Session 2 EMERGING MDROs and Overview of Clostridium



















- Slides: 75

Session 2: EMERGING MDROs and Overview of Clostridium difficile Session 2 1

Objectives of this session Section A: Emerging MDROs • • • Understand the mechanisms of antibiotic resistance Define extended spectrum beta-lactamase organisms Identify multidrug resistant Acinetobacter Identify methods of Acinetobacter spread Define New Delhi Metallo-beta-lactamase Define Klebsiella pneumoniae carbapenemase Section B: Introduction to Clostridium difficile • • • Define C. diff Identify significance in Texas Distinguish diagnosis by severity Session 2 2

Emerging MDROs Section A: In this section we will discuss: • Extended Spectrum Beta-Lactamase producing gram negative bacteria (ESBLs) – MDR Acinetobacter • Carbapenem-resistant Enterobacteriaceae (CRE) – New Delhi Metallo-beta-lactamase (NDM-1) – Klebsiella pneumoniae carbapenemase (KPC) http: //www. slideshare. net/doctorrao/acinetobacter-baumannii Session 2 [for more info] 3

http: //www. fda. gov/animalveterinary/safetyhealth/antimicrobialresistance/ucm 134359. htm

Emerging MDROs Acinetobacter Carbapenem-resistant Enterobacteriaceae (CRE) Session 2 5

Extended-Spectrum Beta-Lactamase producing gram-negative bacteria (ESBLs) • ESBLs refer to many types of bacteria (gramnegative and some gram-positive) that have developed a powerful enzyme that breaks up the structure of some antibiotics, such as penicillins and cephalosporins • ESBL prevalance is unknown, but is increasing – Some reports show that 10 -40% of strains of Escherichia coli and Klebsiella pneumoniae express ESBLs Rupp & Fey. Drugs 2003; 63: 353 -365. Accessed July 6 2014 Session 2 6

MDR Acinetobacter • Infections typically occur in ICU and other healthcare settings with very ill patients • Reasons to be concerned with Acinetobacter – Broad antimicrobial resistance – Can cause large, multi-facility nosocomial outbreaks • Increasing concern since the 1970 s • Widespread public attention in 2004 http: //www. slideshare. net/doctorrao/acinetobacter-baumannii. Accessed June 20, 2014 Session 2 7

From CDC’s ANTIBIOTIC RESISTANCE THREATS in the United States, 2013

MDR Acinetobacter • Between 1/1/02 and 8/31/04, Acinetobacter baumannii infected 102 severely injured service members associated with Operation Iraqi Freedom and Operation Enduring Freedom (Afghanistan) • 5 military treatment facilities were involved • Found to be resistant to : – Imipenem – Amikacin – Ampicillin/sulbactam – Piperacillin/tazobactam – Cefepime http: //www. cdc. gov/mmwr/preview/mmwrhtml/mm 5345 a 1. htm. Accessed July 6, 2014 – Ciprofloxacin Session 2 9

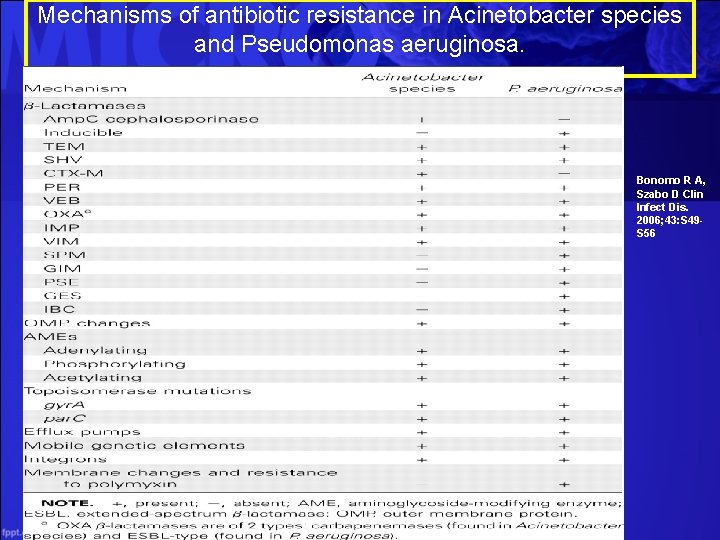
Mechanisms of antibiotic resistance in Acinetobacter species and Pseudomonas aeruginosa. Bonomo R A, Szabo D Clin Infect Dis. 2006; 43: S 49 S 56

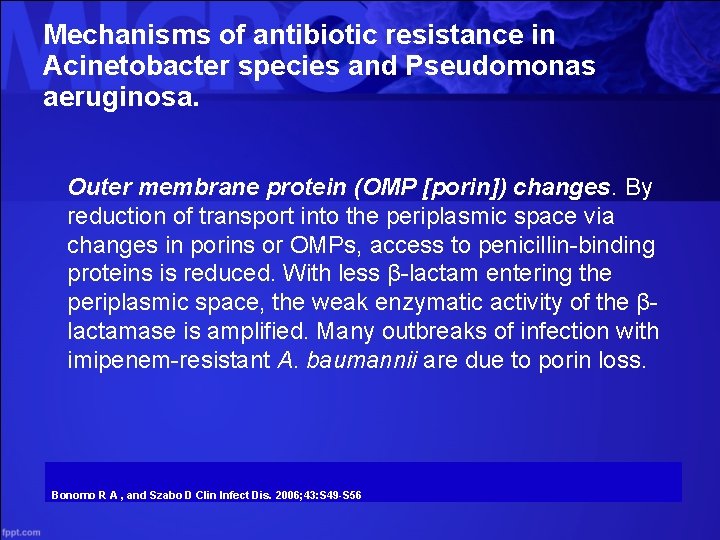
Mechanisms of antibiotic resistance in Acinetobacter species and Pseudomonas aeruginosa. Outer membrane protein (OMP [porin]) changes. By reduction of transport into the periplasmic space via changes in porins or OMPs, access to penicillin-binding proteins is reduced. With less β-lactam entering the periplasmic space, the weak enzymatic activity of the βlactamase is amplified. Many outbreaks of infection with imipenem-resistant A. baumannii are due to porin loss. Bonomo R A , and Szabo D Clin Infect Dis. 2006; 43: S 49 -S 56

