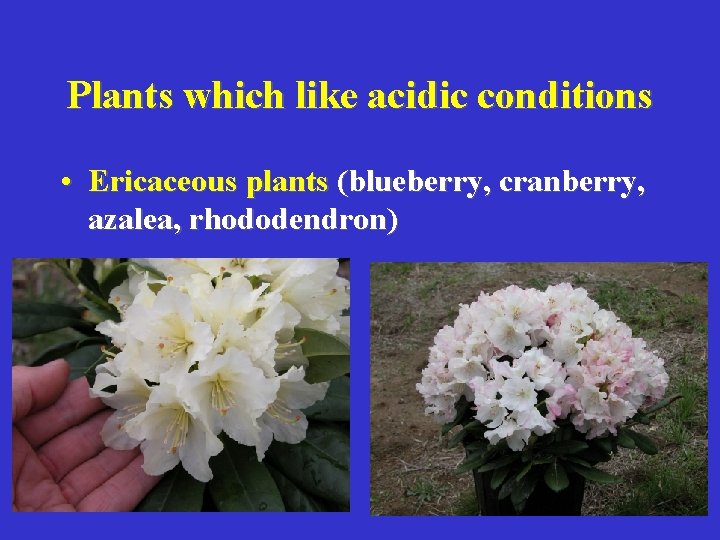
Plants which like acidic conditions • Ericaceous plants (blueberry, cranberry, azalea, rhododendron)

Plants which like acidic conditions • Ericaceous plants (blueberry, cranberry, azalea, rhododendron)

Plants which like acidic conditions • Ericaceous plants (blueberry, cranberry, azalea, rhododendron)

Plants which like acidic conditions • Ericaceous plants (blueberry, cranberry, azalea, rhododendron)

Plants which like acidic conditions • Ericaceous plants (blueberry, cranberry, azalea, rhododendron)

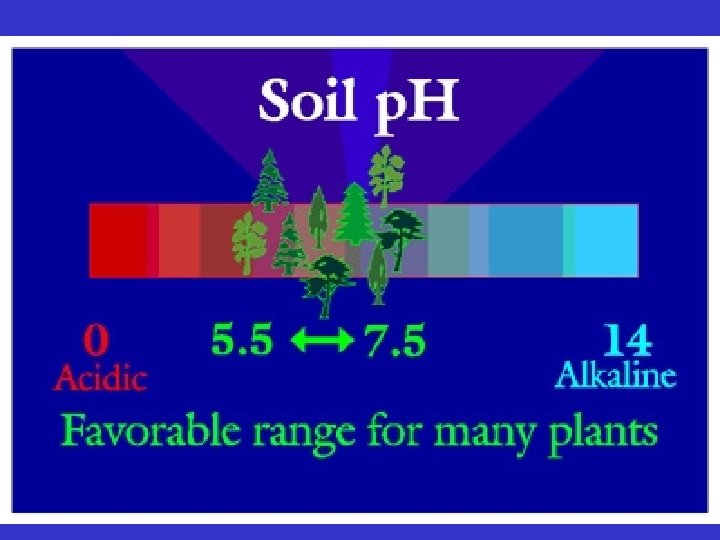
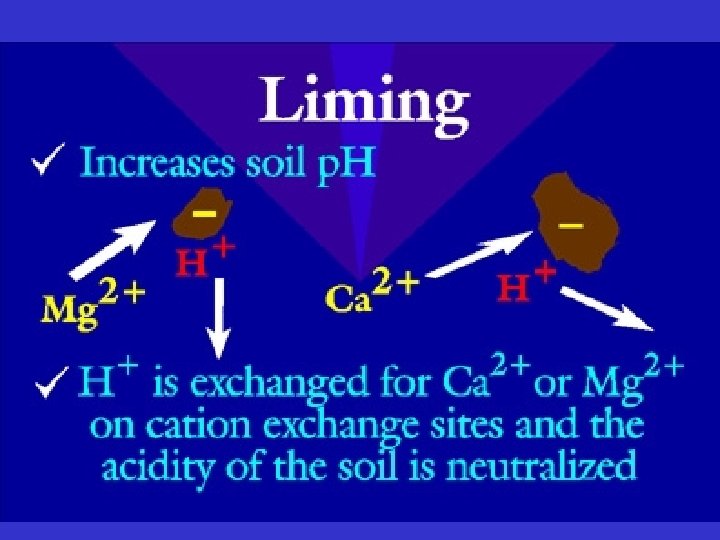
Soil Chemistry • If p. H very low or very high, soil nutrients become unavailable to the plant • Liming - raises the p. H (makes the soil more basic) and is used on acidic soils. – Ca. CO 3 – Mg. CO 3 • Most of Vermont’s soils are acidic • Most tropical soils are acidic due to high rainfall