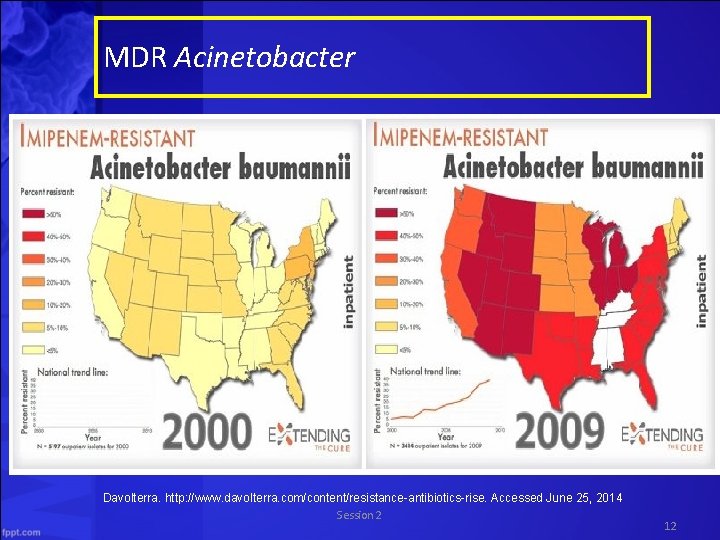
MDR Acinetobacter Davolterra. http: //www. davolterra. com/content/resistance-antibiotics-rise. Accessed June 25, 2014 Session 2 12

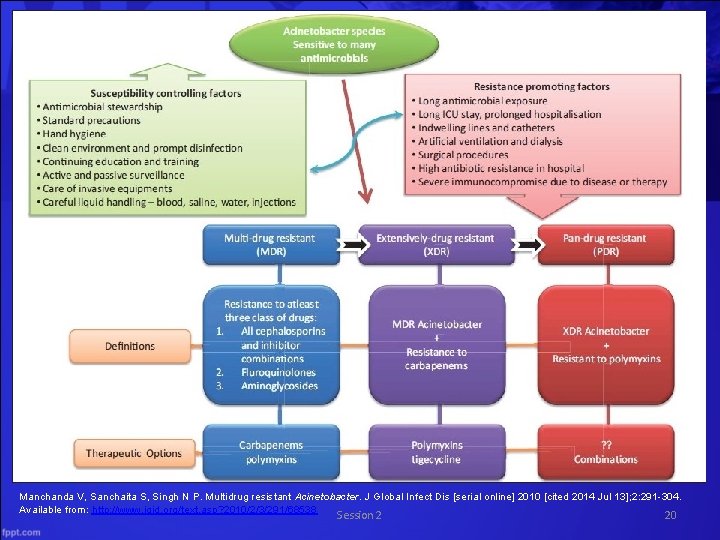
MDR Acinetobacter General Information • The Latin means “motionless” – these organisms lack cilia or flagella • Gram negative bacillus – The Acinetobacter baumannii species causes 80% of the reported infections associated with Acinetobacter • Commonly found in water and soil • Survives on surfaces for weeks http: //www. cdc. gov/HAI/organisms/acinetobacter. html. Accessed July 7, 2014 Manchanda et al. J Glob Infect Dis 2010; 2(3): 291 -304. Session 2 13

Many sources of colonization or infection with MDRA in a hospital environment Examples include: • Hands of the hospital staff • Food (including hospital food) • Tap water • Infusion pumps and Respiratory therapy equipment • Mattresses, pillows, bed curtains and blankets in vicinity of infected patients • Soap dispensers • Fomites like bed rails, stainless steel trolleys, door handles, telephone handles, tabletops • Hospital sink traps and floors Manchanda et al. J Glob Infect Dis 2010; 2(3): 291 -304. Session 2 14

MDR Acinetobacter Risk Factors for MDRA Infection • • • Exposure to antimicrobial agents esp. , carbapenems, colistin Receipt of mechanical ventilation Prolonged length of hospital stay Exposure to an intensive care unit (ICU) Colonization pressure Recent surgery Invasive procedures Underlying severity of illness Environmental contamination Source: Manchanda et al. J Glob Infect Dis 2010; 2(3): 291 -304. Session 2 15

MDR Acinetobacter Transmission • Person-to-person • Contact with contaminated surfaces • Survives for weeks on clothing, bed rails, ventilators, sinks, doorknobs – This makes transmission very difficult to control • Respiratory system is the most prominent route of entry Session 2 http: //www. cdc. gov/HAI/organisms/acinetobacter. html. Accessed July 7, 2014 Manchanda et al. J Glob Infect Dis 2010; 2(3): 291 -304. 16

MDR Acinetobacter Infections • Acinetobacter (especially A. baumannii) can cause the following infections: – Bacteremia – Pneumonia – Meningitis – Urinary tract infections – Wound infections • Has been shown to prolong mechanical ventilation dependence, ICU stay, and hospital stay Maragakis & Perl. Clinical Infectious Diseases 2008; 46(8): 1254 -63. Session 2 17

MDR Acinetobacter Treatment • Carbapenems (imipenem and meropenem) are the treatment of choice for Acinetobacter infections – Unfortunately, CRA isolates are increasingly reported worldwide • Ampicillin-sulbactam – Cure rates approaching 70% – Good patient outcomes are associated with lower severity of illness • Tigecycline – Has bacteriostatic activity against MDRA species – High-level resistance to tigecycline has been detected Maragakis & Perl. Clinical Infectious Diseases – Best reserved for salvage Session therapy 2008; 46(8): 1254 -63. 2 18

MDR Acinetobacter Treatment • Aminoglycosides (tobramycin and amikacin) – Usually used in conjunction with another active antimicrobial agent • Polymyxin B or polymyxin E (colistin) – Reported cure/improvement rates of 57 – 77% – Patient outcomes are dependent upon type and severity of infection Maragakis & Perl. Clinical Infectious Diseases 2008; 46(8): 1254 -63. Session 2 19

Manchanda V, Sanchaita S, Singh N P. Multidrug resistant Acinetobacter. J Global Infect Dis [serial online] 2010 [cited 2014 Jul 13]; 2: 291 -304. Available from: http: //www. jgid. org/text. asp? 2010/2/3/291/68538 Session 2 20

From CDC’s ANTIBIOTIC RESISTANCE THREATS in the United States, 2013

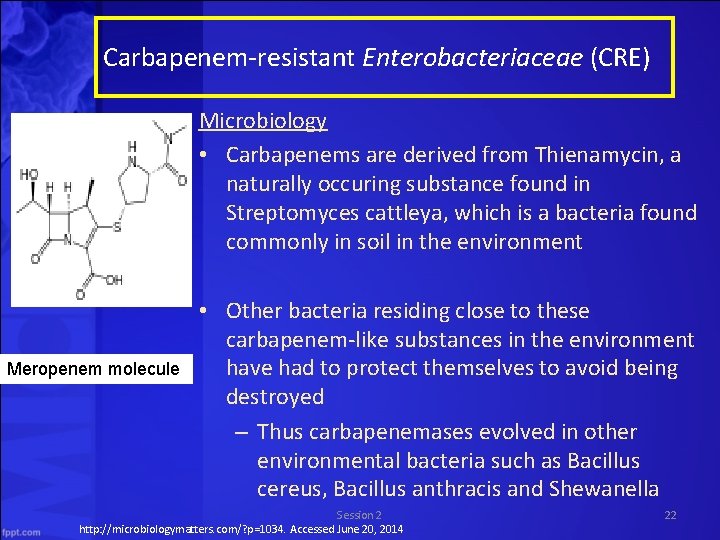
Carbapenem-resistant Enterobacteriaceae (CRE) Microbiology • Carbapenems are derived from Thienamycin, a naturally occuring substance found in Streptomyces cattleya, which is a bacteria found commonly in soil in the environment Meropenem molecule • Other bacteria residing close to these carbapenem-like substances in the environment have had to protect themselves to avoid being destroyed – Thus carbapenemases evolved in other environmental bacteria such as Bacillus cereus, Bacillus anthracis and Shewanella Session 2 http: //microbiologymatters. com/? p=1034. Accessed June 20, 2014 22

Carbapenem-resistant Enterobacteriaceae (CRE) Microbiology • Humans have taken these naturally-occuring compounds, purified and administering them in the form of carbapenem antibiotics, thus ramping up the selection pressure on the human bacterial flora http: //microbiologymatters. com/? p=1034. Accessed June 20, 2014 Session 2 23

Carbapenem-resistant Enterobacteriaceae (CRE) Microbiology • Enterobacteriaceae are a large, group of gram-negative rods – natural habitat is intestinal tract of humans and animals – Includes many genera such as escherichia, shigella, salmonella, enterobacter, klebsiella, serratia, proteus • Human enterobacteriaceae, faced with this selection pressure now “defend” against carbapenems, and have developed resistance genes from the environmental bacteria, most likely by transfer on mobile genetic elements. • Both carbapenems and carbapenemases have probably been around for millions of years. http: //microbiologymatters. com/? p=1034. Accessed June 20, 2014 Session 2 24

New Delhi Metallo-beta-lactamase (NDM 1) • First described in 2009 • NDM 1 is a carbapenemase that is carried on a plasmid along with other genes that confers resistance to nearly all antibiotics • Can be transmitted to other Enterobacteriaceae • Gastrointestinal colonization of humans • Contaminates water sources and environmental surfaces – Can contribute to transmission in the healthcare setting • Because clinical cultures identify only a fraction of patients with CRE, CDC recommends active surveillance to identify CRE colonized patients, once an infection with CRE has been documented Session 2 25

References on NDM 1 Yong D, et al. Characterization of a new metallo-beta-lactamse gene, gla ndm-1, and a novel erythromycin esterase gene carried on a unique genetic structure in Klebsiella pneumoniae sequence type 14 from India. Antimicrob Agents Chemother 2009; 53: 5046 -5054 Walsh TR et al. Dissemination of NDM-1 positive bacteria in the New Delhi environment and its implications for human health: an environmental point prevalence study. Lancet Infect Dis 2001; 11: 355362 Snitkin ES et al. Tracking a hospital outbreak of carbapenem-resistant Klebsiella pneumoniae with whole-genome sequencing. Sci Transl Med 2012; 4: 148 ra 116 Centers for Disease Control and Prevention (CDC). Guidance for Control of Carbapenem-Resistant Enterobacteriaceae (CRE). Atlanta CDC, 2012. http: //www. cdc. gov/hai/pdfs/cre-guidance-508. pdf. Session 2 26

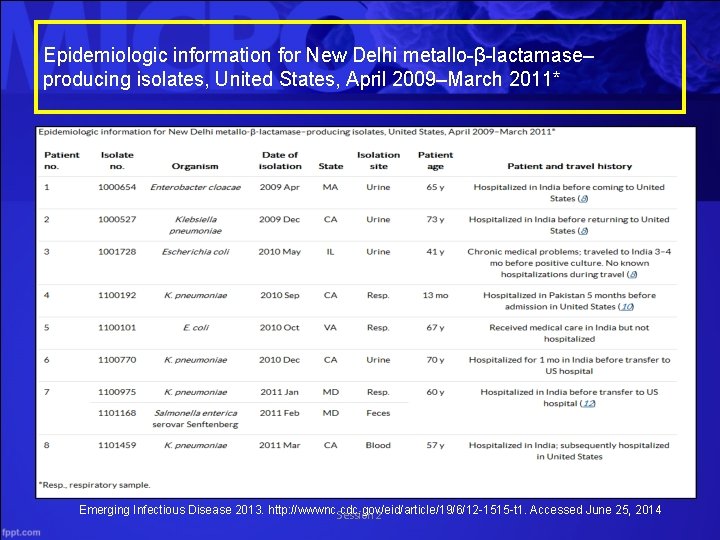
Epidemiologic information for New Delhi metallo-β-lactamase– producing isolates, United States, April 2009–March 2011* Emerging Infectious Disease 2013. http: //wwwnc. cdc. gov/eid/article/19/6/12 -1515 -t 1. Accessed June 25, 2014 Session 2