Liming DON’TS • You should NOT lime your lawn after having applied a fertilizer that contains ammonium nitrate. The ammonium is converted to ammonia gas which can cause burning on the foliage of the grass and surrounding trees and shrubs!

Cliff of limestone


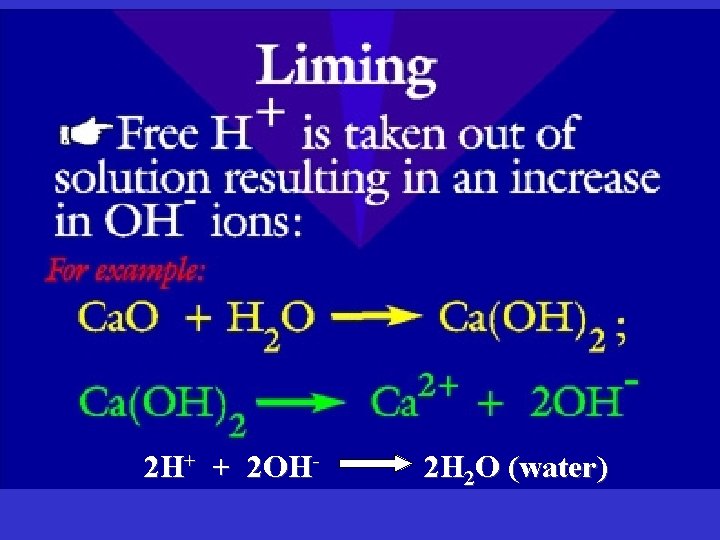
2 H+ + 2 OH- 2 H 2 O (water)

Soil Chemistry • Acidifying the soil or lowering the p. H – Elemental sulfur (effect is temporary) – Ammonium sulfate (effect is temporary) – Aluminum sulfate is also used but not recommended as aluminum is a heavy metal!

Soil Chemistry • Basic soils often cause iron chlorosis in plants • This can be corrected by acidifying the soil or choosing a plant which is tolerant of alkaline soils! • Intervienal chlorosis: often on acid-loving plants like roses, fruit trees, azaleas and rhododendrons




Blue in acidic soils <5. 5 with available aluminum Pink in neutral soils near p. H of 7

 Soil is a mixture of weathered rock and ________.
Soil is a mixture of weathered rock and ________. Showeet com
Showeet com The removal and transport of weathered materials from
The removal and transport of weathered materials from In o captain my captain the ship is metaphor for
In o captain my captain the ship is metaphor for Regolith in soil profile
Regolith in soil profile The process by which natural forces move weathered
The process by which natural forces move weathered Acid and bases properties
Acid and bases properties Ferric alum as a double salt
Ferric alum as a double salt Carbonate salts examples
Carbonate salts examples Write the probable colour of the following salts
Write the probable colour of the following salts Naming esters
Naming esters Pure planet earth
Pure planet earth Oceans
Oceans Chlor-rid industrial cleaning
Chlor-rid industrial cleaning Example of elixir in pharmacy
Example of elixir in pharmacy Soluble and insoluble salts
Soluble and insoluble salts Acidic salt examples
Acidic salt examples To detect the presence of adulterants in sugar
To detect the presence of adulterants in sugar Chapter 19 acids bases and salts answer key
Chapter 19 acids bases and salts answer key Neutral salts
Neutral salts Carbonate salts examples
Carbonate salts examples Bile salts in urine
Bile salts in urine White rush bath salts
White rush bath salts Bile salts name
Bile salts name Zn chemistry
Zn chemistry Antiferomagnetism
Antiferomagnetism Acid and base products
Acid and base products Bile salts in urine
Bile salts in urine Chapter 19 acids bases and salts
Chapter 19 acids bases and salts Bile salts name
Bile salts name Chapter 19 acids bases and salts
Chapter 19 acids bases and salts