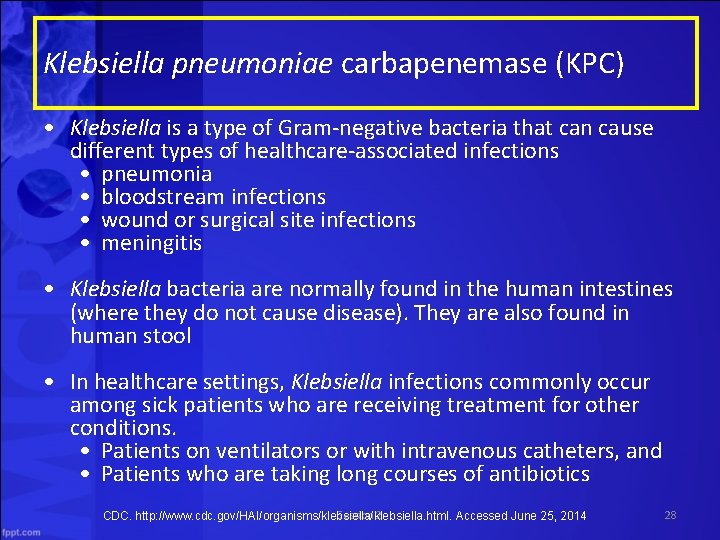
Klebsiella pneumoniae carbapenemase (KPC) • Klebsiella is a type of Gram-negative bacteria that can cause different types of healthcare-associated infections • pneumonia • bloodstream infections • wound or surgical site infections • meningitis • Klebsiella bacteria are normally found in the human intestines (where they do not cause disease). They are also found in human stool • In healthcare settings, Klebsiella infections commonly occur among sick patients who are receiving treatment for other conditions. • Patients on ventilators or with intravenous catheters, and • Patients who are taking long courses of antibiotics Session 2 CDC. http: //www. cdc. gov/HAI/organisms/klebsiella. html. Accessed June 25, 2014 28

Klebsiella pneumoniae carbapenemase (KPC) • Healthy people usually do not get Klebsiella infections • Increasingly, Klebsiella bacteria are developing antimicrobial resistance, especially to carbapenems. Klebsiella pneumoniae carbapenemase is one such mechanism of resistance Session 2 CDC. http: //www. cdc. gov/HAI/organisms/klebsiella. html. Accessed June 25, 2014 29

Klebsiella pneumoniae carbapenemase (KPC) • Infections caused by KPCs are becoming an increasingly significant problem worldwide since the first detection of these enzymes greater than a decade ago • Because carbapenems are the treatment of choice, along with an increasing incidence of fluoroquinolone resistance among Enterobacteriaceae, there has been an increased reliance on carbapenems in clinical practice Session 2 Arnold RS et al. http: //www. ncbi. nlm. nih. gov/pmc/articles/PMC 3075864/ Accessed June 25, 2014 30

Klebsiella pneumoniae carbapenemase (KPC) • In 2001, the first KPC-producing K pneumoniae isolate was reported in North Carolina. Although K pneumoniae remains the most prevalent bacterial species carrying KPCs, the enzyme has been identified in several other Gram-negative bacilli • In 2009, the Centers for Disease Control and Prevention (CDC) released a report on KPC-producing bacteria in which the term Carbapenem-Resistant Enterobacteriaceae (CRE) was proposed as more accurate, given the understanding that multiple species of Gram-negative bacteria can harbor the KPC-resistant element Session 2 Arnold RS et al. http: //www. ncbi. nlm. nih. gov/pmc/articles/PMC 3075864/ Accessed June 25, 2014 31

Klebsiella pneumoniae carbapenemase (KPC) Microbiological Testing for KPC-producing Organisms • Misidentification of KPC-producing bacteria is common with standard susceptibility testing – Automated systems identify 7 -87% of KPC-producing K pneumoniae as susceptible to imipenem or meropenem – The great variability that has been observed in carbapenem minimal inhibitory concentrations (MICs) by routine testing is likely related to the phenotypic heterogeneity among isolates, giving the appearance of susceptibility in vitro. Arnold RS et al. http: //www. ncbi. nlm. nih. gov/pmc/articles/PMC 3075864/ Accessed June 25, 2014 Session 2 32

Klebsiella pneumoniae carbapenemase (KPC) Microbiological Testing for KPC-producing Organisms • Misidentification of KPC-producing bacteria is common with standard susceptibility testing – Several groups have made the observation that an ertapenem MIC in the resistant range by standard susceptibility testing may be the most sensitive indicator for presence of a KPC. Arnold RS et al. http: //www. ncbi. nlm. nih. gov/pmc/articles/PMC 3075864/ Accessed June 25, 2014 Session 2 33

Klebsiella pneumoniae carbapenemase (KPC) Microbiological Testing • Misidentification – In two different studies, one involving 33 cases of KPCproducing Enterobacter spp and another involving 28 cases of KPC-producing K pneumoniae, determination of ertapenem MICs by automated testing identified all cases as ertapenem-resistant. – Ertapenem resistance has been found to be the most sensitive clinical test of KPC production regardless of the method used and is recommended by the CDC Arnold RS et al. http: //www. ncbi. nlm. nih. gov/pmc/articles/PMC 3075864/ Accessed June 25, 2014 Session 2 34

Klebsiella pneumoniae carbapenemase (KPC) • Confirmatory testing for KPC-producing bacteria is recommended in geographical locations where Enterobacteriaceae are noted to have decreased susceptibility to carbapenems or resistance to most noncarbapenem beta-lactams by routine testing • The most easily performed confirmatory test for KPCs is the modified Hodge test, which has been found to be 100% sensitive for the detection of a carbapenemase, although not specific for KPC production Arnold RS et al. http: //www. ncbi. nlm. nih. gov/pmc/articles/PMC 3075864/ Accessed June 25, 2014 Session 2 35

Clinical microbiology costs for methods of active surveillance for Klebsiella pneumoniae CPE (Mathers et al. ) This study assessed the costs and benefits of perirectal screening for CPE colonization at a university hospital and affiliated long-term acute care hospital (LTACH) Methods • Weekly perirectal screening was performed for all patients in selected ICUs and the LTACH, where a persistently high incidence of CPE was witnessed • Weekly surveillance also included inpatient units caring for patients known to be colonized or infected with CPE • All patients newly admitted to the LTACH and patients newly admitted to the hospital with known hospitalizations outside of the U. S. were also screened Mathers et al. ICHE 2014; 35: 350 -355 Session 2 36

Clinical microbiology costs for methods of active surveillance for Klebsiella pneumoniae CPE Three methods were assessed: 1. Direct inoculation of a swab to a chromogenic agar (CA) 2. Selection in broth with plating on CA 3. Broth selection with plating on Mac. Conkey agar (CDC method) CDC microbiologist, Alicia Shams, demonstrates Klebsiella pneumoniae growing on a Mac. Conkey agar plate. Mathers et al. ICHE 2014; 35: 350 -355 Session 2 37

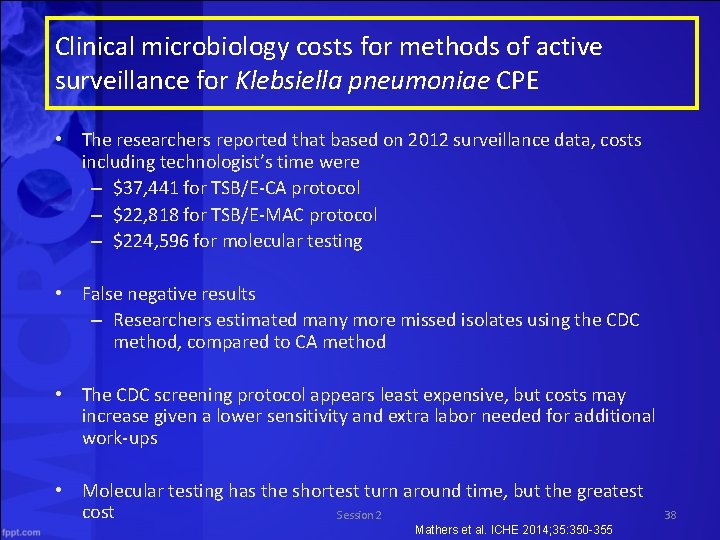
Clinical microbiology costs for methods of active surveillance for Klebsiella pneumoniae CPE • The researchers reported that based on 2012 surveillance data, costs including technologist’s time were – $37, 441 for TSB/E-CA protocol – $22, 818 for TSB/E-MAC protocol – $224, 596 for molecular testing • False negative results – Researchers estimated many more missed isolates using the CDC method, compared to CA method • The CDC screening protocol appears least expensive, but costs may increase given a lower sensitivity and extra labor needed for additional work-ups • Molecular testing has the shortest turn around time, but the greatest cost Session 2 Mathers et al. ICHE 2014; 35: 350 -355 38

Clinical microbiology costs for methods of active surveillance for Klebsiella pneumoniae CPE In conclusion • There is no perfect test for colonization with a CPE • Several factors should be considered – Test performance – Result time – Prevalence – Organism type – Mechanism of resistance – Laboratory capabilities – Cost Mathers et al. ICHE 2014; 35: 350 -355 Session 2 39

KPC Treatment Options • Clinicians increasingly dependent on polymyxins and tigecycline • Some experts suggest that high-dose continuous infusion of a carbapenem may be helpful, though evidence of efficacy is lacking • Research is needed to determine the optimal use of these antibiotics with or without other partially active antibiotics in the treatment of infections caused by KPCproducing bacteria Arnold RS et al. http: //www. ncbi. nlm. nih. gov/pmc/articles/PMC 3075864/ Accessed June 25, 2014 Session 2 40

KPC Treatment Options • Polymyxins achieve concentration-dependent bactericidal killing – often the only agents active against KPCproducing bacteria that achieve adequate levels in the serum to treat serious bloodstream infections – In the past, polymyxins were used infrequently, largely due to their associated nephrotoxicity and neurotoxicity Session 2 Arnold RS et al. http: //www. ncbi. nlm. nih. gov/pmc/articles/PMC 3075864/ 41 Accessed June 25, 2014

KPC Treatment Options • Polymyxins – There is a small amount of evidence of use of polymyxins as monotherapy for the treatment of KPCs. Three bloodstream infections during a Manhattan outbreak caused by KPC-producing K pneumoniae isolates susceptible to polymyxins were treated with polymyxin B, and one survived – Commonly used in combination with other antimicrobials, although there are no prospective data to evaluate the efficacy of this approach Session 2 Arnold RS et al. http: //www. ncbi. nlm. nih. gov/pmc/articles/PMC 3075864/ 42 Accessed June 25, 2014

• Questions? Session 2

TV Quiz! Session 2

Overview of Clostridium difficile Session 2

Introduction to Clostridium difficile Section B: In this section, we will discuss • Significance • Microbiology of C. difficile • Pathology and epidemiology including relationship to antibiotic use • Role of colonized patient & environment in transmission • Lab testing methods • Treatment options Session 2 46


Clostridium difficile Significance • C. difficile colonizes the human intestinal tract after the normal gut flora has been altered by antibiotic therapy and is the causative organism of antibiotic-associated pseudomembranous colitis • Incidence of C. difficile remains at historically high levels AHRQ. Clostridium difficile infections: diagnosis, treatment, and prevention. December 2011. AHRQ Pub No 11(12)-EHC-051 -3. Session 2 48

Clostridium difficile in Texas • CDI is a high priority healthcare associated infection because of the number of case/outbreaks and high costs – In 2009, 18, 739 hospital patients in Texas were diagnosed with CDI – On average, CDI extends length of hospital stay 8 -9 days with $11, 000 to $13, 000 additional cost Session 2 49

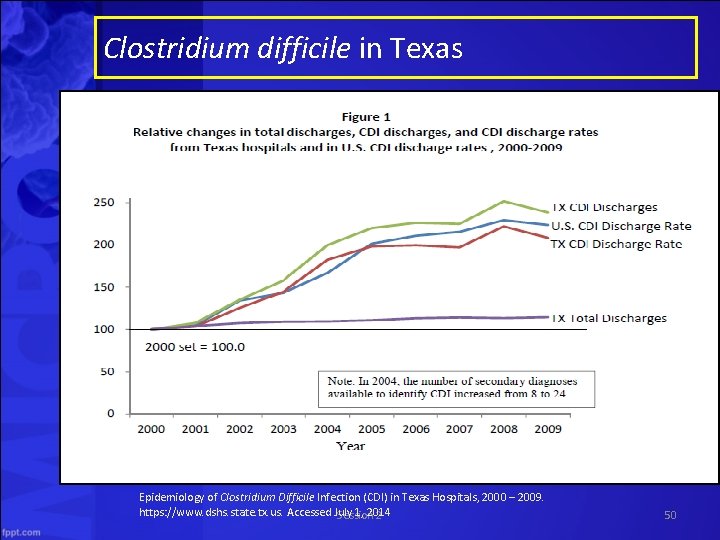
Clostridium difficile in Texas Epidemiology of Clostridium Difficile Infection (CDI) in Texas Hospitals, 2000 – 2009. https: //www. dshs. state. tx. us. Accessed July 1, 2014 Session 2 50

Clostridium difficile Epidemiology • In 1978 identified as the causative pathogen in cases – Attributed largely to clindamycin • Increasing use of penicillins and cephalosporins – Between 1989 and 1992, a strain of C. difficile highly resistant to clindamycin (the "J strain") was implicated in large outbreaks of diarrhea in four hospitals in the United States • The use of clindamycin is a specific risk factor for this strain, and colonization with the clindamycin-resistant strain increases the risk for developing C. difficile–associated diarrhea (CDAD) • From 2003 to 2006, cases became – More frequent, severe, refractory to standard therapy – More likely to relapse than previously described Session 2 51

Clostridium difficile Epidemiology in Texas • Increases in incidence and severity – Emergence of B 1/NAP 1/027, more virulent strain • Increased toxin production • High level resistance to fluoroquinolones • Previously low risk populations experiencing severe CDI • Adaptable to environmental changes • Community wide issue; not just for hospitals or long term care facilities Source: Tx DSHS. Communication July 2, 2014 Session 2 52

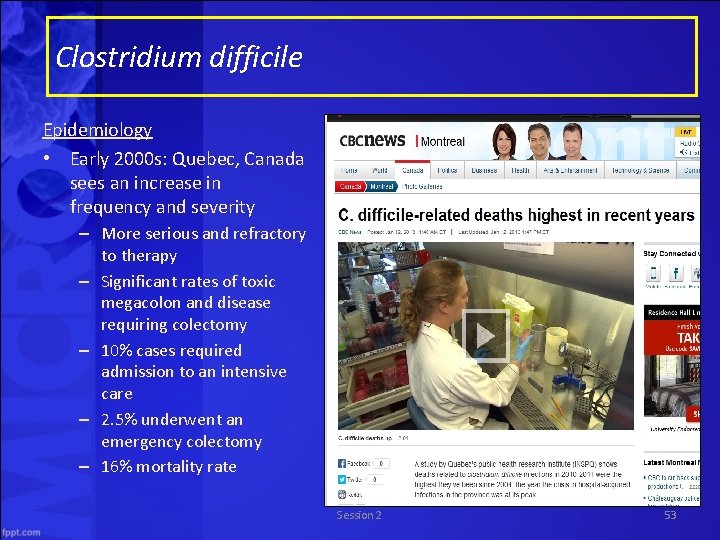
Clostridium difficile Epidemiology • Early 2000 s: Quebec, Canada sees an increase in frequency and severity – More serious and refractory to therapy – Significant rates of toxic megacolon and disease requiring colectomy – 10% cases required admission to an intensive care – 2. 5% underwent an emergency colectomy – 16% mortality rate Session 2 53

Clostridium difficile Role of Colonized Patient & Environment in Transmission • C diff spores are very hardy – Able to survive in the environment for extended periods of time – Resist the effects of alcohol gels and some disinfectants – Spread on healthcare workers’ hands Graphic Source: http: //thehappyhospitalist. blogspot. com/2011/10/c-diff-prevention-in-hospital-explained. html. Accessed June 20, 2014 Session 2 54

Clostridium difficile Lab Testing Methods • Clostridium difficile toxin is very unstable – Degrades at room temperature and may be undetectable within 2 hours after collection of a stool specimen – False-negative results occur when specimens are not promptly tested or kept refrigerated until testing can be done CDC http: //www. cdc. gov/hai/organisms/cdiff_faqs_hcp. html#a 6. Accessed June 20, 2014 Session 2 55

Clostridium difficile Lab Testing Methods • Stool culture – Most sensitive test but frequent false-positive results due to presence of non-toxigenic Clostridium difficile strains • Test isolates for toxin production (i. e. “toxigenic culture”) – Labor intensive – Require appropriate culture environment to grow anaerobic microorganisms – Slow turn-around time (48 -96 hours) CDC http: //www. cdc. gov/hai/organisms/cdiff_faqs_hcp. html#a 6. Accessed June 20, 2014 Session 2 56

Clostridium difficile Lab Testing Methods • Toxin testing – Tissue culture cytotoxicity assay detects toxin B only • Requires technical expertise to perform • Costly • 24 -48 hr for a final result • Specific and sensitive results • Less sensitive than PCR or toxigenic culture CDC http: //www. cdc. gov/hai/organisms/cdiff_faqs_hcp. html#a 6. Accessed June 20, 2014 Session 2 57

Clostridium difficile Lab Testing Methods • Toxin testing – Enzyme immunoassay detects toxin A, toxin B, or both A and B • Most labs employ a toxin B-only or A and B assay • Same-day assays • Relatively inexpensive • Easy to perform • Increasing concerns about relative insensitivity (less than tissue culture cytotoxicity and much less than PCR or toxigenic culture) CDC http: //www. cdc. gov/hai/organisms/cdiff_faqs_hcp. html#a 6. Accessed June 20, 2014 Session 2 58

Clostridium difficile Lab Testing Methods • Molecular tests: FDA-approved Polymerase Chain Reaction (PCR) assays – test for the gene encoding toxin B – highly sensitive and specific for the presence of toxinproducing C. diff CDC http: //www. cdc. gov/hai/organisms/cdiff_faqs_hcp. html#a 6. Accessed June 20, 2014 Session 2 59

PCR testing for C. difficile • The use of more sensitive and rapid testing for CDI diagnosis is critical for the clinical management of patients. • However, healthcare facilities should expect an increase in CDI rates when transitioning to a PCR-based assay and also should emphasize appropriate testing practices to avoid detection of asymptomatic carriers. • A recent study by Belmares et al. reported an almost 2 fold increase in C. difficile hospital rates and monthly C. difficile case load after the transition to PCR-based testing for CDI. Current Status of Clostridium difficile Infection Epidemiology; Clin Infect Dis. (2012) 55 (suppl 2): S 65 -S 70 doi: 10. 1093/cid/cis 319 Session 2 60

Clostridium difficile • In summary, while culturing and testing for toxin remains the gold standard for diagnosis, the need for rapid results has led many labs to rely on immunoassays that detect the presence of toxins in stool samples and recently, genetic tests that detect toxin-related genes – There is no evidence on what method is most costeffective AHRQ. Clostridium difficile infections: diagnosis, treatment, and prevention. December 2011. AHRQ Pub No 11(12)-EHC-051 -3. Session 2 61

Clostridium difficile Treatment Options • Typical manifestations of C. difficile and a positive diagnostic assay should receive antibiotics for treatment. Empiric therapy is appropriate pending results of diagnostic testing if the clinical suspicion is high • Treatment is not indicated in patients who have a positive toxin assay but are asymptomatic Session 2 Up. To. Date 2014. Wolters Kluwer Health. www. uptodate. com. Accessed June 20, 2014 62

Clostridium difficile Non Severe Disease • Initial therapy consists of oral metronidazole or oral vancomycin • Duration of therapy is recommended to be 10 to 14 days • Patients with an underlying infection requiring prolonged duration of antibiotics are at a greater risk for recurrence • Repeat stool assays are NOT warranted during or following treatment in patients who are recovering or are symptom free Session 2 Up. To. Date 2014. Wolters Kluwer Health. www. uptodate. com. Accessed June 20, 2014 63

Clostridium difficile Recurrent Disease • Recurrence – complete abatement of CDI symptoms while on appropriate therapy, followed by subsequent reappearance of diarrhea and other symptoms after treatment has been discontinued – should be distinguished from persistent diarrhea without resolution during initial therapy – 25% of cases treated with metronidazole or vancomycin; patients may experience several episodes of recurrent colitis – often occurs within 1 to 3 weeks after discontinuation of antibiotic therapy, but can occur as late as 2 -3 months AHRQ. Clostridium difficile infections: diagnosis, treatment, and prevention. December 2011. AHRQ Pub No 11(12)-EHC 051 -3. Up. To. Date 2014. Wolters Kluwer Health. www. uptodate. com. Session 2 Accessed June 20, 2014 64

Clostridium difficile Antibiotic Therapy in Recurrence • There are no rigorous studies of management for multiple recurrences of CDI. – Patients may benefit from vancomycin, fidaxomicin, or rifaximin, with or without the use of probiotics AHRQ. Clostridium difficile infections: diagnosis, treatment, and prevention. December 2011. AHRQ Pub No 11(12)-EHC-051 -3. Session 2 Up. To. Date 2014. Wolters Kluwer Health. www. uptodate. com. Accessed June 20, 2014 65

Clostridium difficile Non Antibiotic Therapy • Fecal bacteriotherapy – May be useful for treatment of patients with recurrent CDI. • Probiotic therapy – Studies are inconclusive regarding benefit • Monoclonal antibodies – Adjunctive use of monoclonal antibodies against C. difficile toxins A and B appears to reduce the recurrence rate, but they are not yet available for routine clinical use AHRQ. Clostridium difficile infections: diagnosis, treatment, and prevention. December 2011. AHRQ Pub No 11(12)-EHC-051 -3. Session 2 Up. To. Date 2014. Wolters Kluwer Health. www. uptodate. com. Accessed June 20, 2014 66

Clostridium difficile Severe Disease • Patients with acute C. difficile infection may develop signs of systemic toxicity with or without profuse diarrhea • There is no consensus definition for severe CDI – And no agreement as to the most important clinical indicators to differentiate severity Up. To. Date 2014. Wolters Kluwer Health. www. uptodate. com. Accessed June 20, 2014 Benson L et al. Changing epidemiology of clostridium difficile-associated disease in children. Infect Control Hosp Epidemiol 2007; 28: 1233 -1235 Clements ACA et al. Clostridium difficile PCR ribotype 027: assessing the risks of further worldwide spread. Lancet Infect Dis 2010; 10: 395 -404 Session 2 67

Clostridium difficile Severe Disease • Incidence is difficult to determine – In a Quebec outbreak caused by hypervirulent North American Pulsed Field type 1 (NAP 1), risk of complications was 11% – Increasingly includes low risk • Pregnant women • Children • People with no recent exposure to antimicrobials Up. To. Date 2014. Wolters Kluwer Health. www. uptodate. com. Accessed June 20, 2014 Benson L et al. Changing epidemiology of clostridium difficile-associated disease in children. Infect Control Hosp Epidemiol 2007; 28: 1233 -1235 Clements ACA et al. Clostridium difficile PCR ribotype 027: assessing the risks of further worldwide spread. Lancet Infect Dis 2010; 10: 395 -404 Session 2 68

Clostridium difficile Severe Disease • Treatment includes antibiotic therapy, supportive care, and close monitoring – Surgery may be considered if no improvement & serum lactate is >2. 2 Session 2 Up. To. Date 2014. Wolters Kluwer Health. www. uptodate. com. Accessed June 20, 2014 69

Clostridium difficile Severe Disease • Oral vancomycin is considered first line therapy for severe CDI – Major advantage of vancomycin over metronidazole: vancomycin is not absorbed, so maximal concentrations can act intracolonically at the site of infection – Metronidazole is significantly less expensive, but there is evidence that in severe or refractory cases, vancomycin has a significantly higher cure rate (97% vs 76% with metronidazole) – Fidaxomicin may be considered in patients who cannot tolerate vancomycin, but data are limited Session 2 Up. To. Date 2014. Wolters Kluwer Health. www. uptodate. com. Accessed June 20, 2014 70

Clostridium difficile Severe Disease • Severely ill patients with ileus may have markedly delayed passage of oral antibiotics from the stomach to the colon • Intravenous metronidazole may be beneficial – Intravenous vancomycin has no effect on C. diff colitis since vancomycin is not excreted into the colon Session 2 Up. To. Date 2014. Wolters Kluwer Health. www. uptodate. com. Accessed June 20, 2014 71

Clostridium difficile Fecal Transplants • CDC suggests that restoring ‘normal bacteria’ via fecal transplants may be an effective treatment for recurrent, debilitating CDI • CDC is working with other government agencies to translate microbiome science into practical infection prevention Mc. Donald C. Using fecal transplants to treat recurrent Clostridium difficile infections. CDC. Session 2 http: //blogs. cdc. gov/safehealthcare/2012/01/17/using-fecal-transplants. Accessed June 20, 2014 72

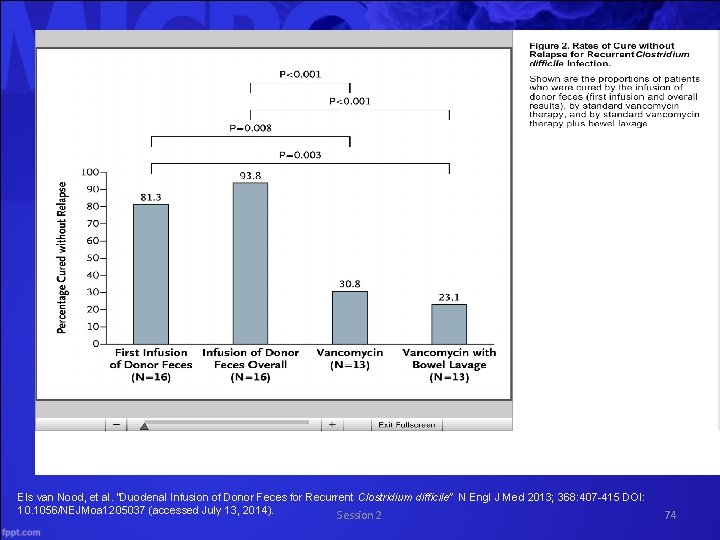
Duodenal Infusion of Donor Feces for Recurrent Clostridium difficile Recurrent refractory CDI • Randomly assigned one of 3 treatment options • Vancomycin and bowel lavage and fecal transplant via nasoduodenal tube • Vancomycin only • Vancomycin and bowel lavage • Primary end point was resolution of diarrhea associated with CDI without relapse for 10 weeks • Stopped early after interim analysis showed overwhelming evidence that transplant was superior Els van Nood, et al. “Duodenal Infusion of Donor Feces for Recurrent Clostridium difficile” N Engl J Med 2013; 368: 407 -415 DOI: 10. 1056/NEJMoa 1205037 (accessed July 13, 2014) Session 2 73

Els van Nood, et al. “Duodenal Infusion of Donor Feces for Recurrent Clostridium difficile” N Engl J Med 2013; 368: 407 -415 DOI: 10. 1056/NEJMoa 1205037 (accessed July 13, 2014). Session 2 74

Questions? Session 2
 Cadaveric spasm and rigor mortis difference
Cadaveric spasm and rigor mortis difference Clostridium botulinum reino
Clostridium botulinum reino Botulinum
Botulinum Morfologi clostridium perfringens
Morfologi clostridium perfringens Medio de cultivo para clostridium perfringens
Medio de cultivo para clostridium perfringens G
G Tetanus
Tetanus Cuantificacion
Cuantificacion Measles vs chicken pox
Measles vs chicken pox Clostridium tetani
Clostridium tetani L
L Lofotrica
Lofotrica Tsi
Tsi Membrane
Membrane Clostridium tetani
Clostridium tetani Microbiology
Microbiology Clostridium perfringens sintomas
Clostridium perfringens sintomas Clostridium sporogenes
Clostridium sporogenes Clostridium welchii
Clostridium welchii Clostridium perfringens ppt
Clostridium perfringens ppt Clostridium spores
Clostridium spores What is deca's mission
What is deca's mission It infrastructure and emerging technologies
It infrastructure and emerging technologies The byzantine empire and emerging europe
The byzantine empire and emerging europe It infrastructure and emerging technologies
It infrastructure and emerging technologies Bridging expanding emerging
Bridging expanding emerging Byzantine empire 1300
Byzantine empire 1300 Emerging technology chapter 2
Emerging technology chapter 2 It infrastructure and emerging technologies
It infrastructure and emerging technologies Chapter 5 it infrastructure and emerging technologies
Chapter 5 it infrastructure and emerging technologies Jeffrey arnett emerging adulthood theory
Jeffrey arnett emerging adulthood theory Emerging media definition
Emerging media definition Emerging database technologies and applications
Emerging database technologies and applications Emerging database technologies and applications
Emerging database technologies and applications The byzantine empire and emerging europe
The byzantine empire and emerging europe Clarivate analytics emerging sources citation index
Clarivate analytics emerging sources citation index Emerging technology synthesis
Emerging technology synthesis Emerging issues in community development
Emerging issues in community development Explain emerging patterns of state politics in india
Explain emerging patterns of state politics in india Emerging adulthood psychosocial development
Emerging adulthood psychosocial development Emerging proficient extending
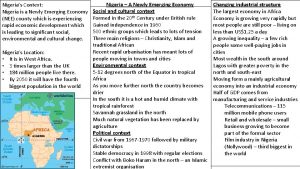
Emerging proficient extending Is nigeria a newly emerging economy
Is nigeria a newly emerging economy Levinson's stages
Levinson's stages Emerging role of finance manager in india
Emerging role of finance manager in india Business intelligence market trends
Business intelligence market trends Introduction to industrial relations
Introduction to industrial relations Emerging adulthood cognitive development
Emerging adulthood cognitive development Emerging technology chapter 5
Emerging technology chapter 5 Arts of emerging europe
Arts of emerging europe Jeffrey arnett emerging adulthood theory
Jeffrey arnett emerging adulthood theory Emerging technologies introduction
Emerging technologies introduction Nhmrc emerging leadership fellow
Nhmrc emerging leadership fellow Emerging technology chapter 4
Emerging technology chapter 4 Chapter 22 life in the emerging urban society
Chapter 22 life in the emerging urban society Soft trends in software engineering
Soft trends in software engineering Emerging issues in taxation
Emerging issues in taxation Key roles for the new big data ecosystem
Key roles for the new big data ecosystem Emerging trends in business intelligence implementation
Emerging trends in business intelligence implementation Atlas of emerging jobs
Atlas of emerging jobs Dubai emerging market
Dubai emerging market Grundwasserreinigung emerging contaminants
Grundwasserreinigung emerging contaminants Emerging trends in strategic management
Emerging trends in strategic management Memory technology in computer architecture
Memory technology in computer architecture World heart federation emerging leaders
World heart federation emerging leaders Swinburne emerging leader
Swinburne emerging leader Renaissance emerging markets fund
Renaissance emerging markets fund Emerging trends in society
Emerging trends in society Challenges of emerging trends in operating systems
Challenges of emerging trends in operating systems Challenges of international business in emerging markets
Challenges of international business in emerging markets Global fund flows
Global fund flows Emerging technology advisors
Emerging technology advisors Emerging trends in disaster mitigation
Emerging trends in disaster mitigation 8 emerging technologies
8 emerging technologies Marketing strategies for emerging markets
Marketing strategies for emerging markets Institutional risks
Institutional risks Risks of doing business in emerging markets
Risks of doing business in emerging markets